A Mediterranean-Style Diet Improves the Parameters for the Management and Prevention of Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
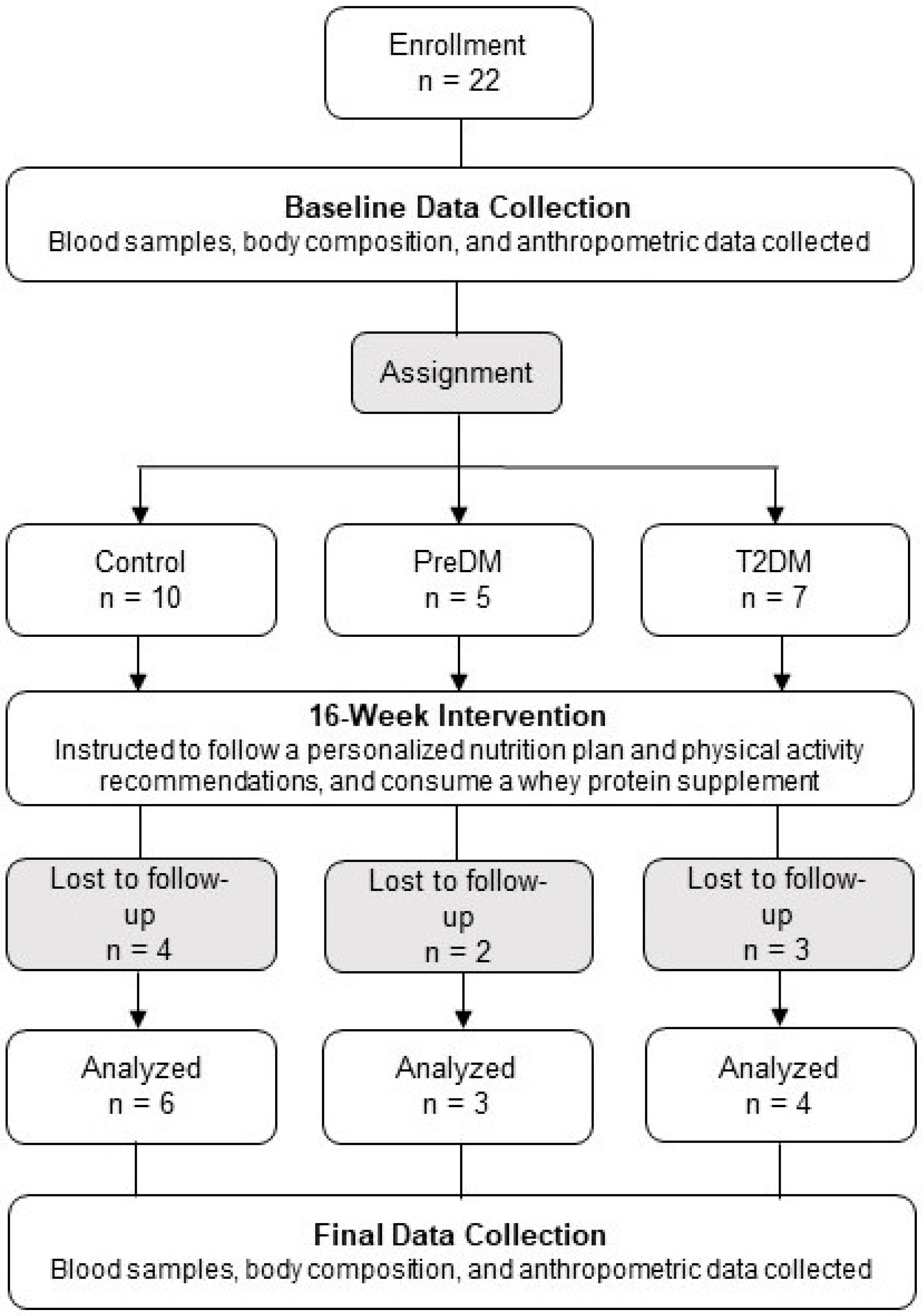
2.1. Participants
2.2. Experimental Protocol
2.3. Analyses of Parameters of Glycemic Control and Systemic Inflammation
2.4. Body Composition and Anthropometric Analyses
2.5. Statistical Analyses
3. Results
3.1. Parameters of Glycemic Control
3.1.1. Fasting Plasma Glucose
3.1.2. Hemoglobin A1c
3.1.3. Fasting Plasma Insulin
3.2. Systemic Inflammation
3.3. Body Composition and Anthropometrics
4. Discussion
4.1. Glycemic Control
4.1.1. Strengths—Considerations
4.1.2. Limitations—Considerations
4.1.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40 (Suppl. S1), S11–S24. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 20 July 2022).
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 2 October 2022).
- Harris, M.F. The metabolic syndrome. Aust. Fam. Physician 2013, 42, 524–527. [Google Scholar] [PubMed]
- Kahn, S.; Hull, R.; Utzschneider, K. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S100–S110. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Rowan-Martin, M.T.; Levin, R.; Fonseca, V.A.; Schmittdiel, J.A.; Herman, W.H.; Aubert, R.E. Determinants of adherence to diabetes medications: Findings from a large pharmacy claims database. Diabetes Care 2015, 38, 604–609. [Google Scholar] [CrossRef]
- Georgoulis, M.; Kontogianni, M.D.; Yiannakouris, N. Mediterranean diet and diabetes: Prevention and treatment. Nutrients 2014, 6, 1406–1423. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.; Tong, T.; Forouhi, N.; Khandelwal, S.; Dorairaj, P.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2016, 146, 920S–927S. [Google Scholar] [CrossRef]
- Adams, R.L.; Broughton, K.S. Insulinotropic Effects of Whey: Mechanisms of Action, Recent Clinical Trials, and Clinical Applications. Ann. Nutr. Metab. 2016, 69, 56–63. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J. Nutr. Biochem. 2013, 24, 1–5. [Google Scholar] [CrossRef]
- Office of Disease Prevention and Health Promotion (OASH). Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. Available online: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf (accessed on 17 May 2022).
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Diabetes Tests & Diagnosis. Available online: https://www.niddk.nih.gov/health-information/diabetes/overview/tests-diagnosis#type2 (accessed on 2 June 2022).
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Du, T.; Xu, Y.; Xu, W.; Chen, X.; Sun, K.; Yu, X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Achana, F.; Troughton, J.; Gray, L.J.; Khunti, K.; Davies, M.J. A Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: A network meta-analysis. J. Hum. Nutr. Diet. 2014, 27, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Kelleher, A.H.; Kristo, A.S. Mediterranean Diet. Encyclopedia 2021, 1, 371–387. [Google Scholar] [CrossRef]
- Murray, A.E.; McMorrow, A.M.; O’Connor, E.; Kiely, C.; Mac Ananey, O.; O’Shea, D.; Egaña, M.; Lithander, F.E. Dietary quality in a sample of adults with type 2 diabetes mellitus in Ireland; a cross-sectional case control study. Nutr. J. 2013, 12, 110. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Calabrese, I.; Rivellese, A.A.; Bonora, E.; Signorini, S.; Perriello, G.; Squatrito, S.; Buzzetti, R.; Sartore, G.; et al. Impact of a Mediterranean Dietary Pattern and Its Components on Cardiovascular Risk Factors, Glucose Control, and Body Weight in People with Type 2 Diabetes: A Real-Life Study. Nutrients 2018, 10, 1067. [Google Scholar] [CrossRef]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef]
- Khan, R.; Chua, Z.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 20 July 2022).
- Kotzakioulafi, E.; Bakaloudi, D.R.; Chrysoula, L.; Theodoridis, X.; Antza, C.; Tirodimos, I.; Chourdakis, M. High Versus Low Adherence to the Mediterranean Diet for Prevention of Diabetes Mellitus Type 2: A Systematic Review and Meta-Analysis. Metabolites 2023, 13, 779. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Gortmaker, S.L.; Hu, F.B.; Kanaya, A.M.; Kandula, N.R.; Sun, Q.; Bhupathiraju, S.N. A South Asian Mediterranean-style diet is associated with favorable adiposity measures and lower diabetes risk: The MASALA cohort. Obesity 2023, 31, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Maykish, A.; Sikalidis, A.K. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. J. Pers. Med. 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
| Group | ||||
|---|---|---|---|---|
| Control n = 10 | PreDM n = 5 | T2DM n = 7 | p-Value | |
| Age (years) † | 44.6 ± 10.3 | 51.8 ± 11.5 | 46.9 ± 14.8 | 0.565 |
| Sex (% female) | 60 | 40 | 57.1 | 0.781 |
| Body Mass Index (BMI) † | 28.6 ± 5.86 | 30.7 ± 6.46 | 31.6 ± 5.30 | 0.569 |
| Group | ||||
|---|---|---|---|---|
| Control | PreDM | T2DM | ||
| Fasting plasma glucose (FPG; mg/dL) | Baseline: | 82.6 ± 7.66 a | 106.0 ± 9.11 b | 126.1 ± 26.98 b |
| Week 16: | 81.7 ± 5.72 | 94.0 ± 7.55 | 106.5 ± 31.17 | |
| p-value 1: | 0.790 | 0.065 | 0.232 | |
| Hemoglobin A1c (HbA1c; %) | Baseline: | 5.2 ± 0.29 a | 5.7 ± 0.30 a | 6.8 ± 1.20 b |
| Week 16: | 5.1 ± 0.34 A | 5.7 ± 0.06 A,B | 6.0 ± 0.75 B | |
| p-value 1: | 0.224 | 1.000 | 0.391 | |
| Fasting Plasma Insulin (FPI; mU/L) | Baseline: | 8.4 ± 4.35 | 10.7 ± 5.86 | 17.5 ± 12.27 $ |
| Week 16: | 5.4 ± 2.93 | 7.0 ± 4.59 | 10.8 ± 7.25 | |
| p-value 1: | 0.162 | 0.209 | 0.216 | |
| Group | ||||
|---|---|---|---|---|
| Control | PreDM | T2DM | ||
| High-sensitivity C-reactive protein (hs-CRP; mg/L) | Baseline: | 2.4 ± 2.10 | 3.6 ± 3.62 | 5.7 ± 6.30 |
| Week 16: | 1.5 ± 0.84 | 2.2 ± 3.02 | 4.0 ± 3.70 | |
| p-value 1: | 0.408 | 0.414 | 0.862 | |
| Interleukin-6 (IL-6; pg/mL) | Baseline: | 1.8 ± 2.47 | 2.6 ± 1.74 | 3.8 ± 3.85 |
| Week 16: | 1.2 ± 0.50 | 2.0 ± 1.39 | 4.1 ± 4.21 | |
| p-value 1: | 0.814 | 0.877 | 0.718 | |
| Tumor necrosis factor-alpha (TNF-α; pg/mL) | Baseline: | 1.0 ± 0.33 | 1.3 ± 0.19 | 1.4 ± 0.51 |
| Week 16: | 1.2 ± 0.20 | 1.1 ± 0.28 | 1.3 ± 0.39 | |
| p-value 1: | 0.749 | 0.395 | 0.492 | |
| Group | ||||
|---|---|---|---|---|
| Control | PreDM | T2DM | ||
| Body Weight (lbs) | Baseline: | 191.7 ± 49.15 | 206.4 ± 41.31 | 198.9 ± 47.54 |
| Week 16: | 167.1 ± 43.28 | 196.5 ± 40.82 | 171.8 ± 49.92 | |
| p-value 1: | 0.030 | 0.603 | 0.135 | |
| Waist Circumference (WC; inches) | Baseline: | 39.8 ± 6.28 | 42.0 ± 7.89 | 42.7 ± 6.45 |
| Week 16: | 37.2 ± 6.62 | 38.8 ± 8.01 | 37.4 ± 5.31 | |
| p-value 1: | 0.239 | 0.431 | 0.058 | |
| Body Mass Index (BMI) | Baseline: | 28.6 ± 5.86 | 30.7 ± 6.46 | 31.6 ± 5.30 |
| Week 16: | 26.2 ± 5.90 | 28.4 ± 5.19 | 28.6 ± 5.48 | |
| p-value 1: | 0.043 | 0.592 | 0.136 | |
| Body Fat (percent region, BF%) | Baseline: | 36.6 ± 6.46 | 34.7 ± 8.40 | 38.4 ± 4.93 |
| Week 16: | 34.6 ± 8.00 | 30.6 ± 7.73 | 36.3 ± 5.78 | |
| p-value 1: | 0.003 | 0.986 | 0.199 | |
| Visceral Adipose Tissue (VAT; mass, lbs) | Baseline: | 2.5 ± 1.54 | 3.73 ± 2.49 | 4.35 ± 2.24 |
| Week 16: | 1.83 ± 1.24 | 2.82 ± 2.92 | 2.99 ± 2.14 | |
| p-value 1: | 0.127 | 0.148 | 0.188 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derrick, S.A.; Nguyen, S.T.; Marthens, J.R.; Dambacher, L.L.; Sikalidis, A.K.; Reaves, S.K. A Mediterranean-Style Diet Improves the Parameters for the Management and Prevention of Type 2 Diabetes Mellitus. Medicina 2023, 59, 1882. https://doi.org/10.3390/medicina59101882
Derrick SA, Nguyen ST, Marthens JR, Dambacher LL, Sikalidis AK, Reaves SK. A Mediterranean-Style Diet Improves the Parameters for the Management and Prevention of Type 2 Diabetes Mellitus. Medicina. 2023; 59(10):1882. https://doi.org/10.3390/medicina59101882
Chicago/Turabian StyleDerrick, Stefani A., Sarah T. Nguyen, Jordan R. Marthens, Leah L. Dambacher, Angelos K. Sikalidis, and Scott K. Reaves. 2023. "A Mediterranean-Style Diet Improves the Parameters for the Management and Prevention of Type 2 Diabetes Mellitus" Medicina 59, no. 10: 1882. https://doi.org/10.3390/medicina59101882
APA StyleDerrick, S. A., Nguyen, S. T., Marthens, J. R., Dambacher, L. L., Sikalidis, A. K., & Reaves, S. K. (2023). A Mediterranean-Style Diet Improves the Parameters for the Management and Prevention of Type 2 Diabetes Mellitus. Medicina, 59(10), 1882. https://doi.org/10.3390/medicina59101882








