Colon Bowel Preparation in the Era of Artificial Intelligence: Is There Potential for Enhancing Colon Bowel Cleansing?
Abstract
:1. Introduction
2. Summary of the Current Recommendations
3. Type of Bowel Cleansing Solutions
4. Assessment of Bowel Cleansing
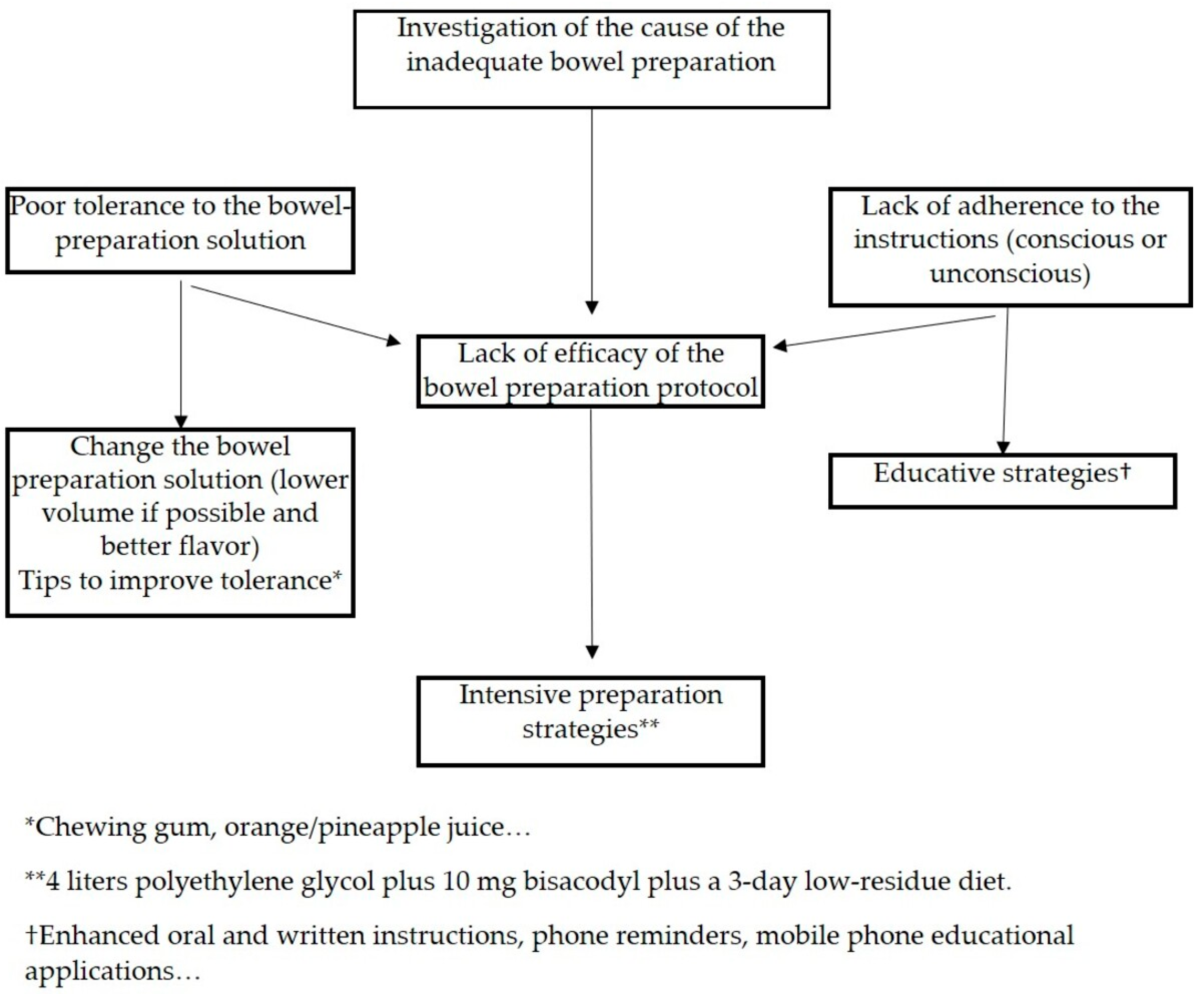
5. Predictors of Poor Bowel Cleansing
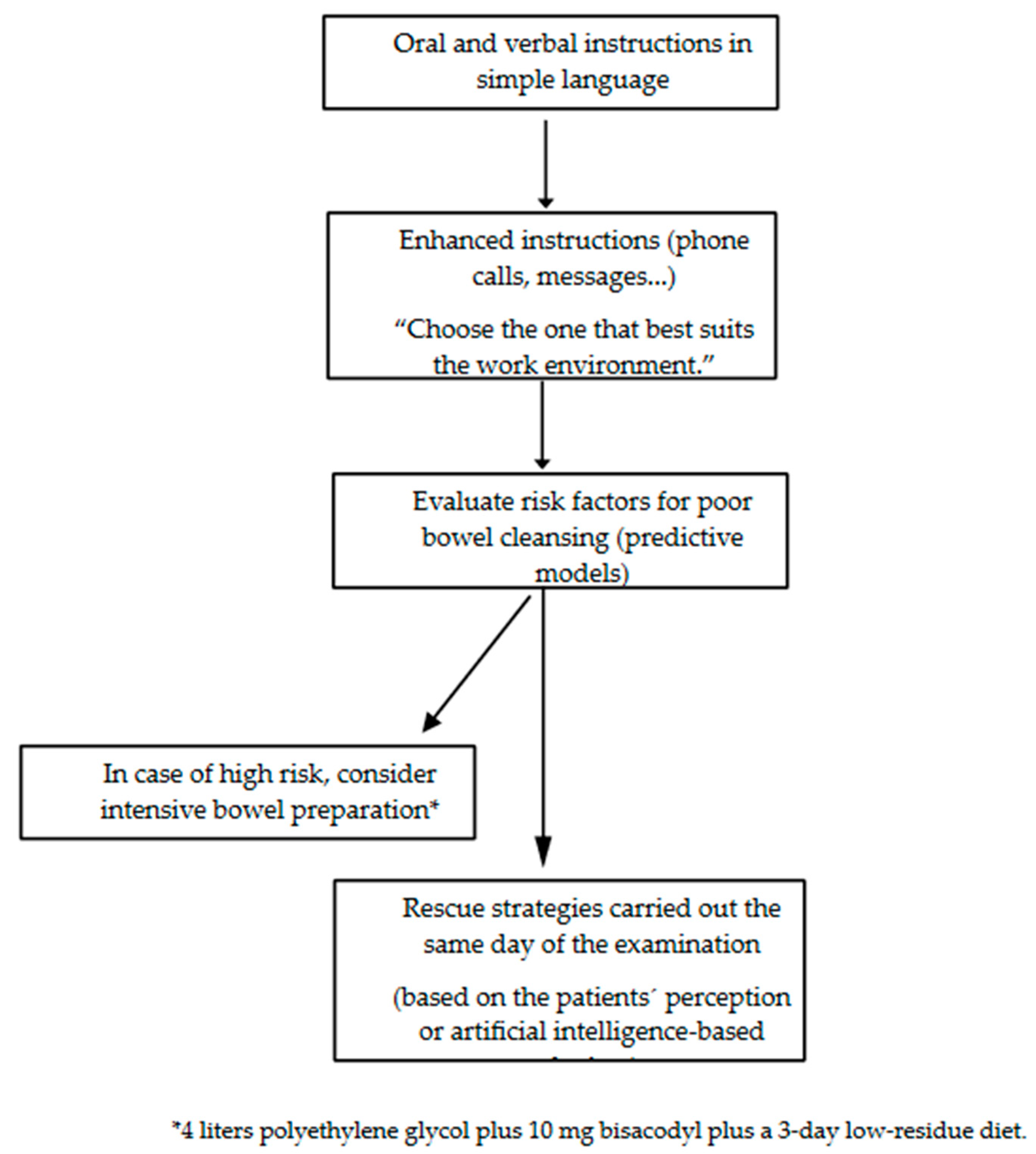
6. Strategies for Improving Bowel Cleansing
6.1. Strategies for Increasing Tolerance
6.2. Strategies for Decreasing Incompliance
- -
- Individual or group informative sessions: These sessions are conducted by trained health care personnel, and in them, a patient receives instructions regarding dietary aspects, the type and administration of the evacuating solution, and precautions to be taken with the home treatment. The results are conflicting in the published studies [58,59].
- -
- Printed educational materials: Using brochures or pamphlets that combine text with illustrative images or drawings about good or poor colon cleansing, lesions were detected based on colonic cleanliness and permitted or prohibited foods. The distribution of this material had positive effects on cleansing quality in most of the studies [60,61].
- -
- Audiovisual material: Educational videos can enhance understanding through the use of simple words, illustrations, and video clips. Some RCTs have compared this strategy to the standard practice, with two studies observing better colon cleansing quality in the intervention groups [62].
- -
- Phone calls or text messages: Through telephone communication, the importance of colonic preparation, the method of following the diet, and taking the evacuating solution are emphasized, along with addressing doubts and providing reminders of scheduled appointments. Such RCTs have demonstrated better colonic cleansing quality in patients assigned to intervention groups [63].
- -
- Mobile applications and social networks: Mobile phones and social networks have become significant sources of medical information. RCTs have evaluated colon cleansing quality in patients who used smartphone applications detailing the information about the colonoscopy preparation, with explanatory images and/or videos, compared to the utility of receiving oral and written instructions [64]. Colonic cleansing quality was superior in the intervention groups in these studies [64,65].
6.3. Intensified Interventions
6.4. Rescue Strategies
7. Role of Artificial Intelligence in Improving Bowel Cleansing
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Atkin, W.; Wooldrage, K.; Parkin, D.M.; Wooldrage, K.; Parkin, D.M.; Kralj-Hans, I.; MacRae, E.; Shah, U.; Duffy, S.; Cross, A.J.; et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: The UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017, 389, 1299–1311. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect, of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Thomas-Gibson, S.; Bugajski, M.; Bretthauer, M.; Rees, C.J.; Dekker, E.; Hoff, G.; Jover, R.; Suchanek, S.; Ferlitsch, M.; et al. Performance measures for lower gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2017, 49, 378–397. [Google Scholar]
- Hassan, C.; East, J.; Radaelli, F.; Spada, C.; Benamouzig, R.; Bisschops, R.; Bretthauer, M.; Dekker, E.; Dinis-Ribeiro, M.; Ferlitsch, M.; et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 775–794. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.D.; Brookes, M.; Lee, T.J.; Rogers, P.; Sharp, L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: A National Endoscopy Database Analysis. Gut 2021, 70, 537–543. [Google Scholar] [CrossRef]
- Johnson, D.A.; Barkun, A.N.; Cohen, L.B.; Dominitz, J.A.; Kaltenbach, T.; Martel, M.; Robertson, D.J.; Boland, C.R.; Giardello, F.M.; Lieberman, D.A.; et al. Optimizing adequacy of bowel cleansing for colonoscopy: Recommendations from the US multi-society task force on colorectal cancer. Gastroenterology 2014, 147, 903–924. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Gonzalez, M.A.; Flores, L.; Roux, J.A.; Seoane, A.; Pedro-Botet, J.; Carot, L.; Fernandez-Clotet, A.; Raga, A.; Pantaleon, M.A.; Barranco, L.; et al. Efficacy of a multifactorial strategy for bowel preparation in diabetic patients undergoing colonoscopy: A randomized trial. Endoscopy 2016, 48, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.M.; Manam, R.; Hoen, H.; Chalasani, N. Predictors of inadequate bowel preparation for colonoscopy. Am. J. Gastroenterol. 2001, 96, 1797–1802. [Google Scholar] [CrossRef]
- Saltzman, J.R.; Cash, B.D.; Pasha, S.F.; Early, D.S.; Muthusamy, V.R.; Khashab, M.A.; Chathadi, K.V.; Fanelli, R.D.; Chandrasekhara, V.; Lightdale, J.R.; et al. Bowel preparation before colonoscopy. Gastrointest. Endosc. 2015, 81, 781–794. [Google Scholar] [CrossRef]
- Hernandez, G.; Gimeno-Garcia, A.Z.; Quintero, E. Strategies to optimise the quality of bowel cleansing. Gastroenterol. Hepatol. 2019, 42, 326–338. [Google Scholar] [CrossRef]
- Yang, H.J.; Park, D.I.; Park, S.K.; Kim, S.; Lee, T.; Jung, Y.; Eun, C.S.; Han, D.S. A Randomized Controlled Trial Comparing Colonoscopic Enema with Additional Oral Preparation as a Salvage for Inadequate Bowel Cleansing Before Colonoscopy. J. Clin. Gastroenterol. 2019, 53, e308–e315. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Garcia, A.Z.; Hernandez-Perez, A.; Nicolas-Perez, D.; Hernandez-Guerra, M. Artificial Intelligence Applied to Colonoscopy: Is It Time to Take a Step Forward? Cancers 2023, 15, 2193. [Google Scholar] [CrossRef]
- Martel, M.; Barkun, A.N.; Menard, C.; Restellini, S.; Kherad, O.; Vanasse, A. Split-Dose Preparations Are Superior to Day-Before Bowel Cleansing Regimens: A Meta-analysis. Gastroenterology 2015, 149, 79–88. [Google Scholar] [CrossRef]
- Bucci, C.; Rotondano, G.; Hassan, C.; Rea, M.; Bianco, M.A.; Cipolletta, L.; Ciacci, C.; Marmo, R. Optimal bowel cleansing for colonoscopy: Split the dose! A series of meta-analyses of controlled studies. Gastrointest. Endosc. 2014, 80, 566–576.e562. [Google Scholar] [CrossRef]
- Horton, N.; Garber, A.; Hasson, H.; Lopez, R.; Burke, C.A. Impact of Single- vs. Split-Dose Low-Volume Bowel Preparations on Bowel Movement Kinetics, Patient Inconvenience, and Polyp Detection: A Prospective Trial. Am. J. Gastroenterol. 2016, 111, 1330–1337. [Google Scholar] [CrossRef]
- Pohl, J.; Halphen, M.; Kloess, H.R.; Fischbach, W. Impact of the quality of bowel cleansing on the efficacy of colonic cancer screening: A prospective, randomized, blinded study. PLoS ONE 2015, 10, e0126067. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, F.; Paggi, S.; Hassan, C.; Senore, C.; Fasoli, R.; Anderloni, A.; Buffoli, F.; Savarese, M.F.; Spinzi, G.; Rex, D.K.; et al. Split-dose preparation for colonoscopy increases adenoma detection rate: A randomised controlled trial in an organised screening programme. Gut 2017, 66, 270–277. [Google Scholar] [CrossRef]
- Avalos, D.J.; Castro, F.J.; Zuckerman, M.J.; Keihanian, T.; Berry, A.C.; Nutter, B.; Sussman, D.A. Bowel Preparations Administered the Morning of Colonoscopy Provide Similar Efficacy to a Split Dose Regimen: A Meta Analysis. J. Clin. Gastroenterol. 2018, 52, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Azmi, N.; Mahadeva, S.; Goh, K.L. Split-dose vs same-day reduced-volume polyethylene glycol electrolyte lavage solution for morning colonoscopy. World J. Gastroenterol. 2014, 20, 14488–14494. [Google Scholar] [CrossRef]
- Kotwal, V.S.; Attar, B.M.; Carballo, M.D.; Lee, S.S.; Kaura, T.; Go, B.; Zhang, H.; Trick, W.E. Morning-only polyethylene glycol is noninferior but less preferred by hospitalized patients as compared with split-dose bowel preparation. J. Clin. Gastroenterol. 2014, 48, 414–418. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Jamal, M.M.; Nguyen, E.T.; Puli, S.R.; Bechtold, M.L. Low-residue versus clear liquid diet before colonoscopy: A meta-analysis of randomized, controlled trials. Gastrointest. Endosc. 2016, 83, 499–507.e491. [Google Scholar] [CrossRef] [PubMed]
- Song, G.M.; Tian, X.; Ma, L.; Yi, L.J.; Shuai, T.; Zeng, Z.; Zeng, X.T. Regime for Bowel Preparation in Patients Scheduled to Colonoscopy: Low-Residue Diet or Clear Liquid Diet? Evidence from Systematic Review with Power Analysis. Medicine 2016, 95, e2432. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Garcia, A.Z.; de la Barreda, H.R.; Reygosa, C.; Hernandez, A.; Mascareno, I.; Nicolas-Perez, D.; Jimenez, A.; Lara, A.J.; Alarcon-Fernandez, O.; Hernandez-Guerra, M.; et al. Impact of a 1-day versus 3-day low-residue diet on bowel cleansing quality before colonoscopy: A randomized controlled trial. Endoscopy 2019, 51, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Machlab, S.; Martinez-Bauer, E.; Lopez, P.; Pique, N.; Puig-Divi, V.; Junquera, F.; Lira, A.; Brullet, E.; Selva, A.; Garcia-Iglesias, P.; et al. Comparable quality of bowel preparation with single-day versus three-day low-residue diet: Randomized controlled trial. Dig. Endosc. 2021, 33, 797–806. [Google Scholar] [CrossRef]
- Scaglione, G.; Oliviero, G.; Labianca, O.; Bianco, M.A.; Granata, R.; Ruggiero, L.; Iovino, P. One-Day versus Three-Day Low-Residue Diet and Bowel Preparation Quality before Colonoscopy: A Multicenter, Randomized, Controlled Trial. Dig. Dis. 2023, 41, 708–718. [Google Scholar] [CrossRef]
- Taveira, F.; Areia, M.; Elvas, L.; Alves, S.; Brito, D.; Saraiva, S.; Cadime, A.T. A 3-day low-fibre diet does not improve colonoscopy preparation results compared to a 1-day diet: A randomized, single-blind, controlled trial. United Eur. Gastroenterol. J. 2019, 7, 1321–1329. [Google Scholar] [CrossRef]
- Restellini, S.; Kherad, O.; Menard, C.; Martel, M.; Barkun, A.N. Do adjuvants add to the efficacy and tolerance of bowel preparations? A meta-analysis of randomized trials. Endoscopy 2018, 50, 159–176. [Google Scholar]
- Hassan, C.; Bretthauer, M.; Kaminski, M.F.; Polkowski, M.; Rembacken, B.; Saunders, B.; Benamouzig, R.; Holme, O.; Green, S.; Kuiper, T.; et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013, 45, 142–150. [Google Scholar] [CrossRef]
- Beck, D.E. Bowel preparation for colonoscopy. Clin. Colon. Rectal Surg. 2010, 23, 10–13. [Google Scholar] [CrossRef]
- Jin, Z.; Lu, Y.; Zhou, Y.; Gong, B. Systematic review and meta-analysis: Sodium picosulfate/magnesium citrate vs. polyethylene glycol for colonoscopy preparation. Eur. J. Clin. Pharmacol. 2016, 72, 523–532. [Google Scholar] [CrossRef]
- Spadaccini, M.; Frazzoni, L.; Vanella, G.; East, J.; Radaelli, F.; Spada, C.; Fuccio, L.; Benamouzig, R.; Bisschops, R.; Bretthauer, M.; et al. Efficacy and Tolerability of High- vs Low-Volume Split-Dose Bowel Cleansing Regimens for Colonoscopy: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 1454–1465.e1414. [Google Scholar] [CrossRef]
- Maida, M.; Macaluso, F.S.; Sferrazza, S.; