Preventing the Progression of Myopia in Children—A Review of the Past Decade
Abstract
:1. Introduction
2. Materials and Methods
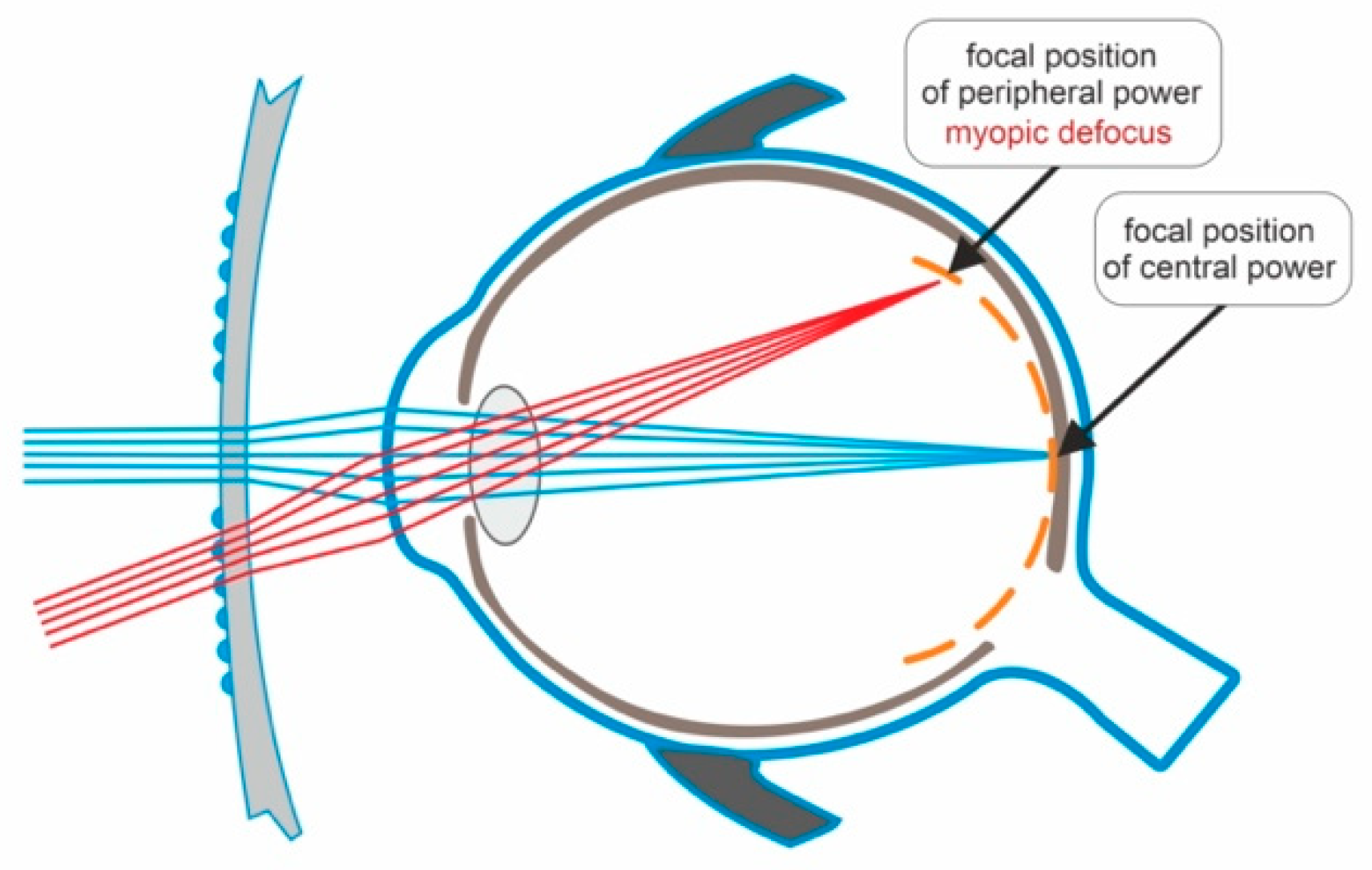
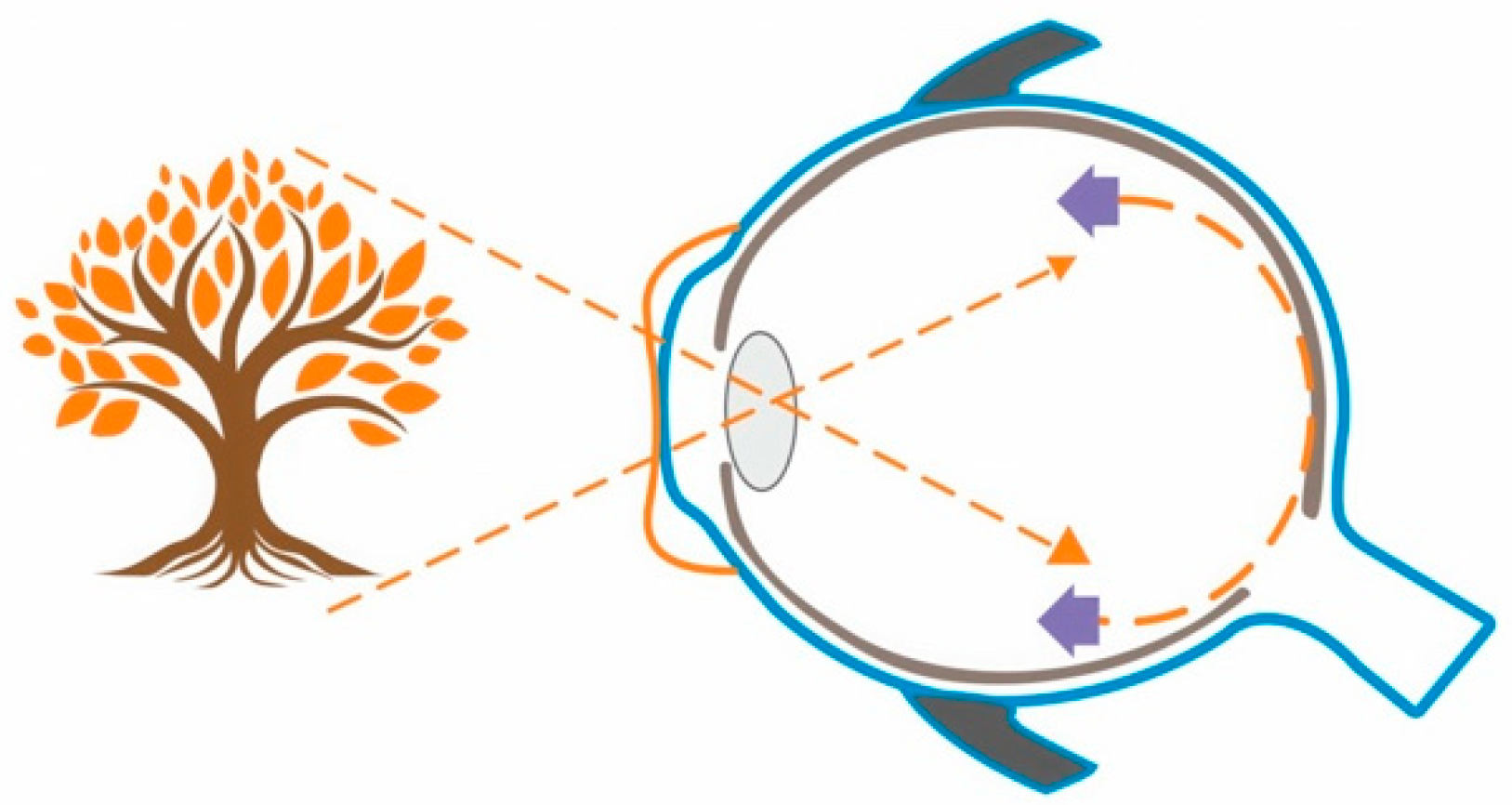
3. The Efficacy and Safety of Defocus-Incorporated Multiple-Segment Spectacle Lenses in the Prevention of Myopia Progression
- Group 1 (36 children) wore defocus-incorporated multiple-segment spectacle lenses (DIMSsl) for the entire 6-year duration;
- Group 2 (14 children) wore DIMSsl for the first 3.5 years and then switched to single-vision lenses (SVsl);
- Group 3 (22 children) wore SVsl for the first 2 years and then switched to DIMSsl for the remaining 4 years;
- Group 4 (18 children) wore SVsl for the first 2 years, then DIMSsl for 1.5 years, and finally switched back to SVsl for the last 2.5 years.
- Group 1: SER −3.04 ± 0.89 D/−3.69 ± 1.42 D;
- Group 2: SER −2.98 ± 1.13 D/−4.28 ± 1.15 D;
- Group 3: SER −2.68 ± 0.88 D/−3.92 ± 1.18 D;
- Group 4: SER −2.65 ± 1.18 D/−3.87 ± 1.53 D.
- Group 1: AXL 24.68 ± 0.76 mm/25.28 ± 0.81 mm;
- Group 2: AXL 25.00 ± 0.80 mm/25.71 ± 0.69 mm;
- Group 3: AXL 24.62 ± 0.79 mm/25.43 ± 1.01 mm;
- Group 4: AXL 24.42 ± 0.86 mm/25.14 ± 0.87 mm.
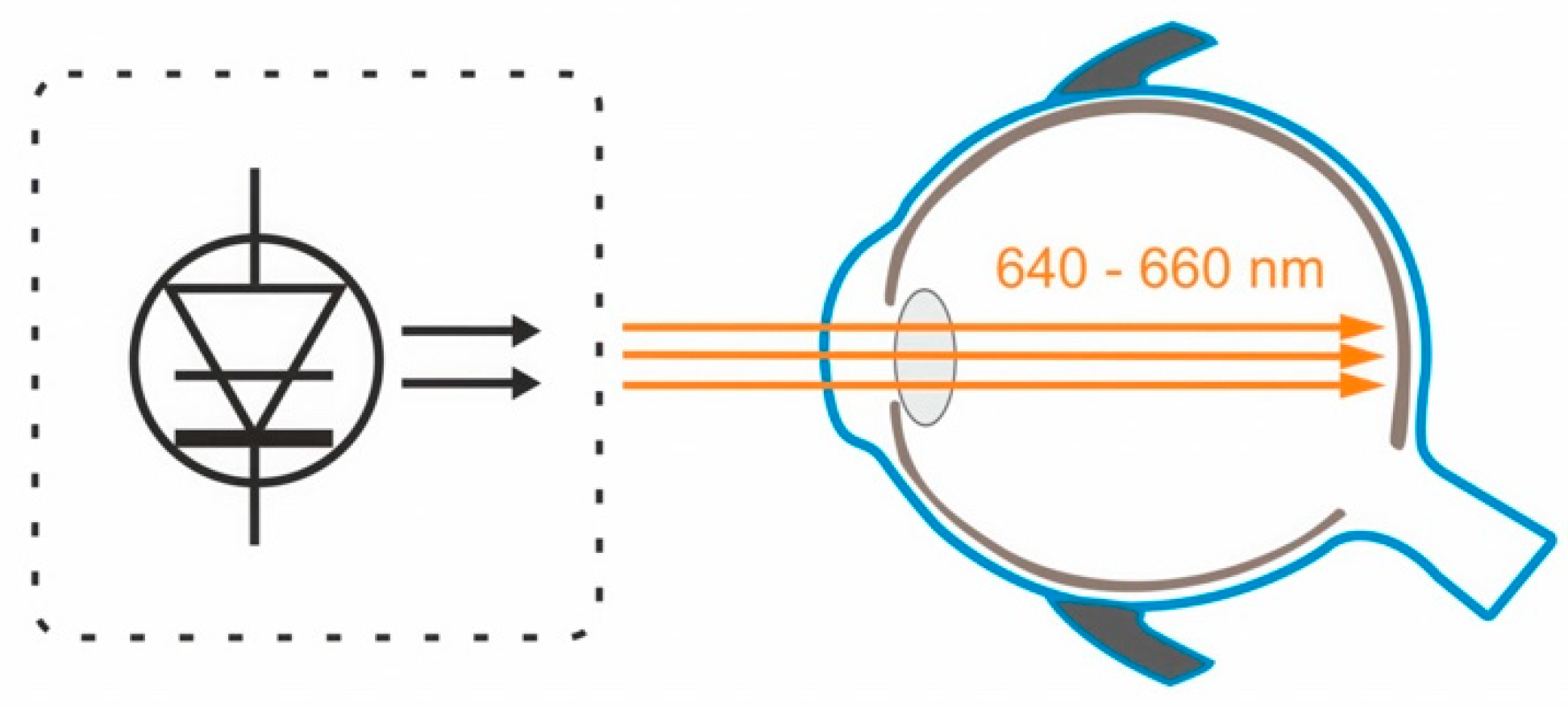
4. The Efficacy and Safety of Low-Intensity Red-Light Therapy in the Prevention of Myopia Progression
5. The Efficacy and Safety of the Combination of Orthokeratology and Low-Dose Atropine 0.01%
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foster, P.J.; Jiang, Y. Epidemiology of myopia. Eye 2014, 28, 202–208. [Google Scholar] [CrossRef]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Matsui, K.; Lai, T.Y.Y.; Lai, C.-C.; Cheung, C.M.G. Updates of pathologic myopia. Prog. Retin. Eye Res. 2016, 52, 156–187. [Google Scholar] [CrossRef] [PubMed]
- Mutti, D.O.; Mitchell, G.L.; Moeschberger, M.L.; Jones, L.A.; Zadnik, K. Parental myopia, near work, school achievement, and children’s refractive error. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3633–3640. [Google Scholar]
- Hammond, C.J.; Snieder, H.; Gilbert, C.E.; Spector, T.D. Genes and environment in refractive error: The twin eye study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1232–1236. [Google Scholar]
- Lingham, G.; Mackey, D.A.; Lucas, R.; Yazar, S. How does spending time outdoors protect against myopia? A review. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef]
- Goss, D.A.; Wickham, M.G. Retinal-image mediated ocular growth as a mechanism for juvenile onset myopia and for emmetropization. A literature review. Doc. Ophthalmol. Adv. Ophthalmol. 1995, 90, 341–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wen, D.; Wang, Q.; McAlinden, C.; Flitcroft, I.; Chen, H.; Saw, S.M.; Chen, H.; Bao, F.; Zhao, Y.; et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology 2016, 123, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Tang, W.C.; Lee, P.H.; Zhang, H.Y.; Qi, H.; Hasegawa, K.; To, C.H. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: Results of a 3-year follow-up study. Br. J. Ophthalmol. 2022, 106, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.Y.; Tang, W.C.; Qi, H.; Radhakrishnan, H.; Hasegawa, K.; To, C.H.; Charman, W.N. Effect of Defocus Incorporated Multiple Segments Spectacle Lens Wear on Visual Function in Myopic Chinese Children. Transl. Vis. Sci. Technol. 2020, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.Y.; Tang, W.C.; Tse, D.Y.-Y.; Lee, R.P.K.; Chun, R.K.M.; Hasegawa, K.; Qi, H.; Hatanaka, T.; To, C.H. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2020, 104, 363–368. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Tang, W.C.; Zhang, H.Y.; Lee, P.H.; Tse, D.Y.Y.; Qi, H.; Vlasak, N.; To, C.H. Long-term myopia control effect and safety in children wearing DIMS spectacle lenses for 6 years. Sci. Rep. 2023, 13, 5475. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y.; Huang, D.; Yang, J.; Fan, C.; Chen, C.; Li, J.; Wang, Q.; Li, S.; Jiang, B.; et al. The Efficacy of Defocus Incorporated Multiple Segments Lenses in Slowing Myopia Progression: Results from Diverse Clinical Circumstances. Ophthalmology 2023, 130, 542–550. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Lam, C.S.Y.; Tang, W.C.; Lee, P.H.; Tse, D.Y.; To, C.H. Changes in relative peripheral refraction in children who switched from single-vision lenses to Defocus Incorporated Multiple Segments lenses. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2023, 43, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Lam, C.S.Y.; Tang, W.C.; Leung, M.; To, C.H. Defocus Incorporated Multiple Segments Spectacle Lenses Changed the Relative Peripheral Refraction: A 2-Year Randomized Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2020, 61, 53. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Liao, Y.; Zhou, W.; Li, Q.; Wang, J.; Tang, J.; Pei, Y.; Wang, X. Low-intensity red-light therapy in slowing myopic progression and the rebound effect after its cessation in Chinese children: A randomized controlled trial. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2023, 261, 575–584. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, R.; Chen, X.; Zhang, J.; Bulloch, G.; Lin, X.; Wu, X.; Li, J. Efficacy Comparison of Repeated Low-Level Red Light and Low-Dose Atropine for Myopia Control: A Randomized Controlled Trial. Transl. Vis. Sci. Technol. 2022, 11, 33. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, Z.; Xu, H.; He, M. Myopia Control Effect of Repeated Low-Level Red-Light Therapy in Chinese Children: A Randomized, Double-Blind, Controlled Clinical Trial. Ophthalmology 2023, 130, 198–204. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Zhu, Z.; Xiang, K.; Zhang, X.; Zhang, B.; Chen, J.; Yang, J.; Du, L.; Niu, C.; et al. Effect of Repeated Low-level Red Light on Myopia Prevention Among Children in China With Premyopia: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e239612. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Z.; Tan, X.; Kong, X.; Zhong, H.; Zhang, J.; Xiong, R.; Yuan, Y.; Zeng, J.; Morgan, I.G.; et al. Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology 2022, 129, 509–519. [Google Scholar] [CrossRef]
- Xiong, R.; Zhu, Z.; Jiang, Y.; Kong, X.; Zhang, J.; Wang, W.; Kiburg, K.; Yuan, Y.; Chen, Y.; Zhang, S.; et al. Sustained and rebound effect of repeated low-level red-light therapy on myopia control: A 2-year post-trial follow-up study. Clin. Exp. Ophthalmol. 2022, 50, 1013–1024. [Google Scholar] [CrossRef]
- Xiong, R.; Zhu, Z.; Jiang, Y.; Wang, W.; Zhang, J.; Chen, Y.; Bulloch, G.; Yuan, Y.; Zhang, S.; Xuan, M.; et al. Longitudinal Changes and Predictive Value of Choroidal Thickness for Myopia Control after Repeated Low-Level Red-Light Therapy. Ophthalmology 2023, 130, 286–296. [Google Scholar] [CrossRef]
- Jiang, J.; Long, W.; Hu, Y.; Zhao, F.; Zhao, W.; Zheng, B.; Feng, Z.; Li, Z.; Yang, X. Accommodation and vergence function in children using atropine combined with orthokeratology. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2023, 46, 101704. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, N.; Konno, Y.; Hamada, N.; Kanda, Y.; Shimmura-Tomita, M.; Kaburaki, T.; Kakehashi, A. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: A 2-year randomised trial. Sci. Rep. 2020, 10, 12750. [Google Scholar] [CrossRef]
- Kinoshita, N.; Konno, Y.; Hamada, N.; Kanda, Y.; Shimmura-Tomita, M.; Kakehashi, A. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: First year results. Jpn. J. Ophthalmol. 2018, 62, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Ng, A.L.; Cheng, G.P.; Woo, V.C.; Cho, P. Combined 0.01% atropine with orthokeratology in childhood myopia control (AOK) study: A 2-year randomized clinical trial. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2023, 46, 101723. [Google Scholar] [CrossRef]
- Tan, Q.; Ng, A.L.; Choy, B.N.; Cheng, G.P.; Woo, V.C.; Cho, P. One-year results of 0.01% atropine with orthokeratology (AOK) study: A randomised clinical trial. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2020, 40, 557–566. [Google Scholar] [CrossRef]
- Vincent, S.J.; Tan, Q.; Ng, A.L.K.; Cheng, G.P.M.; Woo, V.C.P.; Cho, P. Higher order aberrations and axial elongation in combined 0.01% atropine with orthokeratology for myopia control. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2020, 40, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Wang, N. Combined Orthokeratology with Atropine for Children with Myopia: A Meta-Analysis. Ophthalmic Res. 2021, 64, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Du, L.; Ji, N.; Li, B.; Pang, X.; Li, X.; Ma, N.; Huang, C.; Fu, A. Combination of orthokeratology lens with 0.01% atropine in slowing axial elongation in children with myopia: A randomized double-blinded clinical trial. BMC Ophthalmol. 2022, 22, 438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Z.; Hu, Y.; Jiang, J.; Long, W.; Cui, D.; Chen, W.; Yang, X. Short-term effects of atropine combined with orthokeratology (ACO) on choroidal thickness. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2021, 44, 101348. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Sorsby, A. THE CONTROL OF SCHOOL MYOPIA. Br. Med. J. 1933, 2, 730–733. [Google Scholar] [CrossRef] [PubMed]
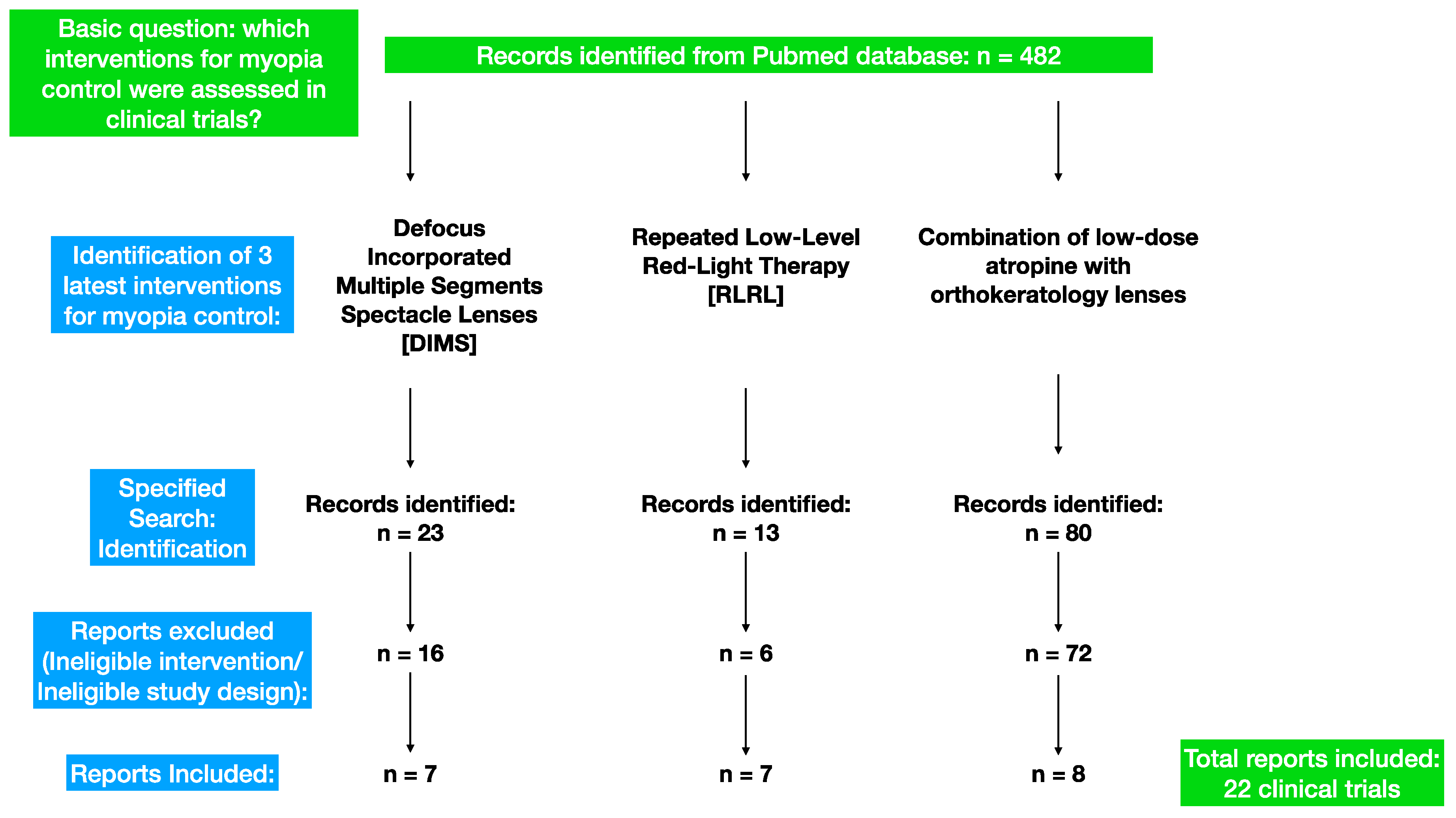
| Date of Search: | May–June 2023 |
|---|---|
| Database searched: | PubMed |
| Target items: | Journal papers |
| Years covered by search: | 2011–2023 |
| Language: | English |
| Search terms used: | DIMS AND myopia control, Orthokeratology AND atropine AND myopia control, low-level red-light therapy AND myopia control. |
| Article: | Design: | Evaluated on: | Results: |
|---|---|---|---|
| Lam et al. 2020 [12] | Prospective, randomized, double-masked. Cycloplegic refraction and axial length measured at baseline and each 6-month interval over 2 years. | 183 myopic children (Range: −1.0–5.0 D) Aged: 8–13 years old | DIMSsl group: myopic progression—0.41 ± 0.06 D (52% slower than SVsl) Axial length elongation—0.21 ± 0.02 mm (62% less than SVsl) SVsl group: myopic progression—0.85 ± 0.08 D Axial length elongation: 0.55 ± 0.02 mm |
| Zhang et al. 2020 [16] | Prospective, randomized, double-masked. Cycloplegic central refraction, peripheral refraction (at 6 retinal points (10°, 20°, and 30° nasally and temporally), and axial length measured at baseline and each 6-month interval over 2 years. | 183 myopic children (Range: −1.0–5.0 D) Aged: 8–13 years old | DIMSsl group: constant and symmetrical RPR profile. SVsl group: asymmetrical peripheral myopic shifts between the nasal and temporal retina. |
| Lam et al. 2020 [11] | Prospective, randomized, double-masked. Distance and near best-corrected visual acuity measured monocularly; distance and near phoria; Monocular and binocular amplitude of accommodation; Lag of accommodation; Stereopsis—measured at baseline and each six-month interval over 2 years. | 160 myopic children (Range: −1.0–5.0 D) Aged: 8–13 years old | After 2 years:
|
| Lam et al. 2022 [10] | Prospective, not randomized. 1-year follow-up, spherical equivalent refraction, and axial length measured every 6 months. Historical control group from clinical records of the optometry clinic. | 128 children: DIMS continuation n = 65 children, SVsl switched to DIMS n = 55 | Myopic progression (spherical equivalent refraction) over 3 years: DIMSsl—−0.52± 0.69 D SVsl to DIMSsl—0.92 ± 0.81 D Axial length elongation over 3 years: DIMSsl—0.31 ± 0.26 mm SVsl to DIMSsl—0.57 ± 0.33 mm |
| Zhang et al. 2023 [15] | Prospective, not randomized. 1-year follow-up, Cycloplegic central refraction, peripheral refraction (at 6 retinal points (10°, 20°, and 30° nasally and temporally), and axial length measured at baseline and every 6-month interval for 3 years. | 128 children: DIMS continuation n = 65 SVsl switched to DIMS after 2 years n = 55 | DIMS group: constant and symmetrical PRP profile SVsl in the first 2 years: significant increases in hyperopic RPR at 20° nasal. After switching to DIMSsl in the third year significant reductions in hyperopic RPR at 20 N (mean difference: −1.14 ± 1.93 D, p < 0.0001) and 30 N (mean difference: −1.07 ± 1.17 D, p < 0.0001) |
| Lam et al. 2023 [13] | Prospective 6-year follow up. Measured values: Axial length, Cycloplegic refraction. | 90 myopic children completed the study. Mean age at the enrollment was about 10 years old. Group 1: DIMSsl 6 years n = 36 Group 2: DIMSsl 3.5 years, SVsl 2.5 years n = 14 Group 3: SVsl 2 years, DIMSsl 4 years n = 22 Group 4: SVsl 2 years, DIMSsl 1.5 years, SVsl 2.5 years n = 18 | SER at baseline/at 6 years for each group: Gr. 1 −3.04 ± 0.89/−3.69 ± 1.42 D Gr. 2 −2.98 ± 1.13/−4.28 ± 1.15 D Gr. 3 −2.68 ± 0.88/−3.92 ± 1.18 D Gr. 4 −2.65 ± 1.18/−3.87 ± 1.53 D AXL at baseline/at 6 years for each group: Gr. 1: 24.68 ± 0.76/25.28 ± 0.81 mm Gr. 2: 25.00 ± 0.80/25.71 ± 0.69 mm Gr. 3: 24.62 ± 0.79/25.43 ± 1.01 mm Gr. 4: 24.42 ± 0.86/25.14 ± 0.87 mm |
| Liu et al. 2023 [14] | Retrospective. Propensity score matching strategy. | Myopic patients 6–16 years old 3639 patients wearing DIMSsl 6838 patients wearing SVsl After PSM: 2240 pairs with one-year follow-up 735 pairs with two-year follow-up | Myopia progression in the first year: DIMSsl, –0.50 ± 0.43 D; SVsl, –0.77 ± 0.58 D; p < 0.001 Myopia progression in the second year: DIMS, –0.88 ± 0.62 D; SV, –1.23 ± 0.76 D; p < 0.001 |
| Article: | Design: | Evaluated on: | Results: |
|---|---|---|---|
| Jiang et al. 2022 [21] | Multicenter, randomized, parallel-group, single-blind clinical trial Cycloplegic refraction and axial length measured at baseline and 1-, 3-, 6-, and 12-month follow-up visits. | 264 children 8–13 years old with myopia from −1.0 to −5.0 D (cycloplegic SER). 117 RLRL group 129 SVsl group | Adjusted 12-month axial elongation: RLRL: 0.13 mm (95% CI, 0.09–0.17 mm) SVsl: 0.38 mm (95% CI 0.34–0.42 mm) Adjusted 12-month SER: RLRL: −0.20 D (95% CI, −0.29 to −0.11 D) SVsl: −0.79 D (95% CI, −0.88 to −0.69 D) |
| Dong et al. 2023 [19] | Prospective, randomized, double-blind, controlled clinical trial, Cycloplegic refraction and axial length measured at baseline and at six months. | 112 Chinese myopic children 7–12 years old. RLRL group n = 56 Sham device control group n = 55 | Mean SER change over 6 months: RLRL: −0.06 ± 0.03 D Sham device: −0.11 ± 0.33 D Mean AXL changes over 6 months: RLRL: −0.02 ± 0.11 D Sham device: −0.13 ± 0.10 D No treatment-related adverse events were reported |
| Xiong et al. 2022 [22] | Prospective, post-trial follow-up study/real-world study (RWS). Cycloplegic refraction and axial length measured at 24 months from the beginning of RCT. | 114 children who completed a real-world study (after completing 1-year RCT the participants were invited to voluntarily participate an RWS). SVS-SVS group n = 41 SVS-RLRL group n = 10 RLRL-SVS group n = 52 RLRL-RLRL group n = 11 | Over a 2-year period mean AXL change: SVS-SVS: −0.28 ± 0.14 mm SVS-RLRL: −0.05 ± 0.24 mm RLRL-SVS: −0.42 ± 0.20 mm RLRL-RLRL: −0.12 ± 0.16 mm Over a 2-year period mean SER change: SVS-SVS: −0.54 ± 0.39 D SVS-RLRL: −0.09 ± 0.55 D RLRL-SVS: −0.91 ± 0.48 D RLRL-RLRL: −0.20 ± 0.56 D A modest rebound effect was noted after treatment cessation. |
| Xiong et al. [23] | Secondary analysis of data from multicenter RCT. Values measured at 1, 3, 6, and 12 months: Changes in macular choroidal thickness (mCT) assessed by SS-OCT, Visual acuity, Axial Length, SER, and treatment compliance. Additionally: their associations with myopia control. | 120 children: RLRL group n = 60 SVS n = 60 | Changes in the mCT from baseline for the RLRL group: 1 month: 14,755 μm 3 months: 5286 μm 6 months: 1543 μm 12 months: 9089 μm SVS group: 1 month: 1111 μm 3 months: 8212 μm 6 months: 10,190 μm 12 months: 10,407 μm Models including only mCT changes at 3 months had acceptable predictive discrimination of good myopia control over 12 months. |
| Chen et al. 2022 [18] | Prospective, single-masked, single-center randomized controlled trial. Primary outcome: change in AXL Secondary outcome: change in SER followed at 1, 3, 6, and 12 months. | 62 children 7 to 15 years old. Repeated Low-Level Red light (RLRL) group n = 31 Low-dose Atropine (LDA) group n = 31 | Mean one-year change in AXL: RLRL: 0.08 mm (95% CI, 0.03–0.14 mm) LDA: 0.33 mm (95% CI 0.27–0.38 mm) Mean 1-year change in SER: RLRL: −0.03 D (95% CI, −0.01 to −0.08 D) LDA: −0.57 D (95% CI, −0.40 to −0.73 D) |
| Chen et al. 2022 [17] | Prospective, randomized, controlled clinical trial. Phase 1—treatment phase (intervention: two sessions per day lasting 3 min)–12 month Phase 2—washout phase—LRL cessation. Ophthalmic examinations at 3, 6, 9, 12, and 15 months. The outcomes: Axial length (AL), spherical equivalent refraction (SER), subfoveal choroidal thickness (SFCT), and accommodative function. | 102 children 6–13 years old. Low-intensity red-light (LRL) group n = 51 Single-focus spectacles (SFS) group n =51 At 12 months completed: 46 LRL and 40 SFS | AXL elongation at 12 months: LRL: 0.01 mm (95% CI 0.05–0.07 mm) SFS: 0.39 mm (95% CI 0.33–0.45 mm) SER progression at 12 months: LRL: 0.05 D (95% CI 0.08–0.19 D) SFS: 0.64 D (95%CI 0.78–0.51 D) Changes in SFCT in the LRL group: thickening in the first 3 months, relative stability in the following months. SFS: progressive thinning of the SFCT Accommodative function assessed with: AA amplitude of accommodation AR accommodative response AF accommodative facility PRA positive relative accommodation NRA negative relative accommodation Main outcome: AR and PRA in the LRL group were more negative than in the SFS group. |
