Anticoagulation Status and Left Atrial Appendage Occlusion Indications in Hospitalized Cardiology Patients with Atrial Fibrillation: A Hellenic Cardiorenal Morbidity Snapshot (HECMOS) Sub-Study
Abstract
:1. Introduction
2. Materials and Methods
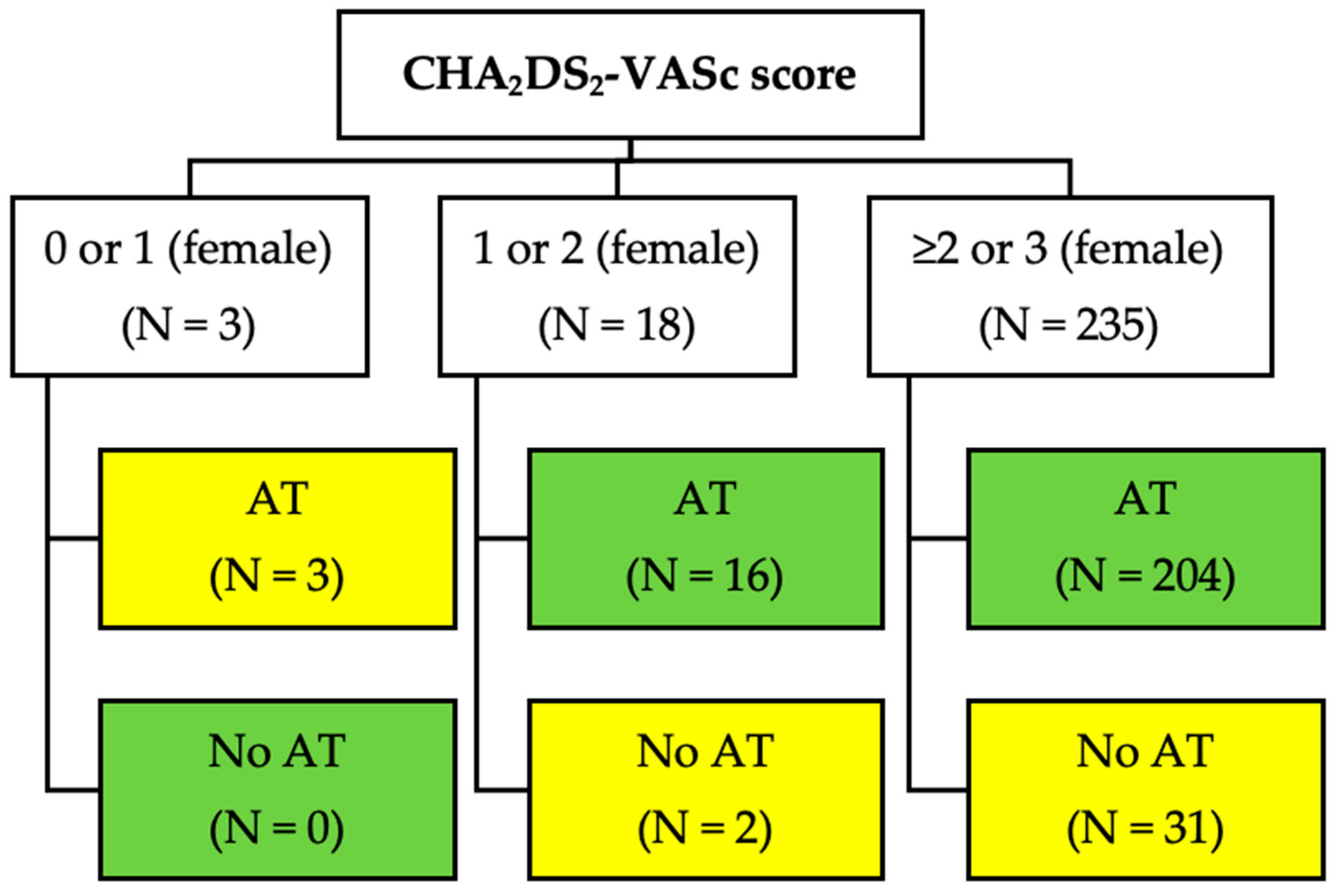
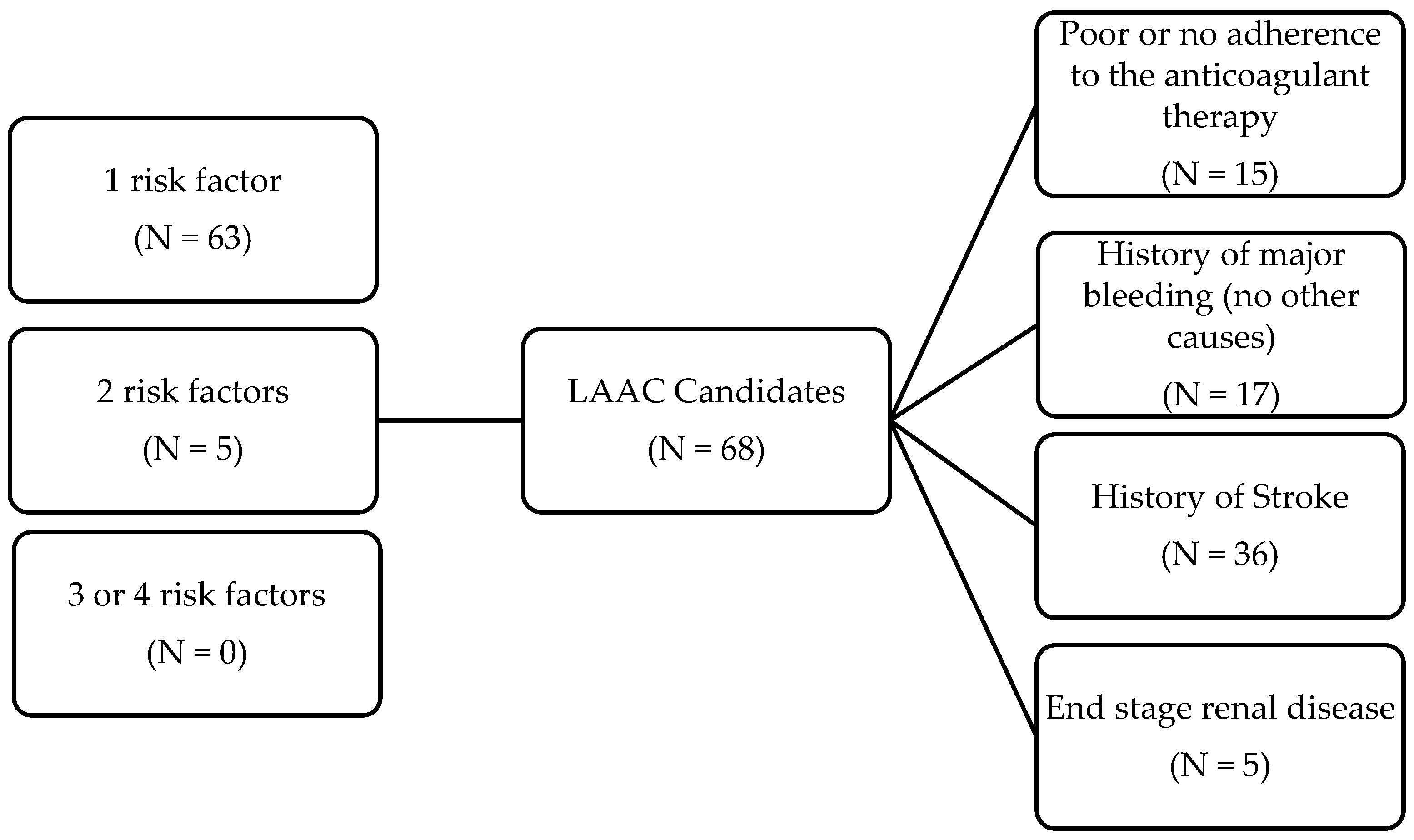
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef]
- Lamassa, M.; Di Carlo, A.; Pracucci, G.; Basile, A.M.; Trefoloni, G.; Vanni, P.; Spolveri, S.; Baruffi, M.C.; Landini, G.; Ghetti, A.; et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: Data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke 2001, 32, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Kartas, A.; Samaras, A.; Vasdeki, D.; Dividis, G.; Fotos, G.; Paschou, E.; Forozidou, E.; Tsoukra, P.; Kotsi, E.; Goulas, I.; et al. Flaws in Anticoagulation Strategies in Patients With Atrial Fibrillation at Hospital Discharge. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.E.; Tsioufis, C.; Konstantinidis, D.; Iliakis, P.; Leontsinis, I.; Tousoulis, D. Anticoagulation in Deep Venous Thrombosis: Current Trends in the Era of Non- Vitamin K Antagonists Oral Anticoagulants. Curr. Pharm. Des. 2020, 26, 2692–2702. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Alkhouli, M.; Reddy, V. Left Atrial Appendage Occlusion for The Unmet Clinical Needs of Stroke Prevention in Nonvalvular Atrial Fibrillation. Mayo Clin. Proc. 2019, 94, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, M.; Vidal, X.; Ballarin, E.; Rottenkolber, M.; Schmiedl, S.; Grave, B.; Huerta, C.; Martin-Merino, E.; Montero, D.; Leon-Muñoz, L.M.; et al. Adherence to Direct Oral Anticoagulants in Patients With Non-Valvular Atrial Fibrillation: A Cross-National Comparison in Six European Countries (2008–2015). Front. Pharmacol. 2021, 12, 682890. [Google Scholar] [CrossRef]
- Papakonstantinou, P.E.; Asimakopoulou, N.I.; Papadakis, J.A.; Leventis, D.; Panousieris, M.; Mentzantonakis, G.; Hoda, E.; Panagiotakis, S.; Gikas, A. Frailty Status Affects the Decision for Long-Term Anticoagulation Therapy in Elderly Patients with Atrial Fibrillation. Drugs Aging 2018, 35, 897–905. [Google Scholar] [CrossRef]
- Alkhouli, M.; Ellis, C.R.; Daniels, M.; Coylewright, M.; Nielsen-Kudsk, J.E.; Holmes, D.R. Left Atrial Appendage Occlusion. JACC Adv. 2022, 1, 100136. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Alkhouli, M. The History of the Left Atrial Appendage Occlusion. Card. Electrophysiol. Clin. 2020, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vrana, E.; Kartas, A.; Samaras, A.; Vasdeki, D.; Forozidou, E.; Liampas, E.; Karvounis, H.; Giannakoulas, G.; Tzikas, A. Indications for percutaneous left atrial appendage occlusion in hospitalized patients with atrial fibrillation. J. Cardiovasc. Med. (Hagerstown Md.) 2022, 23, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Leontsinis, I.; Farmakis, D.; Avramidis, D.; Andrikou, E.; Valatsou, A.; Gartzonikas, E.; Doundoulakis, I.; Zarifis, I.; Karpouzis, I.; Kafkala, K.; et al. Cardiorenal multimorbidity in hospitalized cardiology patients: The Hellenic Cardiorenal Morbidity Snapshot (HECMOS) study. Hell. J. Cardiol. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Maddox, T.M.; Kennedy, K.F.; Katz, D.F.; Marzec, L.N.; Lubitz, S.A.; Gehi, A.K.; Turakhia, M.P.; Marcus, G.M. Oral Anticoagulant Therapy Prescription in Patients With Atrial Fibrillation Across the Spectrum of Stroke Risk: Insights from the NCDR PINNACLE Registry. JAMA Cardiol. 2016, 1, 55–62. [Google Scholar] [CrossRef]
- Yu, A.Y.X.; Malo, S.; Svenson, L.W.; Wilton, S.B.; Hill, M.D. Temporal Trends in the Use and Comparative Effectiveness of Direct Oral Anticoagulant Agents Versus Warfarin for Nonvalvular Atrial Fibrillation: A Canadian Population-Based Study. J. Am. Heart Assoc. 2017, 6, e007129. [Google Scholar] [CrossRef]
- Marzec, L.N.; Wang, J.; Shah, N.D.; Chan, P.S.; Ting, H.H.; Gosch, K.L.; Hsu, J.C.; Maddox, T.M. Influence of Direct Oral Anticoagulants on Rates of Oral Anticoagulation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 2475–2484. [Google Scholar] [CrossRef]
- Admassie, E.; Chalmers, L.; Bereznicki, L.R. Changes in Oral Anticoagulant Prescribing for Stroke Prevention in Patients With Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 1133–1138. [Google Scholar] [CrossRef]
