Age-Based Comparative Analysis of Colorectal Cancer Colonoscopy Screening Findings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Experimental Protocol
2.3. Colonoscopy Procedure
2.4. Morphological Analyses
2.5. Statistical Analyses
3. Results
3.1. Patient Demographic Profiling
3.2. Colonoscopy Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gender n (%) | Total n = 1653 | Patient Group Age | p between Groups <0.01 | |
|---|---|---|---|---|
| <50 (n = 1021) | ≥50 (n = 632) | |||
| Female Male | 997 (60.3%) 656 (39.7%) | 609 (59.6%) 412 (40.4%) | 389 (61.6%) 243 (38.4%) | <0.01 <0.01 |
| Group of Patients | Colon Region | ||||||
|---|---|---|---|---|---|---|---|
| Number of Polyps | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
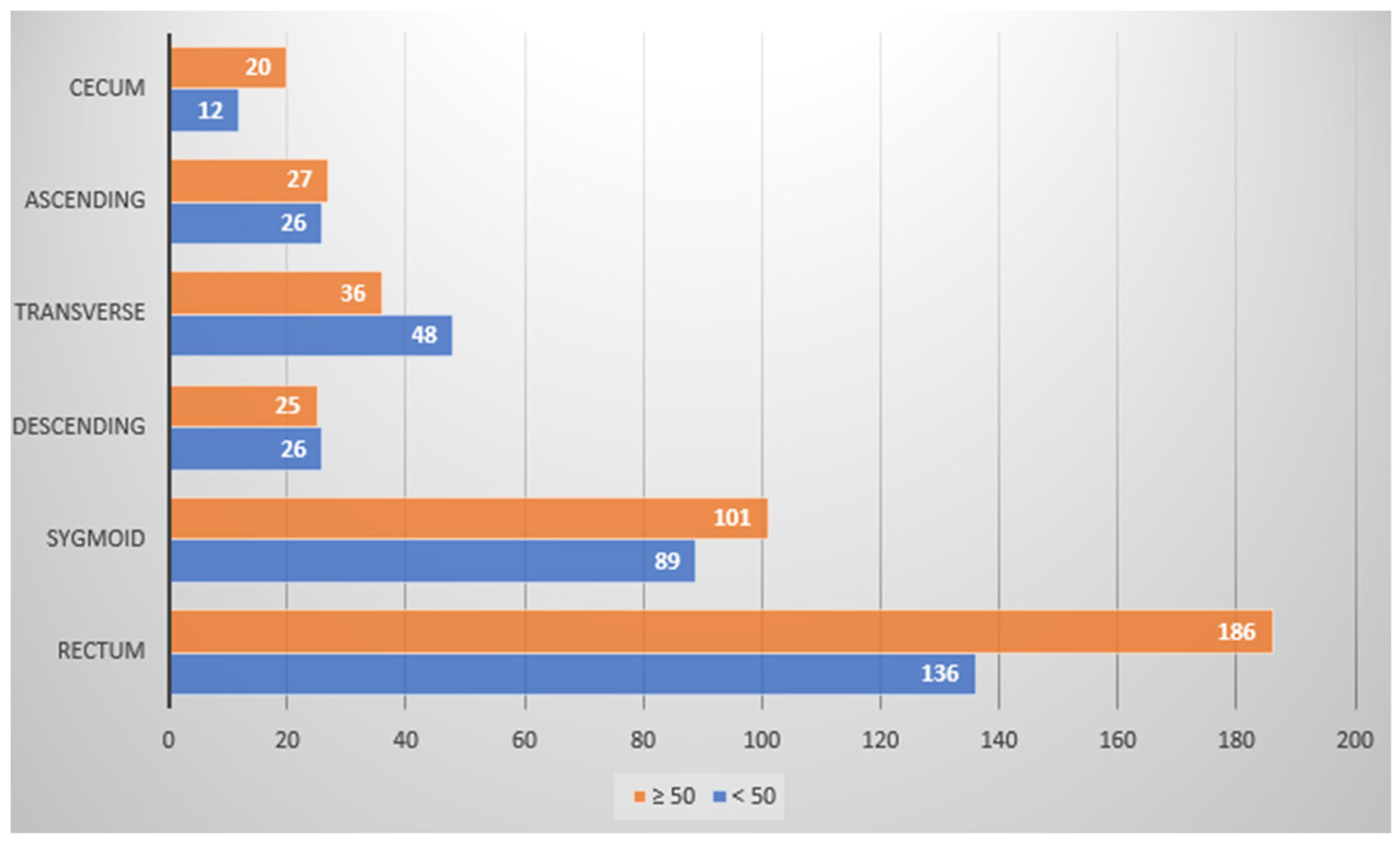
| <50 | 429 (42%) | 178 (41.5%) | 113 (26.3%) | 34 (7.9%) | 58 (13.5%) | 33 (7.7%) | 13 (3%) |
| n = 1021 | |||||||
| ≥50 | 483 (76%) | 220 (45.5%) | 117 (24.2%) | 35 (7.2%) | 45 (9.3%) | 39 (8.1%) | 27 (5.6%) |
| n = 632 | |||||||
| Total number of polyps | 912 (55.2%) | 398 (43.6%) | 230 (25.2%) | 69 (7.6%) | 103 (11.3%) | 72 (7.9%) | 40 (4.4%) |
| p | <0.001 | <0.001 | <0.001 | 0.029 | 0.23 | 0.004 | <0.001 |
| All Patients (n = 1653) | <50 (n = 1021) | ≥50 (n = 632) | |
|---|---|---|---|
| Total number of patients with polyps | 732 | 337 | 395 |
| Patients with 1 polyp | 316 | 189 | 127 |
| Patients with 2 polyps | 243 | 74 | 169 |
| Patients with 3 polyps | 84 | 46 | 38 |
| Patients with 4 polyps | 64 | 21 | 43 |
| Patients with 5 polyps | 22 | 7 | 15 |
| Patients with 8 polyps | 2 | 0 | 2 |
| Patients with 14 polyps | 1 | 0 | 1 |
| Polyp dimensions | <5 mm | 6–9 mm | >10 mm |
| Polyp quantity | 462 | 269 | 181 |
| Patients with Hyperplastic Polyps | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 234 | |||||||
| Patients <50 years | 88 | 46 | 11 | 4 | 9 | 15 | 3 |
| (8.6%) | (52.3%) | (12.5%) | (4.5%) | (10.2%) | (17%) | (3.4%) | |
| Patients ≥50 years | 146 | 78 | 24 | 8 | 13 | 17 | 6 |
| (23.1%) | (53.4%) | (16.4%) | (5.5%) | (8.9%) | (11.6%) | (4.1%) | |
| p | <0.001 | <0.001 | <0.001 | 0.042 | 0.043 | 0.08 | 0.078 |
| Hyperplastic Polyps | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 341 | |||||||
| In patients <50 years | 154 | 82 | 29 | 7 | 15 | 18 | 3 |
| (15.1%) | (53.2%) | (18.8%) | (4.54%) | (9.7%) | (11.7%) | (1.9%) | |
| In patients ≥50 years | 187 | 94 | 32 | 12 | 18 | 22 | 9 |
| (29.6%) | (50.3%) | (17.1%) | (6.4%) | (9.6%) | (11.8%) | (4.8%) | |
| p | <0.001 | <0.001 | 0.02 | 0.025 | 0.051 | 0.027 | 0.009 |
| Patients with Serrated Polyps | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 36 | |||||||