| He et al. 2023 [20] | Prospective, randomized clinical trial, parallel groups in 10 primary schools in Shanghai. Intervention group: RLRL twice a day 5 days per week each session lasting 3 min. Primary outcome: 12-month incidence rate of myopia (SER smaller or equal—0.5 D). Secondary outcomes: SER changes, Axial length, vision function, and optical tomography scan results over 12 months. | 139 children with premyopia, primary school Grade 1–4. SER −0.5–0.5 diopter, at least one parent with SER smaller or equal −3.0 D. | 12-month incidence of myopia in the RLRL group: 40.8% (49 of 120) in the control group: 61.3% (68 of 111). The RLRL intervention significantly reduced SER and AXL (myopic shifts). No visual acuity or structural damage was observed on OCT in the intervention group. |
| Article: | Design: | Evaluated on: | Results: |
|---|---|---|---|
| Tan et al. 2020 [28] | Prospective, randomized, single-masked clinical trial. Intervention: instillation of 0.01% atropine eye-drop once a day in each eye and nightly wear of 4-zone ortho-k lenses or nightly wear of 4-zone ortho-k lenses alone. After baseline 3 monthly visits for atropine prescription and ocular health monitoring. Cycloplegic examinations took place every six months. Measured parameters: refractive error, visual acuity, pupil size, amplitude of accommodation, intraocular pressure, corneal topography, axial length. | Chinese children aged 6–11 years old 29 finished 1 year trial in atropine and orthokeratology group (AOK) 30 finished 1 year trial in orthokeratology only group (OK) | Overall axial elongation in the AOK group: 0.07 (SD 0.16) mm In OK group: 0.16 (SD 0.15) mm A significant difference between groups was observed only during the first 6 months Mesopic and photopic pupil size in the AOK group: 0.64 (SD: 0.48) mm; 0.36 (SD: 0.34) mm In OK group: 0.10 (SD: 0.50) mm and 0.02 (SD: 0.28) mm |
| Tan et al. 2023 [27] | Prospective, randomized, single-masked clinical trial. Intervention: instillation of 0.01% atropine eye-drop once a day in each eye and nightly wear of 4-zone ortho-k lenses or nightly wear of 4-zone ortho-k lenses alone. Data collection visits took place one month after commencement and every six months later. Measurements included: refractive error, visual acuity, pupil size, and choroidal thickness (before cycloplegia). | Chinese children aged 6–11 years old 34 finished 2-year trial in the atropine and orthokeratology (AOK) group 35 finished a 2-year trial in the orthokeratology-only group (OK). | Overall axial elongation in the AOK group: 0.17 (SD 0.03) mm In OK group: 0.34 (SD 0.03) mm. Mesopic and photopic pupil size in the AOK group: 0.70 (SD: 0.09) mm; 0.78 (SD: 0.07) mm In OK group: 0.31 (SD: 0.09) mm and 0.23 (SD: 0.07) mm. Thickening of the choroid: AOK group—22.6 (SD: 3.5) μm OK group—−9.0 (SD: 3.5) μm. Adverse events: higher incidence of photophobia in the AOK group. |
| Kinoshita et al. 2018 [26] | Prospective, randomized clinical trial. A total of participants wore OK lenses for 3 months. Afterward, they were randomly assigned to: Group 1 receiving OK and atropine 0.01% Group 2 receiving only OK Every 3 months measurements of the AXL. | 41 Japanese children 8–12 years old. SER from −1.0 to −6.0 diopters. | Axial length over 1 year: Group 1: 0.09 ± 0.12 mm Group 2: 0.19 ± 0.15 mm |
| Kinoshita et al. 2020 [25] | Prospective, interventional, parallel-group randomized clinical trial. Participants were randomly assigned to: Combination group (orthokeratology and 0.01% atropine) Monotherapy group (orthokeratology). Measured values: axial length, corneal endothelial cell density, intraocular pressure, uncorrected distant and near visual acuity, refraction, and corneal topography. | 80 Japanese children 8–12 years old. SER from −1.0 to −6.0 diopters. 73 completed a 2-year study. | Over 2 years axial length increase: Combination gr. −0.29 ± 0.20 mm Monotherapy gr. −0.40 ± 0.23 mm AXL increase in the subgroup with initial SER from −1.0 to −3.0: Combination gr. −0.30 ± 0.22 Monotherapy gr. −0.48 ± 0.22. With Initial SER from −3.01 to −6.0: Combination gr. −0.27 ± 0.15 Monotherapy gr. −0.25 ± 0.17. |
| Jiang et al. 2023 [24] | Prospective, randomized clinical trial. Divided into four groups: combination group (OK lenses and 0.01% atropine), OK group (OK lenses and placebo eyedrops), atropine group (0.01% atropine and spectacles), and control group (placebo eyedrops, spectacles). Measurements at baseline and after 3 months: subjective refraction, accommodative amplitude, negative and positive relative accommodation, accommodative facility, accommodative lag, horizontal phoria, horizontal fusion vergence, AC/A ratio. | 62 participants aged from 8 to 12 years old with SER from −1.0 to −6.0 completed the study. | After 3 months:
|
| Zhao et al. 2021 [32] | Prospective, randomized, controlled trial. Group 1: 0.01% atropine and orthokeratology n = 39, Gr. 2: atropine 0.01% and single-vision glasses n = 42 Gr. 3: orthokeratology and placebo n = 36 Gr. 4: placebo and single-vision glasses n = 37 Measurements at baseline and after one month of intervention included: Subfoveal choroidal thickness, ocular biometrics, autorefraction, and best-corrected visual acuity. | 154 children 8–12 years old, SER from −1.0 to −6.0 diopters. | SFChT changes: Gr. 1: 14.12 ± 12.88 μm Gr. 2: 5.49 ± 9.38 μm Gr. 3: 9.43 ± 9.14 μm Gr. 4: −4.81 ± 9.93 μm |
| Vincent et al. 2020 [29] | Prospective, randomized clinical trial. Assignation to OK treatment (n = 28), or OK combined with 0.01% atropine (n = 25). Measurements: photopic and scotopic pupil diameters and higher order aberrations axial length at baseline and at six months. | Children age 6–11 years old. SER from −1.0 to −4.0 diopter | Photopic pupil diameter in the AOK group: 14% larger than baseline. Axial elongation in the AOK group vs. in the OK group: 0.01 ± 0.12 mm vs. 0.05 ± 0.08 mm. In the AOK group AXL correlated with an increase in photopic pupil diameter and with some HOA metrics. The correlations mentioned above were not observed in the OK group. |
| Yu et al. 2022 [31] | Prospective, randomized, double-blind, clinical trial. 30 participants: orthokeratology lenses and 0.01% atropine. 30 participants: orthokeratology lenses and placebo eyedrops. Primary outcome: change in axial length (AXL). Secondary outcome: change in pupil diameter (PD) and accommodative amplitude (AMP). Measurements at 4-month intervals. | 60 Chinese myopic (SER from −1.0 to −4.0 diopters) children age 8–12 years old. | After 12 months: AXL in combination group: 0.10 ± 0.14 mm In the control group: 0.20 ± 0.15 mm—significant differences only in the first four months! AMP in both groups was stable in comparison to the baseline. PD in the control group remained stable to baseline. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnękowicz-Augustyn, E.; Teper, S.; Wylęgała, E. Preventing the Progression of Myopia in Children—A Review of the Past Decade. Medicina 2023, 59, 1859. https://doi.org/10.3390/medicina59101859
Wnękowicz-Augustyn E, Teper S, Wylęgała E. Preventing the Progression of Myopia in Children—A Review of the Past Decade. Medicina. 2023; 59(10):1859. https://doi.org/10.3390/medicina59101859
Chicago/Turabian StyleWnękowicz-Augustyn, Emilia, Sławomir Teper, and Edward Wylęgała. 2023. "Preventing the Progression of Myopia in Children—A Review of the Past Decade" Medicina 59, no. 10: 1859. https://doi.org/10.3390/medicina59101859
APA StyleWnękowicz-Augustyn, E., Teper, S., & Wylęgała, E. (2023). Preventing the Progression of Myopia in Children—A Review of the Past Decade. Medicina, 59(10), 1859. https://doi.org/10.3390/medicina59101859








