Abstract
Lymphedema is a progressive condition. Its therapy aims to reduce edema, prevent its progression, and provide psychosocial aid. Nonsurgical treatment in advanced stages is mostly insufficient. Therefore—in many cases—surgical procedures, such as to restore lymph flow or excise lymphedema tissues, are the only ways to improve patients’ quality of life. Imaging modalities: Lymphoscintigraphy (LS), near-infrared fluorescent (NIRF) imaging—also termed indocyanine green (ICG) lymphography (ICG-L)—ultrasonography (US), magnetic resonance lymphangiography (MRL), computed tomography (CT), photoacoustic imaging (PAI), and optical coherence tomography (OCT) are standardized techniques, which can be utilized in lymphedema diagnosis, staging, treatment, and follow-up. Conclusions: The combined use of these imaging modalities and self-assessment questionnaires deliver objective parameters for choosing the most suitable surgical therapy and achieving the best possible postoperative outcome.
Keywords:
lymphedema; lympho-venous anastomosis (LVA); lymphoscintigraphy (LS); near-infrared fluorescent (NIRF) imaging; indocyanine green (ICG); ultrasonography (US); magnetic resonance lymphangiography (MRL); computed tomography (CT); photoacoustic imaging (PAI); optical coherence tomography (OCT) 1. Introduction
Lymphedema is a progressive condition with protein-rich interstitial fluid accumulation due to impaired lymphatic drainage [1,2,3,4,5,6].
Its symptoms vary on a scale well presented in the International Society of Lymphology (ISL) staging system [7] (Appendix A). Recent studies have indicated that ISL staging, based solely on clinical signs, cannot describe lymphedema severity precisely, and it does not correlate well with quality of life (QoL) [7]. Therefore, the combination of various lymphedema evaluations provides a more accurate diagnosis, and imaging modalities are of the utmost importance.
Lymphedema assessment can be carried out using the following:
- Objective measurements [5]: limb volume measurement by perometry/CT/water dis-placement, skin tonometry, bioimpedance spectroscopy (BIS, L-dex score), bioimpedance analysis (BIA), and tissue dielectric constant (TDC);
- Imaging modalities (see Section 2);
- Self-assessment questionnaires: lymphedema life impact scale (LLIS) [8], lower limb functional index (LLFI) [9], lower extremity functional scale (LEFS) [10], disabilities of the arm, shoulder, and hand (DASH) [11], International Classification of Functioning, Disability and Health (ICF) [12], and general quality of life (QoL) [5,7,13,14].
With more precise and earlier diagnosis, individualized lymphedema treatment will be able to efficiently reduce edema volume, prevent further fluid accumulation, and provide psychosocial support.
The gold standard treatment is complex physical therapy, also called complete decongestive therapy/CDT (manual lymphatic drainage, exercise, use of non-elastic wrappings and compressive garments, skin care) [2,4], intermittent pneumatic compression [2,15], compressive garments [16], heat therapy [2,4,17,18], and extracorporeal shock-wave treatment [19]. Pharmacological agents supporting lymph drainage are studied; currently, there is no specific, FDA/EMA-approved drug [6,18]. If any of the conservative therapies fail, an exact lymphedema evaluation will provide more information to design an individualized surgical treatment. In this regard, imaging modalities are crucial for diagnosis, staging, intraoperative imaging, and follow-up. Surgical therapy consists of conventional and microsurgical procedures, and it aims to increase lymph outflow of the swollen areas and—in the severest cases—to debulk the amount of excess tissue [5]. Lymph drainage can be enhanced (1) by anastomoses between the lymphatic and venous systems via direct lympho-venous anastomoses (LVA) or lymph-node-venous anastomoses (LNVA) [20]—in multiple centers as a preventative measure (LYMPHA); (2) by restoring lymph vessel patency in locally interrupted lymph systems with lympho-lymphatic anastomoses (LLA) or a venous segment interposition (LVLA) [21]; or (3) by vascularized lymph node (VLNT), vessel (VLVT) or system (LYST) transfer. Liposuction—possibly lymph vessel sparing— and surgical resection (“debulking”) aim to decrease the amount of excess fat, fibrotic subcutaneous tissue, and skin.
In this paper, the authors describe all current imaging modalities for diagnosis, therapy planning, and follow-up documentation and provide information on each of their indications for their usage, functionality, practicability, and limitations.
2. Imaging Modalities
2.1. Ultrasonography (US)
Ultrasonography (US) is the modality of choice to exclude venous components in lymphedema. Furthermore, it is a valuable tool for assessing lymphedema and tissue composition, identifying veins and lymphatic vessels suitable for LVA (Figure 1), and can also be useful for lymphatic tissue transfer by providing information on flap anatomy [22]. The latter examinations are frequently performed using high-frequency (HFUS) and ultra-high-frequency (UHFUS) (48–70 MHz) probes.
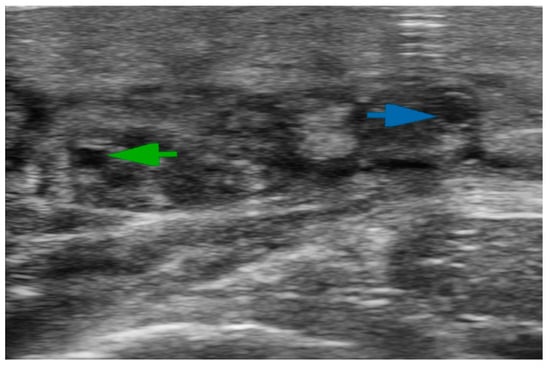
Figure 1.
Ultra-high-frequency ultrasound (48 MHz probe) accurately reveals lymphatic vessels and neighboring veins. The green arrow indicates the lymphatic collector; the blue arrow shows the vein.
Increased dermal thickness and tissue stiffness due to fibrosis are characteristic of chronic lymphedema and can be examined via conventional B-mode US and ultrasound elastography [23], respectively. Sonographic localization of fluid and solid predominant regions in the lymphedematous limb is crucial since we expect the most volume reduction by positioning physiologic procedures (such as LVA surgeries and lymphatic tissue transfers) in the regions of excessive fluid accumulation. Preoperatively identifying adjacent superficial comparable-diameter venules [24] and ectasis-type lymph vessels—according to normal, ectasis, contraction, sclerosis type (NECST) classification [25]—significantly increases the success rate of LVA operations [24,25,26,27].
Contrast-enhanced ultrasonography (CEUS) is the most recent sonographic tool for detecting sentinel lymph nodes and lymphatic vessels [28,29,30]. CEUS, HFUS, and UHFUS are effective means of planning LVA procedures by revealing candidate venules, the most optimal regions, and lymphatic vessels, and are—in many cases—superior alternatives to ICG lymphography [28,31,32,33].
The limitations of the US are a steep learning curve, dependency on the examiner’s skill, and the low tissue penetration of UHFUS (23.5 mm for 48 MHz and 10 mm for 70 MHz [34]).
2.2. Lymphoscintigraphy (LS)
Lymphoscintigraphy (LS) is a nuclear medicine modality, and it has been the gold standard for confirming the diagnosis of lymphedema for several decades. It is a procedure where a gamma-ray-emitting technetium 99 m labeled compound is injected intradermally/subcutaneously/subfascially, and its distribution is subsequently detected with a gamma camera. (Figure 2) Its limitations are the lack of standardized protocol, inferior spatial resolution, the presence of ionizing radiation, and accessibility. The lack of standardized protocol differences in the selection of the radiotracer, dose, injection site, acquisition time, and dynamic or static acquisition after rest or exercise deters the cross-center reproducibility of the lymphoscintigraphic results [35].
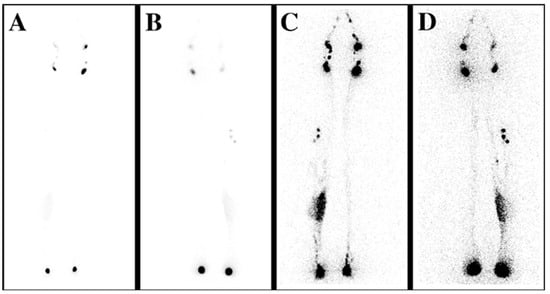
Figure 2.
Lymphoscintigraphy (LS) reveals the lymphatic flow insufficiency. (A) Anteroposterior (AP) transmission LS scan shows the early distribution of the tracer; proximal lymph nodes can be observed. (B) Posteroanterior (PA) scan, early distribution. (C) AP LS scan demonstrates the late distribution of the isotope, showing lymphatic retention on the medial calf on the right side. (D) PA scan, late distribution.
There are multiple 99 mTc-labeled tracers, such as albumin-nanocolloid, sulfur colloid, phytate, antimony sulfide, and so forth, with different particle sizes [35,36,37,38]. Furthermore, 50–70 nm is considered the optimal diameter since such particles can enter the lymphatic system but not the blood capillaries [38]. This value ranges between 10 and 100 nm, according to another publication [37].
Lymphoscintigraphy delivers both qualitative and quantitative interpretations. It describes the lymphatic morphology—recommended with colloidal tracers, such as the number of proximal lymph nodes, the quantity and course of lymphatic vessels, the presence of collateral lymphatic flow, and the characteristics of dermal backflow [35]. The measurement of the tracer uptake in the proximal lymph nodes, tracer clearance from the injection site, and appearance in the blood are the quantitative parameters of lymphoscintigraphy. Comparison between the affected and unaffected limb, if possible, provides invaluable data, both in qualitative and quantitative examinations. The relevance of quantitative lymphoscintigraphy is questionable in everyday use since the diagnosis of lymphedema can be confirmed via qualitative lymphoscintigraphy, the quantitative measurements are time-consuming, and their results are often inconsistent [39].
Greater sensitivity and better three-dimensional spatial resolution can be achieved by combining LS with single-photon emission computed tomography (LS-SPECT) or computed tomography [40].
The available lymphoscintigraphic staging systems aiming to help decision making and follow-up are largely intricate for everyday interpretation, but they can be useful for specific indications. They do not have a clear relation to lymphedema severity and treatment outcome. The most recent LS staging system is the Taiwan Lymphoscintigraphy Staging (TLS), based on the visualization of (1) proximal/intermediate lymph nodes, (2) linear lymphatic vessels, and (3) dermal backflow; LS acquisition protocol is detailed in [40] (Figure 3). (TLS differentiates lymphedema into three patterns and seven stages: L0, normal drainage; P1-3, partial obstruction; T4-6, total obstruction.) Cheng’s Lymphedema Grading helps lymphedema surgical treatment-related decision making and is based on imaging (TLS, ICG findings) and clinical signs (duration of symptoms, circumferential and CT-based volumetry, and frequency of cellulitis) [41]; see Table 1.
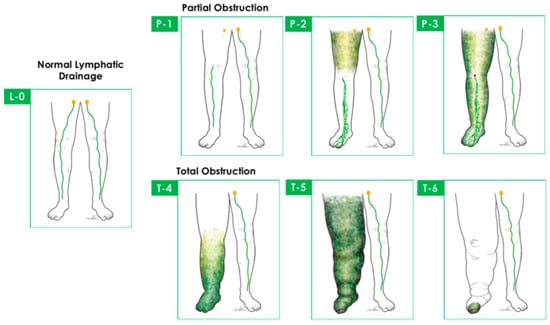
Figure 3.
Taiwan Lymphoscintigraphy Staging differentiates lymphedema into 3 patterns and 7 stages: L0, normal drainage; P1–3, partial obstruction; T4–6, total obstruction. Cited with permission [41].

Table 1.
Cheng’s Lymphedema Grading System helps lymphedema surgical treatment–related decision-making and is based on imaging and clinical signs [41].
2.3. Indocyanine Green Lymphography (ICG-L)
Indocyanine green (ICG) lymphography (ICG-L), also termed near-infrared fluorescent (NIRF) imaging, is a mainstay tool in many centers for diagnosing and assessing lymphedema. NIRF can detect superficial lymphatics (imaging depth: 1–2 cm) pre-, intra-, and postoperatively in real time. Therefore, it is a helpful tool for reconstructive surgery planning, intraoperative imaging, mapping, reverse mapping, and lymphatic vessel-sparing liposuction [5,42].
ICG is a fluorescent, water-soluble dye with a molecular weight of 774.96 Da and great affinity to plasma lipoproteins (maximum absorption wavelength: 790 nm; maximum emission wavelength: 835 nm). It has a low tissue and vascular wall permeability due to its binding to larger molecular weight plasma proteins (intensely to HDL, moderately to LDL) [43]. It was developed by Kodak Research Laboratories for examining cardiac output, but it gained relevance in assessing liver function, cerebral circulation, and retinal circulation. This sodium-iodine-containing chromodiagnostic solution is excreted in the liver and considered safe intravenously, and if extravasated, with a low incidence of complications; however, it should be used with caution in patients with liver and kidney disease and in dialyzed patients, and it should be omitted in patients with iodine sensitivity, thyroid tumor, or hyperactivity. It is FDA approved for intravenous use (in lymphography, ICG is used off-label) and was classified as pregnancy-risk category C; however, some studies contradict its toxicity for the fetus [44,45,46].
NIRF protocols vary in different centers. It is commonly administered in or around the lymphedematous area or in the second and fourth interdigital space of the extremities. The lymphatic uptake and flow can be promoted using the fill and flush technique [47] or active movement. Early distribution can be examined right after administration; the late plateau phase can be imaged 2 to 18 h after the injection. Patent, non-leaking lymphatic vessels are depicted as linear patterns, dilated lymphatics show as a splash, minor lymphatic extravasation is visualized as stardust, and extensive extravasation is expressed as a diffuse dermal backflow pattern.
A qualitative staging system was introduced based on the ICG dermal backflow patterns [48,49,50,51], as shown in Table 2 and Table 3. Though ICG staging does not correlate well with the ISL stage, bioimpedance spectroscopy findings, limb volume difference, and LLIS, it is a highly sensitive method to diagnose lymphatic dysfunction, even in a subclinical phase [14,51].