| Patients <50 years | 12 | 0 | 2 | 2 | 5 | 1 | 2 |
| (1.2%) | (16.7%) | (16.7%) | (41.7%) | (8.3%) | (16.7%) | ||
| Patients ≥50 years | 24 | 3 | 3 | 4 | 6 | 3 | 5 |
| (3.8%) | (12.5%) | (12.5%) | (16.7%) | (25%) | (12.5%) | (20.8%) | |
| p | <0.001 | 0.046 | 0.377 | 0.211 | 0.352 | 0.159 | 0.114 |
| Serrated Polyps | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 52 | |||||||
| In patients <50 years | 17 | 0 | 2 | 3 | 6 | 3 | 3 |
| (1.7%) | (11.8%) | (17.6%) | (35.3%) | (17.6%) | (17.6%) | ||
| In patients ≥50 years | 35 | 3 | 3 | 6 | 7 | 8 | 8 |
| (5.5%) | (8.6%) | (8.6%) | (17.1%) | (20%) | (22.9%) | (22.9%) | |
| p | <0.001 | 0.056 | 0.377 | 0.093 | 0.263 | 0.026 | 0.026 |
| Patients with LGD Adenomas | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 285 | |||||||
| Patients <50 years | 167 | 58 | 51 | 19 | 29 | 6 | 4 |
| (16.3%) | (34.7%) | (30.5%) | (11.3%) | (17.4%) | (3.6%) | (2.4%) | |
| Patients ≥50 years | 118 | 53 | 37 | 9 | 11 | 3 | 5 |
| (18.7%) | (44.9%) | (31.4%) | (7.6%) | (9.3%) | (2.5%) | (4.2%) | |
| p | 0.226 | 0.033 | 0.449 | 0.504 | 0.157 | 0.762 | 0.284 |
| LGD Adenomas | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 327 | |||||||
| In patients <50 years | 181 | 62 | 54 | 22 | 32 | 7 | 4 |
| (17.7%) | (34.3%) | (29.8%) | (12.2%) | (17.7%) | (3.9%) | (2.2%) | |
| In patients ≥50 years | 146 | 67 | 43 | 12 | 14 | 5 | 5 |
| (23.1%) | (45.9%) | (29.5%) | (8.2%) | (9.6%) | (3.4%) | (3.4%) | |
| p | 0.008 | <0.001 | 0.203 | 0.722 | 0.27 | 0.806 | 0.284 |
| Patients with HGD Polyps | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 158 | |||||||
| Patients <50 years | 64 | 29 | 23 | 1 | 5 | 4 | 2 |
| n = 1021 | (6.3%) | (45.3%) | (35.9%) | (1.6%) | (7.8%) | (6.25%) | (3.1%) |
| Patients ≥50 years | 94 | 46 | 33 | 4 | 6 | 3 | 2 |
| n = 632 | (14.9%) | (48.9%) | (35.1%) | (4.3%) | (6.4%) | (3.2%) | (2.1%) |
| p | <0.001 | <0.001 | 0.001 | 0.054 | 0.264 | 0.785 | 0.628 |
| HGD Polyps | Total | Rectum | Sigmoid | Descending | Transverse | Ascending | Cecum |
|---|---|---|---|---|---|---|---|
| n = 173 | |||||||
| In patients <50 years | 71 | 31 | 26 | 2 | 5 | 5 | 2 |
| n = 1021 | (6.6%) | (43.7%) | (36.6%) | (2.8%) | (7%) | (7%) | (2.8%) |
| In patients ≥50 years | 102 | 50 | 35 | 5 | 6 | 3 | 3 |
| n = 632 | (16.1%) | (49%) | (34.35) | (4.9%) | (5.9%) | (2.9%) | (2.9%) |
| p | <0.001 | <0.001 | 0.002 | 0.07 | 0.264 | 0.966 | 0.316 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dobson, B.; Ng Liet Hing, C.; Cooper, M.; Lu, C.T.; Nolan, G.; Von Papen, M. Increasing Rate of Colorectal Cancer in Younger Patients: A Review of Colonoscopy Findings in Patients under 50 at a Tertiary Institution. ANZ J. Surg. 2020, 90, 2484–2489. [Google Scholar] [CrossRef] [PubMed]
- Strum, W.B.; Boland, C.R. Clinical and Genetic Characteristics of Colorectal Cancer in Persons under 50 Years of Age: A Review. Dig. Dis. Sci. 2019, 64, 3059–3065. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, M.-M.; Hou, J.-G. Molecular and Cellular Pathways in Colorectal Cancer: Apoptosis, Autophagy and Inflammation as Key Players. Scand. J. Gastroenterol. 2022, 57, 1279–1290. [Google Scholar] [CrossRef]
- Rickelt, S.; Condon, C.; Mana, M.; Whittaker, C.A.; Pfirschke, C.; Roper, J.; Patil, D.T.; Brown, I.; Mattia, A.R.; Zukerberg, L.; et al. Agrin in the Muscularis Mucosa Serves as a Biomarker Distinguishing Hyperplastic Polyps from Sessile Serrated Lesions. Clin. Cancer Res. 2020, 26, 1277–1287. [Google Scholar] [CrossRef]
- Chen, T.-H.; Hsu, H.-C.; You, J.-F.; Lai, C.-C.; Tsou, Y.-K.; Hsu, C.-L.; Fann, C.S.J.; Chien, R.-N.; Chang, M.-L. Extracellular Nicotinamide Phosphoribosyltransferase as a Surrogate Marker of Prominent Malignant Potential in Colonic Polyps: A 2-Year Prospective Study. Cancers 2023, 15, 1702. [Google Scholar] [CrossRef]
- Günel, N.; Yamac, D.; Akcali, Z.; Taneri, F.; Oguz, M. The Clinicopathologic Characteristics of Colorectal Cancer in Patients under 50 Years of Age: Experience of an Oncology Center. Tumori 2001, 87, 74–77. [Google Scholar] [CrossRef]
- Huntley, C.; Torr, B.; Sud, A.; Rowlands, C.F.; Way, R.; Snape, K.; Hanson, H.; Swanton, C.; Broggio, J.; Lucassen, A.; et al. Utility of Polygenic Risk Scores in UK Cancer Screening: A Modelling Analysis. Lancet Oncol. 2023, 24, 658–668. [Google Scholar] [CrossRef]
- Zaborowski, A.M. REACCT Collaborative Colorectal Cancer in the Young: Research in Early Age Colorectal Cancer Trends (REACCT) Collaborative. Cancers 2023, 15, 2979. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-W.; Zhou, Y.-L.; Dai, W.-X.; Chen, H.-M.; Zhou, C.-B.; Zhu, C.-Q.; Ma, X.-Y.; Pan, S.-Y.; Cui, Y.; Xu, J.; et al. Noninvasive Predictive Models Based on Lifestyle Analysis and Risk Factors for Early-Onset Colorectal Cancer. J. Gastroenterol. Hepatol. 2023, 38, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Waye, J.D. What Is a Gold Standard for Colon Polyps? Gastroenterology 1997, 112, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.A.; Noureddine, M. Colorectal Cancer Screening: An Updated Review of the Available Options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef]
- Leslie, A.; Carey, F.A.; Pratt, N.R.; Steele, R.J.C. The Colorectal Adenoma-Carcinoma Sequence. Br. J. Surg. 2002, 89, 845–860. [Google Scholar] [CrossRef]
- Yi, J.M.; Dhir, M.; Van Neste, L.; Downing, S.R.; Jeschke, J.; Glöckner, S.C.; de Freitas Calmon, M.; Hooker, C.M.; Funes, J.M.; Boshoff, C.; et al. Genomic and Epigenomic Integration Identifies a Prognostic Signature in Colon Cancer. Clin. Cancer Res. 2011, 17, 1535–1545. [Google Scholar] [CrossRef]
- Pancione, M.; Remo, A.; Colantuoni, V. Genetic and Epigenetic Events Generate Multiple Pathways in Colorectal Cancer Progression. Patholog. Res. Int. 2012, 2012, 509348. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Vilahur, N.; Bianchini, F.; Guha, N.; Straif, K. International Agency for Research on Cancer Handbook Working Group the IARC Perspective on Colorectal Cancer Screening. N. Engl. J. Med. 2018, 378, 1734–1740. [Google Scholar] [CrossRef]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef]
- Murphy, C.C.; Sandler, R.S.; Sanoff, H.K.; Yang, Y.C.; Lund, J.L.; Baron, J.A. Decrease in Incidence of Colorectal Cancer among Individuals 50 Years or Older After Recommendations for Population-Based Screening. Clin. Gastroenterol. Hepatol. 2017, 15, 903–909.e6. [Google Scholar] [CrossRef] [PubMed]
- Sifaki-Pistolla, D.; Poimenaki, V.; Fotopoulou, I.; Saloustros, E.; Mavroudis, D.; Vamvakas, L.; Lionis, C. Significant Rise of Colorectal Cancer Incidence in Younger Adults and Strong Determinants: 30 Years Longitudinal Differences between under and over 50s. Cancers 2022, 14, 4799. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, X.-N.; Wang, B.-G.; Pan, Y.; Liu, N.; Wang, D.-C.; Hao, X.-S. Prognostic Factors of Young Patients with Colon Cancer after Surgery. World J. Gastroenterol. 2006, 12, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Spaander, M.C.W.; Zauber, A.G.; Syngal, S.; Blaser, M.J.; Sung, J.J.; You, Y.N.; Kuipers, E.J. Young-Onset Colorectal Cancer. Nat. Rev. Dis. Primers 2023, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Participants in the Paris Workshop the Paris Endoscopic Classification of Superficial Neoplastic Lesions: Esophagus, Stomach, and Colon: 30 November to 1 December 2002. Gastrointest. Endosc. 2003, 58, S3–S43. [CrossRef]
- Hewett, D.G.; Kaltenbach, T.; Sano, Y.; Tanaka, S.; Saunders, B.P.; Ponchon, T.; Soetikno, R.; Rex, D.K. Validation of a Simple Classification System for Endoscopic Diagnosis of Small Colorectal Polyps Using Narrow-Band Imaging. Gastroenterology 2012, 143, 599–607.e1. [Google Scholar] [CrossRef]
- Ahadi, M.; Sokolova, A.; Brown, I.; Chou, A.; Gill, A.J. The 2019 World Health Organization Classification of Appendiceal, Colorectal and Anal Canal Tumours: An Update and Critical Assessment. Pathology 2021, 53, 454–461. [Google Scholar] [CrossRef]
- IBM. SPSS Statistics-Overview. Available online: https://www.ibm.com/products/spss-statistics (accessed on 7 June 2023).
- Sedjo, R.L.; Byers, T.; Barrera, E.; Cohen, C.; Fontham, E.T.H.; Newman, L.A.; Runowicz, C.D.; Thorson, A.G.; Thun, M.J.; Ward, E.; et al. A Midpoint Assessment of the American Cancer Society Challenge Goal to Decrease Cancer Incidence by 25% between 1992 and 2015. CA Cancer J. Clin. 2007, 57, 326–340. [Google Scholar] [CrossRef]
- Winawer, S.J.; Fletcher, R.H.; Miller, L.; Godlee, F.; Stolar, M.H.; Mulrow, C.D.; Woolf, S.H.; Glick, S.N.; Ganiats, T.G.; Bond, J.H.; et al. Colorectal Cancer Screening: Clinical Guidelines and Rationale. Gastroenterology 1997, 112, 594–642. [Google Scholar] [CrossRef]
- Rex, D.K.; Petrini, J.L.; Baron, T.H.; Chak, A.; Cohen, J.; Deal, S.E.; Hoffman, B.; Jacobson, B.C.; Mergener, K.; Petersen, B.T.; et al. Quality Indicators for Colonoscopy. Am. J. Gastroenterol. 2006, 101, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Rundle, A.G.; Lebwohl, B.; Vogel, R.; Levine, S.; Neugut, A.I. Colonoscopic Screening in Average-Risk Individuals Ages 40 to 49 vs 50 to 59 Years. Gastroenterology 2008, 134, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Issaka, R.B.; Chan, A.T.; Gupta, S. AGA Clinical Practice Update on Risk Stratification for Colorectal Cancer Screening and Post-Polypectomy Surveillance: Expert Review. Gastroenterology 2023, 165, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.N.; Castro, F.; Golawala, M.; Chen, R. Detection of Colorectal Neoplasia by Colonoscopy in Average-Risk Patients Age 40–49 versus 50–59 Years. Dig. Dis. Sci. 2011, 56, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Hazewinkel, Y.; de Wijkerslooth, T.R.; Stoop, E.M.; Bossuyt, P.M.; Biermann, K.; van de Vijver, M.J.; Fockens, P.; van Leerdam, M.E.; Kuipers, E.J.; Dekker, E. Prevalence of Serrated Polyps and Association with Synchronous Advanced Neoplasia in Screening Colonoscopy. Endoscopy 2014, 46, 219–224. [Google Scholar] [CrossRef]
- Provenzale, D.; Garrett, J.W.; Condon, S.E.; Sandler, R.S. Risk for Colon Adenomas in Patients with Rectosigmoid Hyperplastic Polyps. Ann. Intern. Med. 1990, 113, 760–763. [Google Scholar] [CrossRef]
- Hassan, C.; Pickhardt, P.J.; Marmo, R.; Choi, J.R. Impact of Lifestyle Factors on Colorectal Polyp Detection in the Screening Setting. Dis. Colon. Rectum 2010, 53, 1328–1333. [Google Scholar] [CrossRef]
- Anderson, J.C.; Butterly, L.F.; Robinson, C.M.; Weiss, J.E.; Amos, C.; Srivastava, A. Risk of Metachronous High-Risk Adenomas and Large Serrated Polyps in Individuals with Serrated Polyps on Index Colonoscopy: Data from the New Hampshire Colonoscopy Registry. Gastroenterology 2018, 154, 117–127.e2. [Google Scholar] [CrossRef]
- Vennelaganti, S.; Cuatrecasas, M.; Vennalaganti, P.; Kennedy, K.F.; Srinivasan, S.; Patil, D.T.; Plesec, T.; Lanas, A.; Hörndler, C.; Andraws, N.; et al. Interobserver Agreement among Pathologists in the Differentiation of Sessile Serrated from Hyperplastic Polyps. Gastroenterology 2021, 160, 452–454.e1. [Google Scholar] [CrossRef]
- Farris, A.B.; Misdraji, J.; Srivastava, A.; Muzikansky, A.; Deshpande, V.; Lauwers, G.Y.; Mino-Kenudson, M. Sessile Serrated Adenoma: Challenging Discrimination from Other Serrated Colonic Polyps. Am. J. Surg. Pathol. 2008, 32, 30–35. [Google Scholar] [CrossRef]
- Atkin, W.; Wooldrage, K.; Parkin, D.M.; Kralj-Hans, I.; MacRae, E.; Shah, U.; Duffy, S.; Cross, A.J. Long Term Effects of Once-Only Flexible Sigmoidoscopy Screening after 17 Years of Follow-Up: The UK Flexible Sigmoidoscopy Screening Randomised Controlled Trial. Lancet 2017, 389, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Strum, W. Colorectal Adenomas. N. Engl. J. Med. 2016, 375, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Kolb, J.M.; Hu, J.; DeSanto, K.; Gao, D.; Singh, S.; Imperiale, T.; Lieberman, D.A.; Boland, C.R.; Patel, S.G. Early-Age Onset Colorectal Neoplasia in Average-Risk Individuals Undergoing Screening Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterology 2021, 161, 1145–1155.e12. [Google Scholar] [CrossRef] [PubMed]

| Colonoscopy Finding | Prevalent Cohort (Years Old) | Prevalent Colon Location |
|---|---|---|
| Overall polyp formation | ≥50 | Rectum |
| Hyperplastic polyps | <50 | Rectum |
| Serrated polyps | ≥50 | Ascending colon/cecum |
| LGD adenomas | ≥50 | Rectum |
| HGD adenomas | ≥50 | Sigmoid colon/rectum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkoite, I.; Tolmanis, I.; Abu Meri, H.; Polaka, I.; Mezmale, L.; Lejnieks, A. Age-Based Comparative Analysis of Colorectal Cancer Colonoscopy Screening Findings. Medicina 2023, 59, 2017. https://doi.org/10.3390/medicina59112017
Vilkoite I, Tolmanis I, Abu Meri H, Polaka I, Mezmale L, Lejnieks A. Age-Based Comparative Analysis of Colorectal Cancer Colonoscopy Screening Findings. Medicina. 2023; 59(11):2017. https://doi.org/10.3390/medicina59112017
Chicago/Turabian StyleVilkoite, Ilona, Ivars Tolmanis, Hosams Abu Meri, Inese Polaka, Linda Mezmale, and Aivars Lejnieks. 2023. "Age-Based Comparative Analysis of Colorectal Cancer Colonoscopy Screening Findings" Medicina 59, no. 11: 2017. https://doi.org/10.3390/medicina59112017
APA StyleVilkoite, I., Tolmanis, I., Abu Meri, H., Polaka, I., Mezmale, L., & Lejnieks, A. (2023). Age-Based Comparative Analysis of Colorectal Cancer Colonoscopy Screening Findings. Medicina, 59(11), 2017. https://doi.org/10.3390/medicina59112017