- Proietti, M.; Laroche, C.; Opolski, G.; Maggioni, A.P.; Boriani, G.; Lip, G.Y.H. ‘Real-world’ atrial fibrillation management in Europe: Observations from the 2-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace 2017, 19, 722–733. [Google Scholar] [CrossRef]
- Cullen, M.W.; Kim, S.; Piccini, J.P., Sr.; Ansell, J.E.; Fonarow, G.C.; Hylek, E.M.; Singer, D.E.; Mahaffey, K.W.; Kowey, P.R.; Thomas, L.; et al. Risks and benefits of anticoagulation in atrial fibrillation: Insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 461–469. [Google Scholar] [CrossRef]
- Kourlaba, G.; Stefanou, G.; Tsalamandris, S.; Oikonomou, E.; Papageorgiou, G.; Nikas, N.; Tousoulis, D.; Maniadakis, N. Incidence and cost of bleeding events requiring hospitalization in patients with atrial fibrillation treated with acenocoumarol in Greece. Hell. J. Cardiol. 2021, 62, 234–240. [Google Scholar] [CrossRef]
- Yang, E. A clinician’s perspective: Novel oral anticoagulants to reduce the risk of stroke in nonvalvular atrial fibrillation--full speed ahead or proceed with caution? Vasc. Health Risk Manag. 2014, 10, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Labori, F.; Persson, J.; Bonander, C.; Jood, K.; Svensson, M. Cost-effectiveness analysis of left atrial appendage occlusion in patients with atrial fibrillation and contraindication to oral anticoagulation. Eur. Heart J. 2022, 43, 1348–1356. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. Europace 2020, 22, 184. [Google Scholar] [CrossRef]
- Tzikas, A.; Freixa, X.; Llull, L.; Gafoor, S.; Shakir, S.; Omran, H.; Giannakoulas, G.; Berti, S.; Santoro, G.; Kefer, J.; et al. Patients with intracranial bleeding and atrial fibrillation treated with left atrial appendage occlusion: Results from the Amplatzer Cardiac Plug registry. Int. J. Cardiol. 2017, 236, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Cruz-González, I.; González-Ferreiro, R.; Freixa, X.; Gafoor, S.; Shakir, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; et al. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). Results from the Amplatzer Cardiac Plug registry. Rev. Esp. De Cardiol. 2020, 73, 28–34. [Google Scholar] [CrossRef]
- Potpara, T.S.; Ferro, C.J.; Lip, G.Y.H. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nat. Rev. Nephrol. 2018, 14, 337–351. [Google Scholar] [CrossRef]