Table 2.
ICG lymphography staging based on dermal backflow patterns in lower extremity [48,49].

Table 3.
ICG lymphography staging based on dermal backflow patterns in upper extremity [52].
Quantifying ICG lymphography can be performed by measuring transit times [53] and contractility [54]. However, these quantitative ICG procedures have not gained widespread clinical acceptance.
Indocyanine green lymphography (ICG-L) is useful for planning LVA procedures; microscope-integrated ICG-L can facilitate intraoperative dissection and ensure the patency of the anastomosis (Figure 4 and Figure 5).
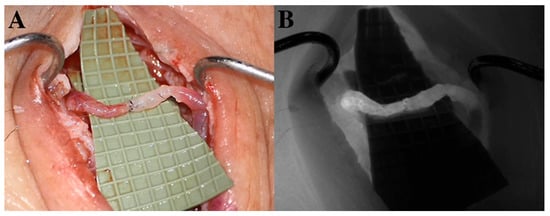
Figure 4.
(A) Lymphovenous anastomosis (LVA). An ecstatic lymphatic vessel (left side) is anastomosed to a reflux-free vein (right side). Wash-out of blood can be observed from the distal part of the vein to the patent venous valve. (B) Microscope-integrated intraoperative ICG-L ensures the patency of lympho-venosus anastomosis.
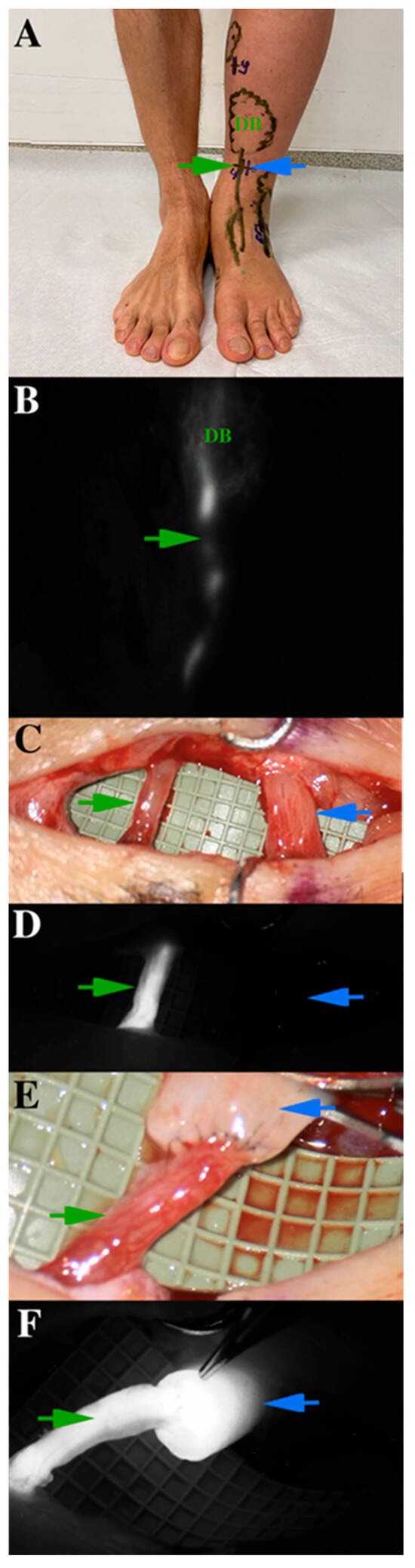
Figure 5.
Presentation of indocyanine green (ICG) lymphography on a specific lymphatic vessel, which is for lymphovenous anastomosis operation. (A) ICG lymphography markings on the skin (the green arrow indicates the lymphatic vessel, the blue arrow shows at the vein). Linear and dermal backflow (DB) patterns are indicated differently. (B) ICG lymphography patterns on the same region. (C) The previously detected lymphatic collector is dissected and verified under microscope. (D) Microscope-integrated ICG lymphography shows the functional lymphatic vessel. (E) The anastomosis is made with 11-0 non-absorbable monofil sutures. (F) Microscope-integrated ICG lymphography ensures the patency of the anastomosis and excludes any leakage. Lymphatic flow washes out blood from the vein.
The limitations of ICG imaging are its small detection depth, the suboptimal parameters of the ICG molecule (low quantum yield, poor stability, self-quenching), and its iodine content [37]. Furthermore, it can visualize just those parts that drain from the injection site.
2.4. Magnetic Resonance Imaging Lymphography (MRL)
Magnetic resonance imaging lymphography (MRL) is a highly sensitive tool for diagnosing fluid accumulation and adipose hypertrophy. It reveals the location of lymphatics, depicts the extent of dermal backflows, and is useful in identifying venous obstruction and occult metastases in 3D without radiation exposure, as shown in Figure 6 [37,51,55,56].
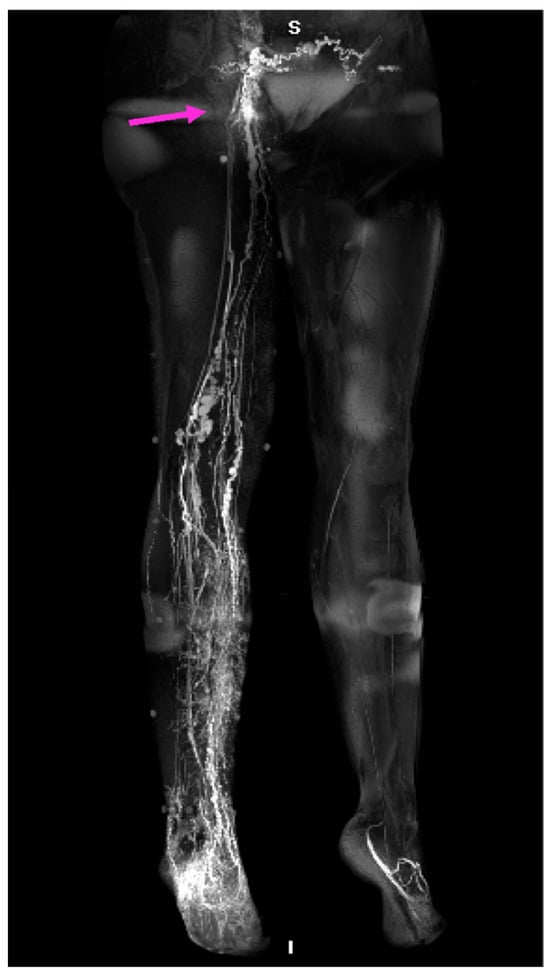
Figure 6.
MRL of a patient with lower limb primary lymphedema. Hyperplasic lymphatic vessels and inguinal lymph nodes (arrow) of the right lower extremity are visible [55].
Non-contrast MRI lymphography uses heavily T2-weighted sequences to highlight signals from the accumulated fluid and depress the signal from solid tissues. It has been utilized to visualize central lymphatics efficiently [57]. Chemical exchange saturation transfer (CEST) MRI uses the protons of amide groups in protein-rich interstitial environments as a contrast, thus being specific for protein-rich interstitial fluid accumulation in lymphedema [58]. For the further enhancement of lymphatic specificity of non-contrast MRI, arterial spin labeling was attempted to assess lymphatic flow velocity [37,56,59].
Contrast-enhanced magnetic resonance lymphography starts with T2-weighted pre-tracer imaging. Subsequently, a gadolinium-based tracer is injected interstitially, and the positive enhancement signal is detected on T1-weighted images. The contrast agent can be administered intranodal, as with X-ray lymphography, for a better visualization of the central or hepatic lymphatics [37]. The Gd-based MR contrast agents have a low molecular weight (<1 kDa). Therefore, they are absorbed by blood vessels and lymphatic vessels, posing difficulty in differentiating between these vascular systems on contrast-based MRL images. Differences between the anatomy of these systems, the delayed uptake in lymphatics compared to blood vasculature revealed on a series of dynamic images, or a second MRI with an intravenous administration of the tracer (delayed MR lymphogram) can help the identification. Furthermore, the so-called dark-blood technique helps differentiate lymphatics and blood vessels by applying an iron-based tracer intravenously and a Gd-based tracer intradermally. The iron-based contrast agent suppresses the venous enhancement [60].
MRL is a valuable tool in confirming the diagnosis of lymphedema and a supportive tool in lymphatic surgery planning [60]. It helps differentiate between fluid- or solid-predominant areas; therefore, it supports identifying the areas that may benefit from reconstructive surgery or excess-tissue removal. Furthermore, it is an invaluable tool in lymphatic vessel and vein mapping before LVA procedures and in donor- and recipient-site assessment in free lymphatic tissue transfer.
The limitations of MRL include its affordability, extensive exposure time, the risk of contrast allergy, and contraindicated utilization in patients with severe kidney disease or metal implants. Furthermore, the low specificity of the contrast agent for lymphatics due to the low molecular weight of the tracer makes selective MR lymphography difficult and emphasizes the need for improved lymphatic-specific tracers [37].
An MRL staging system was reported, but its clinical relevance is still under investigation [61].
2.5. Computed Tomography (CT)
Computed tomography and computed tomography angiography have a supportive role in lymphedema diagnostics. They can detect venous etiology and incidental malignancies and are useful in locating perforator vessels during the preparation for lymphatic tissue transfer [62]. CT can evaluate the excess fibrous tissue and the presence and severity of edema; however, it cannot differentiate between lymphedema and edema [56].
2.6. Photoacoustic Imaging (PAI)
Photoacoustic imaging or optoacoustic imaging—a promising imaging modality—combines optical absorption and ultrasound detection (“light in, sound out”). The examined object is illuminated with non-ionizing, short-pulsed light. The absorbed light generates heat and thermal expansion in the illuminated tissue, which in turn creates ultrasound waves. Therefore, this scalable method captures light-absorbing components (e.g., melanin, hemoglobin, ICG) in real time.
Photoacoustic microscopy provides high spatial resolution at the cost of detection depths, while photoacoustic tomography can penetrate several cm deep but provides imaging with lower resolution.
Knowledge of the absorption spectra of the molecules of interest and the background allows multispectral imaging—multispectral optoacoustic tomography (MSOT)—thus imaging the lymphatics and blood vessels simultaneously [37,63,64,65].
Kajita et al. visualized lymphatics 0.2 mm in diameter at a maximal depth of approximately 2 cm with a robust imaging appliance capable of detecting at two wavelengths [64,66], as shown in Figure 7. Giacalone et al. used a handheld device with similar tissue penetration parameters and capability to monitor seven wavelengths for observing the position and contractility of lymphatic vessels, assuring ICG mapping, and selecting incision sites before LVA [63].
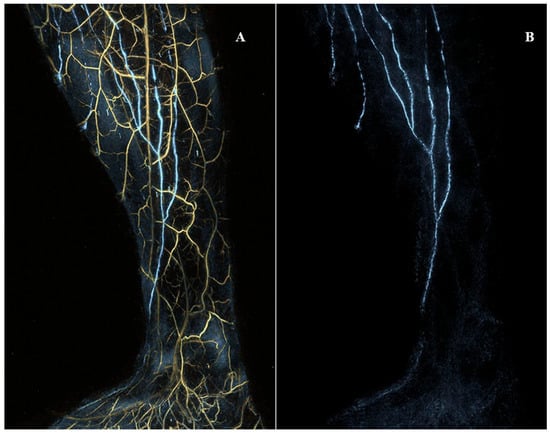
Figure 7.
The medial view of the right lower extremity of a woman in her thirties without any past medical history registered with photoacoustic/optoacoustic lymphangiography. (A) Both venules and lymphatic vessels are shown. (B) Only lymphatic vessels are shown. Cited with permission. [65].
PAI allows the exact visualization of lymphatic vessels without ionizing radiation exposure, in real time, even in the regions of dermal backflows, where the NIRF camera detects just splash or diffuse ICG accumulation.
Despite its limited penetration (approx. 2 cm), PAI is a promising tool and has the potential to gain widespread utilization in medicine.
2.7. Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT), also called laser tomography, is an optical tomographic imaging modality based on low-coherence interferometry; basically, the backscattering is detected from an illuminated object (similarly to B-mode in ultrasound).
OCT was first introduced for ex vivo imaging in 1991 and applied in medicine later in that decade [67,68]. Since then, it has been a mainstay tool in ophthalmology for examining the tissues of the fundus and is becoming a standard tool for atherosclerosis assessment in interventional cardiology [68]. As a potential imaging modality, it was recently introduced in dermatology [69] and lymphology [70]. The probing depth reaches 2 cm in transparent scattering media (e.g., eye) and 1–2 mm in highly scattering tissues (e.g., skin) at a micrometer-precise resolution [67].
In the microscope-integrated OCT system utilized intraoperatively in LVA surgeries by Hayashi et al., this modality has an imaging depth of 2.5 mm and an axial resolution of less than 4 μm [70] and provides information on the number of lymphatic lumens in a dissected area, wall thickness, diameter, luminal obstruction, and valves of lymphatic vessels and improves the patency of LV anastomosis (Figure 8).
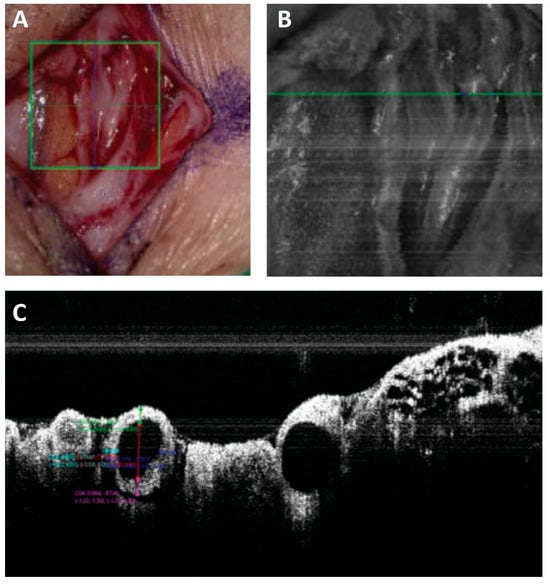
Figure 8.
Lymphatic vessel photographed (A), the green square in A shows the area imaged with a microscope-integrated NIRF camera (B) and the green line in B indicates the position of the crossectional image captured with a microscope-integrated OCT (C). The diameter and wall-thickness of vessels can be measured precisely on the OCT images [70].
Currently, ICG-enhanced lymphatic vessels are considered functional and applicable for LVA. Yang et al. proposed that lymphatic-flow-positive but non-ICG-enhanced lymphatic vessels could be considered functional and suitable for LVA [71]. In this regard, OCT has the potential to supplement the data acquired with ICG and extend the number of candidate lymphatic vessels for LVA.
2.8. Tracer Design and Delivery
Contrast agents with a molecular size of 10–100 nm are considered suitable for lymphatic imaging. The currently approved tracers for human examinations, such as gadolinium-based MRI tracers, isosulphane blue, and ICG fall within this criterion, but they are located at the bottom end of the scale in terms of molecular size. Therefore, their specificity for lymphatic vessels is low [37]. (Interestingly, ICG shows better lymphatic specificity clinically due to its strong affinity for HDL, LDL, and other plasma proteins [43]). In preclinical settings, the encapsulation of the contrast agents in liposomes, micelles, calcium phosphate particles, pre-complexation with polymers, and covalently binding to larger molecules (e.g., PEG) was attempted to increase their lymphatic specificity [37].
Lymphatic contrast agents are administered interstitially (e.g., intradermal injection) or directly into the lymphatic system. There is no standardized protocol for tracer delivery in lymphology practices. One of the most common injection methods is intradermal injection into finger-webs [6]. Commercially available microneedle devices allow pain-free and controllable intradermal tracer injection (e.g., MicronJet 600 by Nanopass Ltd. (Nes Ziona, Israel) [72] and SOFUSA (Sandy Springs, GA, USA) [73]).
3. An Imaging-Based Lymphedema Treatment Protocol
In our tertiary care center specializing in lymphedema treatment, 376 lymphedema surgeries—360 physiological reconstructive surgeries (LVA, VLNT, and 16 debulking operations), liposuction, Charles procedure, and tissue resections—have been performed since October 2017. Considering the scientific data and the distinct characteristics of various imaging methods– shown in Table 4, we developed an algorithm for preoperative assessment.

Table 4.
Imaging modalities for lymphedema evaluation and therapy.
Patients are referred from a physical therapy center, where US, mammography, CT, and MRI are used to exclude any active oncological diseases. LS, with its quantitative measurements, is carried out to confirm the diagnosis of lymphedema. The US is routinely performed to rule out venous outflow abnormalities.
Since our approach is to perform minimal invasive physiological procedures as a first step, if possible, a standardized HFUS or UHFUS examination is performed for screening candidate functional lymphatic vessels and reflux-free veins for LVA surgery in fluid-predominant regions. The LVA operation not only restores lymphatic flow but is a diagnostic procedure, where intraoperative evaluation of the lymphatic vessel state (sclerosis, ectasis, flow, and backflow) and the characteristics of surrounding tissue (fibrosis and dermis thickness) will be carried out, and if necessary, further LVA operation will be planned, based on this intraoperative diagnostic.
In total, 4–6 possible LVA spots are generally marked on the skin for each patient. Multiple lymphosome ICG-L is performed preoperatively in the operating room. We plan skin incisions based on the US and ICG mapping. In case there is an ICG uptake in the dissected lymphatic vessel, a microscope-integrated infrared fluorescence camera facilitates the dissection and ensures the patency of the anastomosis. Should we not find any lymphatic vessels with US or ICG-L for LVA surgery (1%) or LVA surgery cannot ensure a probable positive postoperative outcome, we schedule patients for VLNT operation alone or VLNT and LVA procedures combined.
4. Conclusions
The International Society of Lymphology (ISL) staging system for lymphedema is based just on clinical signs alone and does not provide information necessary for clinical decision-making. Objective measurements (circumference, volume, bioimpedance, dielectric constant), imaging (ultrasound—US; indocyanine green lymphography—ICG-L; lymphoscintigraphy—LS; magnetic resonance imaging—MRI; photoacoustic imaging—PAI; optical coherence tomography—OCT), and self-reporting (lymphedema life impact scale—LLFI; lower limb functional index—LEFS; disabilities of the arm, shoulder, and hand—DASH; international classification of functioning, disabilities and health—ICF; quality of life—QoL) assist in choosing the best therapy for the patient. The preoperative use of US [33], ICG-L [74], MRL [60], LS [39] solely and the combined use of US and ICG-L [27], ICGL and LS [41,75], MRL and ICG-L [55], and ICG-L and LS and MRI and CT [56] has been reported. ICG-L and MRI are superior in diagnosing lymphedema compared to LS or CT [56,57], and the US is applicable as a standalone imaging modality in the localization of LVA-candidate vessels [33]. It is suggested to use combined imaging modalities in lymphedema diagnostics and for establishing a treatment strategy [55,76]. The intraoperative use of ICG-L, PAI, or OCT can be helpful for lymphatic surgeries. Contrast-enhanced ultrasonography (CEUS), chemical exchange saturation transfer (CEST) MRI, multispectral optoacoustic tomography (MSOT), and OCT have the potential to become mainstay tools in lymphedema treatment.
Author Contributions
Conceptualization, C.-H.J.T. and B.M.; validation, C.-H.J.T. and B.M.; investigation, C.-H.J.T., B.M. and B.I.N.; resources, C.-H.J.T.; data curation, B.M. and B.I.N.; writing—original draft preparation, B.I.N.; writing—review and editing, C.-H.J.T., B.M. and B.I.N.; supervision, C.-H.J.T.; project administration, C.-H.J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
International Society of Lymphology Stages classify lymphedema based on clinical signs.
Table A1.
International Society of Lymphology Stages classify lymphedema based on clinical signs.
| ISL Stage | Clinical Signs or Symptoms |
|---|---|
| Stage 0 | No visible swelling. The patient reports sensations associated with swelling, for example, heaviness, tightness, and aching |
| Stage I | Intermittent swelling that resolves overnight or with elevation. Minimal or no pitting. |
| Stage IIa | Visible swelling. Significant pitting. Elevation rarely resolves swelling. |
| Stage IIb | Visible swelling. Tissue firmness is evident in the swollen area. Minimal or no pitting. |
| Stage III | Large, distorted swollen area. No pitting. Very hard skin and tissue, with skin changes including extra folds, discoloration, overgrowths, and lymphorrhea. |

Table A2.
Volume difference assessment [5].
Table A2.
Volume difference assessment [5].
| Level of Severity. | Increase in Limb Volume (%) |
|---|---|
| minimal | 5–10% |
| mild | 10–20% |
| moderate | 20–40% |
| severe | 40% |
References
- Finnane, A.; Hayes, S.C.; Obermair, A.; Janda, M. Quality of Life of Women with Lower-Limb Lymphedema Following Gynecological Cancer. Expert. Rev. Pharm. Outcomes Res. 2011, 11, 287–297. [Google Scholar] [CrossRef]
- Szuba, A.; Rockson, S.G. Lymphedema: Classification, Diagnosis and Therapy. Vasc. Med. 1998, 3, 145–156. [Google Scholar] [CrossRef]
- Partsch, H. Assessment of Abnormal Lymph Drainage for the Diagnosis of Lymphedema by Isotopic Lymphangiography and by Indirect Lymphography. Clin. Dermatol. 1995, 13, 445–450. [Google Scholar] [CrossRef]
- Cemal, Y.; Pusic, A.; Mehrara, B.J. Preventative Measures for Lymphedema: Separating Fact from Fiction. J. Am. Coll. Surg. 2011, 213, 543–551. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The Diagnosis and Treatment of Peripheral Lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Chachaj, A.; Piller, N.; Boccardo, F.; Szuba, A. Lymphedema: General Pathophysiology, Prevention, and Management in Invasive Cancer BT—Cancer Metastasis Through the Lymphovascular System; Leong, S.P., Nathanson, S.D., Zager, J.S., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 261–271. ISBN 978-3-030-93084-4. [Google Scholar]
- Lee, T.S.; Morris, C.M.; Czerniec, S.A.; Mangion, A.J. Does Lymphedema Severity Affect Quality of Life? Simple Question. Challenging Answers. Lymphat. Res. Biol. 2018, 16, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Daniel, T. Validation of the lymphedema life impact scale (LLIS): A condition-specific measurement tool for persons with lymphedema. Lymphology 2015, 48, 128–138. [Google Scholar] [PubMed]
- Gabel, C.P.; Melloh, M.; Burkett, B.; Michener, L.A. Lower Limb Functional Index: Development and Clinimetric Properties. Phys. Ther. 2012, 92, 98–110. [Google Scholar] [CrossRef]
- Binkley, J.M.; Stratford, P.W.; Lott, S.A.; Riddle, D.L. The Lower Extremity Functional Scale (LEFS): Scale Development, Measurement Properties, and Clinical Application. North American Orthopaedic Rehabilitation Research Network. Phys. Ther. 1999, 79, 371–383. [Google Scholar]
- Angst, F. Disabilities of the Arm, Shoulder and Hand Questionnaire (DASH). In Encyclopedia of Quality of Life and Well-Being Research; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1635–1646. [Google Scholar] [CrossRef]
- Stucki, G.; Maksimović, M.; Davidović, D.; Jorga, J. International Classification of Functioning, Disability and Health. Srp. Arh. Celok. Lek. 2007, 135, 371–375. [Google Scholar] [CrossRef]
- Nakane, Y.; Tazaki, M.; Miyaoka, E. WHOQOL User Manual. Iryo Shakai 1999, 9, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Garza, R.M.; Ooi, A.S.H.; Falk, J.; Chang, D.W. The Relationship between Clinical and Indocyanine Green Staging in Lymphedema. Lymphat. Res. Biol. 2019, 17, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.L.; Stout, N.L.; Wanchai, A.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. Intermittent Pneumatic Compression Therapy: A Systematic Review. Lymphology 2012, 45, 13–25. [Google Scholar] [PubMed]
- Mosti, G.; Cavezzi, A. Compression Therapy in Lymphedema: Between Past and Recent Scientific Data. Phlebology 2019, 34, 515–522. [Google Scholar] [CrossRef]
- Liu, N.F.; Olszewski, W. The Influence of Local Hyperthermia on Lymphedema and Lymphedematous Skin of the Human Leg. Lymphology 1993, 26, 28–37. [Google Scholar]
- Chang, T.S.; Gan, J.L.; Fu, K.D.; Huang, W.Y. The Use of 5,6 Benzo-[Alpha]-Pyrone (Coumarin) and Heating by Microwaves in the Treatment of Chronic Lymphedema of the Legs. Lymphology 1996, 29, 106–111. [Google Scholar]
- Joos, E.; Vultureanu, I.; Nonneman, T.; Adriaenssens, N.; Hamdi, M.; Zeltzer, A. Low-Energy Extracorporeal Shockwave Therapy as a Therapeutic Option for Patients with a Secondary Late-Stage Fibro-Lymphedema after Breast Cancer Therapy: A Pilot Study. Lymphat. Res. Biol. 2021, 19, 175–180. [Google Scholar] [CrossRef]
- Pak, C.S.; Suh, H.P.; Kwon, J.G.; Cho, M.-J.; Hong, J.P. Lymph Node to Vein Anastomosis (LNVA) for Lower Extremity Lymphedema. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2059–2067. [Google Scholar] [CrossRef]
- Campisi, C.; Bellini, C.; Campisi, C.; Accogli, S.; Bonioli, E.; Boccardo, F. Microsurgery for Lymphedema: Clinical Research and Long-Term Results. Microsurgery 2010, 30, 256–260. [Google Scholar] [CrossRef]
- Cho, M.J.; Kwon, J.G.; Pak, C.J.; Suh, H.P.; Hong, J.P. The Role of Duplex Ultrasound in Microsurgical Reconstruction: Review and Technical Considerations. J. Reconstr. Microsurg. 2020, 36, 514–521. [Google Scholar] [CrossRef]
- Lee, Y.L.; Huang, Y.L.; Chu, S.Y.; Chan, W.H.; Cheng, M.H.; Lin, Y.H.; Chang, T.Y.; Yeh, C.K.; Tsui, P.H. Characterization of Limb Lymphedema Using the Statistical Analysis of Ultrasound Backscattering. Quant. Imaging Med. Surg. 2020, 10, 48–56. [Google Scholar] [CrossRef]
- Visconti, G.; Salgarello, M.; Hayashi, A. The Recipient Venule in Supermicrosurgical Lymphaticovenular Anastomosis: Flow Dynamic Classification and Correlation with Surgical Outcomes. J. Reconstr. Microsurg. 2018, 34, 581–589. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Kawakami, Y. Ultrasonography for Classifying Lymphatic Sclerosis Types and Deciding Optimal Sites for Lymphatic-Venous Anastomosis in Patients with Lymphoedema. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1274–1281. [Google Scholar] [CrossRef]
- Bianchi, A.; Visconti, G.; Hayashi, A.; Santoro, A.; Longo, V.; Salgarello, M. Ultra-High Frequency Ultrasound Imaging of Lymphatic Channels Correlates with Their Histological Features: A Step Forward in Lymphatic Surgery. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1622–1629. [Google Scholar] [CrossRef]
- Masoodi, Z.; Steinbacher, J.; Tinhofer, I.E.; Czedik-Eysenberg, M.; Mohos, B.; Roka-Palkovits, J.; Huettinger, N.; Meng, S.; Tzou, C.-H.J. “Double Barrel” Lymphaticovenous Anastomosis: A Useful Addition to a Supermicrosurgeon’s Repertoire. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4267. [Google Scholar] [CrossRef]
- Jang, S.; Lee, C.U.; Hesley, G.K.; Knudsen, J.M.; Brinkman, N.J.; Tran, N.V. Lymphatic Mapping Using US Microbubbles before Lymphaticovenous Anastomosis Surgery for Lymphedema. Radiology 2022, 304, 218–224. [Google Scholar] [CrossRef]
- Lahtinen, O.; Vanninen, R.; Rautiainen, S. Contrast-Enhanced Ultrasound: A New Tool for Imaging the Superficial Lymphatic Vessels of the Upper Limb. Eur. Radiol. Exp. 2022, 6, 18. [Google Scholar] [CrossRef]
- Hayashi, A.; Yamamoto, T.; Yoshimatsu, H.; Hayashi, N.; Furuya, M.; Harima, M.; Narushima, M.; Koshima, I. Ultrasound Visualization of the Lymphatic Vessels in the Lower Leg. Microsurgery 2016, 36, 397–401. [Google Scholar] [CrossRef]
- Hayashi, A.; Hayashi, N.; Yoshimatsu, H.; Yamamoto, T. Effective and Efficient Lymphaticovenular Anastomosis Using Preoperative Ultrasound Detection Technique of Lymphatic Vessels in Lower Extremity Lymphedema. J. Surg. Oncol. 2018, 117, 290–298. [Google Scholar] [CrossRef]
- Mohos, B.; Czedik-Eysenberg, M.; Steinbacher, J.; Tinhofer, I.; Meng, S.; Tzou, C.-H.J. Long-Term Use of Ultrasound for Locating Optimal LVA Sites: A Descriptive Data Analysis. J. Reconstr. Microsurg. 2022, 38, 238–244. [Google Scholar] [CrossRef]
- Czedik-Eysenberg, M.; Steinbacher, J.; Obermayer, B.; Yoshimatsu, H.; Hara, H.; Mihara, M.; Tzou, C.-H.J.; Meng, S. Exclusive Use of Ultrasound for Locating Optimal LVA Sites-A Descriptive Data Analysis. J. Surg. Oncol. 2020, 121, 51–56. [Google Scholar] [CrossRef]
- Russo, A.; Reginelli, A.; Lacasella, G.V.; Grassi, E.; Ahmed, M.; Karaboue, A.; Quarto, T.; Busetto, G.M.; Aliprandi, A.; Grassi, R.; et al. Clinical Application of Ultra-High-Frequency Ultrasound. J. Pers. Med. 2022, 12, 1733. [Google Scholar] [CrossRef]
- Szuba, A.; Shin, W.S.; Strauss, H.W.; Rockson, S. The Third Circulation: Radionuclide Lymphoscintigraphy in the Evaluation of Lymphedema. J. Nucl. Med. 2004, 44, 43–57. [Google Scholar]
- Strand, S.E.; Bergqvist, L. Radiolabeled Colloids and Macromolecules in the Lymphatic System. Crit. Rev. Ther. Drug Carr. Syst. 1989, 6, 211–238. [Google Scholar]
- Polomska, A.K.; Proulx, S.T. Imaging Technology of the Lymphatic System. Adv. Drug Deliv. Rev. 2020, 170, 294–311. [Google Scholar] [CrossRef]
- Kramer, E.L. Lymphoscintigraphy: Defining a Clinical Role. Lymphat. Res. Biol. 2004, 2, 32–37. [Google Scholar] [CrossRef]
- Vaqueiro, M.; Gloviczki, P.; Fisher, J.; Hollier, L.H.; Schirger, A.; Wahner, H.W. Lymphoscintigraphy in Lymphedema: An Aid to Microsurgery. J. Nucl. Med. 1986, 27, 1125–1130. [Google Scholar]
- Cabrera, R.N.; Chone, C.T.; Zantut-Wittmann, D.E.; Matos, P.S.; Ferreira, D.M.; Pereira, P.S.; Ribeiro, M.P.; Santos, A.O.; Ramos, C.D.; Crespo, A.N.; et al. The Role of SPECT/CT Lymphoscintigraphy and Radioguided Sentinel Lymph Node Biopsy in Managing Papillary Thyroid Cancer. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 834–841. [Google Scholar] [CrossRef]
- Pappalardo, M.; Cheng, M.H. Lymphoscintigraphy for the Diagnosis of Extremity Lymphedema: Current Controversies Regarding Protocol, Interpretation, and Clinical Application. J. Surg. Oncol. 2020, 121, 37–47. [Google Scholar] [CrossRef]
- Lasso, J.M.; Alonso-Farto, J.C. Indocyanine Green-Guided Liposuction for Patients Presenting with Residual Nonpitting Edema after Lymphovenous Anastomosis. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2482–2492. [Google Scholar] [CrossRef]
- Yoneya, S.; Saito, T.; Komatsu, Y.; Koyama, I.; Takahashi, K.; Duvoll-young, J. Binding Properties of Indocyanine Green in Human Blood. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1286–1290. [Google Scholar]
- Robson, S.C.; Mutch, E.; Boys, R.J.; Woodhouse, K.W. Apparent Liver Blood Flow during Pregnancy: A Serial Study Using Indocyanine Green Clearance. Br. J. Obstet. Gynaecol. 1990, 97, 720–724. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yang, H.; Xu, Y. Maternal-Fetal Transfer of Indocyanine Green: A Systematic Review. J. Matern.-Fetal Neonatal Med. 2022, 35, 8181–8185. [Google Scholar] [CrossRef]
- Staurenghi, G.; Bottoni, F.; Giani, A. Clinical Applications of Diagnostic Indocyanine Green Angiography. Retin. Fifth Ed. 2012, 1, 51–81. [Google Scholar] [CrossRef]
- Belgrado, J.P.; Vandermeeren, L.; Vankerckhove, S.; Valsamis, J.B.; Malloizel-Delaunay, J.; Moraine, J.J.; Liebens, F. Near-Infrared Fluorescence Lymphatic Imaging to Reconsider Occlusion Pressure of Superficial Lymphatic Collectors in Upper Extremities of Healthy Volunteers. Lymphat. Res. Biol. 2016, 14, 70–77. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic Indocyanine Green Lymphography Findings in Lower Extremity Lymphedema: The Generation of a Novel Lymphedema Severity Staging System Using Dermal Backflow Patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsuda, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. The Earliest Finding of Indocyanine Green Lymphography in Asymptomatic Limbs of Lower Extremity Lymphedema Patients Secondary to Cancer Treatment: The Modified Dermal Backflow Stage and Concept of Subclinical Lymphedema. Plast. Reconstr. Surg. 2011, 128, 314e–321e. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Koshima, I. Lymphatic Vessel Diameter in Female Pelvic Cancer-Related Lower Extremity Lymphedematous Limbs. J. Surg. Oncol. 2018, 117, 1157–1163. [Google Scholar] [CrossRef]
- Wiser, I.; Mehrara, B.J.; Coriddi, M.; Kenworthy, E.; Cavalli, M.; Encarnacion, E.; Dayan, J.H. Preoperative Assessment of Upper Extremity Secondary Lymphedema. Cancers 2020, 12, 135. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine Green-Enhanced Lymphography for Upper Extremity Lymphedema: A Novel Severity Staging System Using Dermal Backflow Patterns. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef]
- Unno, N.; Nishiyama, M.; Suzuki, M.; Yamamoto, N.; Inuzuka, K.; Sagara, D.; Tanaka, H.; Konno, H. Quantitative Lymph Imaging for Assessment of Lymph Function Using Indocyanine Green Fluorescence Lymphography. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2008, 36, 230–236. [Google Scholar] [CrossRef]
- Granoff, M.D.; Johnson, A.R.; Lee, B.T.; Padera, T.P.; Bouta, E.M.; Singhal, D. A Novel Approach to Quantifying Lymphatic Contractility during Indocyanine Green Lymphangiography. Plast. Reconstr. Surg. 2019, 144, 1197–1201. [Google Scholar] [CrossRef]
- Pons, G.; Clavero, J.A.; Alomar, X.; Rodríguez-bauza, E.; Tom, L.K.; Masia, J. Preoperative Planning of Lymphaticovenous Anastomosis: The Use of Magnetic Resonance Lymphangiography as a Complement to Indocyanine Green Lymphography. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 884–891. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Araki, J.; Kikuchi, K.; Narushima, M.; Yamamoto, T.; Iida, T.; Yoshimatsu, H.; Murai, N.; Mitsui, K.; et al. Indocyanine Green (ICG) Lymphography Is Superior to Lymphoscintigraphy for Diagnostic Imaging of Early Lymphedema of the Upper Limbs. PLoS ONE 2012, 7, e38182. [Google Scholar] [CrossRef]
- Mills, M.; Van Zanten, M.; Borri, M.; Mortimer, P.S.; Howe, F.A.; Gordon, K.; Ostergaard, P. Systematic Review of Magnetic Resonance Lymphangiography From a Technical Perspective. J. Magn. Reson. Imaging 2021, 53, 1766–1790. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, R.; Donahue, M.J. CEST MRI Quantification Procedures for Breast Cancer Treatment-Related Lymphedema Therapy Evaluation. Magn. Reson. Med. 2019, 83, 1760–1773. [Google Scholar] [CrossRef]
- Rane, S.; Donahue, P.M.C.; Towse, T.; Ridner, S.; Chappell, M.; Jordi, J.; Gore, J.; Donahue, M.J. Clinical Feasibility of Noninvasive Visualization of Lymphatic Flow with Principles of Spin Labeling MR Imaging: Implications for Lymphedema Assessment. Radiology 2013, 269, 893–902. [Google Scholar] [CrossRef]
- Neligan, P.C.; Kung, T.A.; Maki, J.H. MR Lymphangiography in the Treatment of Lymphedema. J. Surg. Oncol. 2017, 115, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Soga, S.; Onishi, F.; Mikoshi, A.; Okuda, S.; Jinzaki, M.; Shinmoto, H. Lower Limb Lymphedema Staging Based on Magnetic Resonance Lymphangiography. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 445–453.e3. [Google Scholar] [CrossRef]
- Masia, J.; Pons, G.; Nardulli, M.L. Combined Surgical Treatment in Breast Cancer-Related Lymphedema. J. Reconstr. Microsurg. 2016, 32, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, G.; Yamamoto, T.; Belva, F.; Hayashi, A. Bedside 3D Visualization of Lymphatic Vessels with a Handheld Multispectral Optoacoustic Tomography Device. J. Clin. Med. 2020, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Kajita, H.; Kishi, K. High-Resolution Imaging of Lymphatic Vessels with Photoacoustic Lymphangiography. Radiology 2019, 292, 35. [Google Scholar] [CrossRef]
- Kajita, H.; Oh, A.; Urano, M.; Takemaru, M.; Imanishi, N.; Otaki, M.; Yagi, T.; Aiso, S.; Kishi, K. Photoacoustic Lymphangiography. J. Surg. Oncol. 2020, 121, 48–50. [Google Scholar] [CrossRef]
- Nagae, K.; Asao, Y.; Sudo, Y.; Murayama, N.; Tanaka, Y.; Ohira, K.; Ishida, Y.; Otsuka, A.; Matsumoto, Y.; Saito, S.; et al. Real-Time 3D Photoacoustic Visualization System with a Wide Field of View for Imaging Human Limbs. F1000Research 2018, 7, 1813. [Google Scholar] [CrossRef]
- Schmitt, J.M. Optical Coherence Tomography: A Review. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Yonetsu, T.; Bouma, B.E.; Kato, K.; Fujimoto, J.G.; Jang, I.K. Optical Coherence Tomography: 15 Years in Cardiology. Circ. J. 2013, 77, 1933–1940. [Google Scholar] [CrossRef]
- Baran, U.; Choi, W.J.; Wang, R.K. Potential Use of OCT-Based Microangiography in Clinical Dermatology. Ski. Res. Technol. 2016, 22, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Yoshimatsu, H.; Visconti, G.; Sujarittanakarn, S.; Giacalone, G.; Hayashi, N.; Yamamoto, T.; Yang, J.C.S.; Hong, J.P. Intraoperative Real-Time Visualization of the Lymphatic Vessels Using Microscope-Integrated Laser Tomography. J. Reconstr. Microsurg. 2021, 37, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.S.; Wu, S.C.; Chiang, M.H.; Lin, W.C.; Hsieh, C.H. Intraoperative Identification and Definition of “Functional” Lymphatic Collecting Vessels for Supermicrosurgical Lymphatico-Venous Anastomosis in Treating Lymphedema Patients. J. Surg. Oncol. 2018, 117, 994–1000. [Google Scholar] [CrossRef]
- Polomska, A.K.; Proulx, S.T.; Brambilla, D.; Fehr, D.; Bonmarin, M.; Brändli, S.; Meboldt, M.; Steuer, C.; Vasileva, T.; Reinke, N.; et al. Minimally Invasive Method for the Point-of-Care Quantification of Lymphatic Vessel Function. JCI Insight 2019, 4, e126515. [Google Scholar] [CrossRef]
- Kwon, S.; Velasquez, F.C.; Rasmussen, J.C.; Greives, M.R.; Turner, K.D.; Morrow, J.R.; Hwu, W.-J.; Ross, R.F.; Zhang, S.; Sevick-Muraca, E.M. Nanotopography-Based Lymphatic Delivery for Improved Anti-Tumor Responses to Checkpoint Blockade Immunotherapy. Theranostics 2019, 9, 8332–8343. [Google Scholar] [CrossRef] [PubMed]
- Narushima, M.; Yamamoto, T.; Ogata, F.; Yoshimatsu, H.; Mihara, M.; Koshima, I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J. Reconstr. Microsurg. 2016, 32, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Mihara, M. Multi-Area Lymphaticovenous Anastomosis with Multi-Lymphosome Injection in Indocyanine Green Lymphography: A Prospective Study. Microsurgery 2019, 39, 167–173. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).