- Kefer, J.; Tzikas, A.; Freixa, X.; Shakir, S.; Gafoor, S.; Nielsen-Kudsk, J.E.; Berti, S.; Santoro, G.; Aminian, A.; Landmesser, U.; et al. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Int. J. Cardiol. 2016, 207, 335–340. [Google Scholar] [CrossRef]
- Banerjee, A.; Benedetto, V.; Gichuru, P.; Burnell, J.; Antoniou, S.; Schilling, R.J.; Strain, W.D.; Ryan, R.; Watkins, C.; Marshall, T.; et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: A population-based study. Heart (Br. Card. Soc.) 2020, 106, 119–126. [Google Scholar] [CrossRef]
- Thakkar, J.; Vasdeki, D.; Tzikas, A.; Meier, B.; Saw, J. Incidence, Prevention, and Management of Periprocedural Complications of Left Atrial Appendage Occlusion. Interv. Cardiol. Clin. 2018, 7, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Badimon, J.J.; Escolar, G.; Zafar, M.U. Factor XI/XIa Inhibition: The Arsenal in Development for a New Therapeutic Target in Cardio- and Cerebrovascular Disease. J. Cardiovasc. Dev. Dis. 2022, 9, 437. [Google Scholar] [CrossRef]
- Verma, A.; Ha, A.C.T.; Kirchhof, P.; Hindricks, G.; Healey, J.S.; Hill, M.D.; Sharma, M.; Wyse, D.G.; Champagne, J.; Essebag, V.; et al. The Optimal Anti-Coagulation for Enhanced-Risk Patients Post-Catheter Ablation for Atrial Fibrillation (OCEAN) trial. Am. Heart J. 2018, 197, 124–132. [Google Scholar] [CrossRef] [PubMed]
| History of Atrial Fibrillation | |
|---|---|
| Classification (N = 256) | |
| Paroxysmal | 97 (37.9%) |
| Permanent | 159 (62.1%) |
| On antiarrhythmic medication (including β-blockers) | 200 (78.1%) |
| Previous cardioversion | 34 (13.2%) |
| History of catheter ablation | 9 (3.6%) |
| Permanent pacemaker | 27 (10.5%) |
| Defibrillator | 9 (3.5%) |
| CRT-D | 4 (1.5%) |
| Comorbidities (N = 256) | |
| Smoker (current) | 19 (7.4%) |
| Hypertension | 189 (73.8%) |
| Diabetes | 88 (34.4%) |
| Chronic kidney disease | 97 (38%) |
| Obstructive sleep apnea | 13 (5.1%) |
| Severe aortic stenosis | 10 (4%) |
| Severe aortic regurgitation | 2 (0.7%) |
| Severe mitral regurgitation | 9 (3.5%) |
| Anticoagulation treatment among patients with good compliance (N = 223) | |
| VKA—good INR control | 25 (11.2%) |
| VKA—poor INR control | 7 (3.1%) |
| Dabigatran | 27 (12.1%) |
| Rivaroxaban | 53 (23.8%) |
| Apixaban | 111 (49.8%) |
| Beyond anticoagulants, are on any other medical treatments which increase bleeding risk? (Yes) (N = 256) | 43 (16.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiachris, D.; Papakonstantinou, P.E.; Doundoulakis, I.; Tsioufis, P.; Botis, M.; Dimitriadis, K.; Leontsinis, I.; Kordalis, A.; Antoniou, C.-K.; Mantzouranis, E.; et al. Anticoagulation Status and Left Atrial Appendage Occlusion Indications in Hospitalized Cardiology Patients with Atrial Fibrillation: A Hellenic Cardiorenal Morbidity Snapshot (HECMOS) Sub-Study. Medicina 2023, 59, 1881. https://doi.org/10.3390/medicina59101881
Tsiachris D, Papakonstantinou PE, Doundoulakis I, Tsioufis P, Botis M, Dimitriadis K, Leontsinis I, Kordalis A, Antoniou C-K, Mantzouranis E, et al. Anticoagulation Status and Left Atrial Appendage Occlusion Indications in Hospitalized Cardiology Patients with Atrial Fibrillation: A Hellenic Cardiorenal Morbidity Snapshot (HECMOS) Sub-Study. Medicina. 2023; 59(10):1881. https://doi.org/10.3390/medicina59101881
Chicago/Turabian StyleTsiachris, Dimitris, Panteleimon E. Papakonstantinou, Ioannis Doundoulakis, Panagiotis Tsioufis, Michail Botis, Kyriakos Dimitriadis, Ioannis Leontsinis, Athanasios Kordalis, Christos-Konstantinos Antoniou, Emmanouil Mantzouranis, and et al. 2023. "Anticoagulation Status and Left Atrial Appendage Occlusion Indications in Hospitalized Cardiology Patients with Atrial Fibrillation: A Hellenic Cardiorenal Morbidity Snapshot (HECMOS) Sub-Study" Medicina 59, no. 10: 1881. https://doi.org/10.3390/medicina59101881
APA StyleTsiachris, D., Papakonstantinou, P. E., Doundoulakis, I., Tsioufis, P., Botis, M., Dimitriadis, K., Leontsinis, I., Kordalis, A., Antoniou, C.-K., Mantzouranis, E., Iliakis, P., Vlachakis, P. K., Gatzoulis, K. A., & Tsioufis, K. (2023). Anticoagulation Status and Left Atrial Appendage Occlusion Indications in Hospitalized Cardiology Patients with Atrial Fibrillation: A Hellenic Cardiorenal Morbidity Snapshot (HECMOS) Sub-Study. Medicina, 59(10), 1881. https://doi.org/10.3390/medicina59101881











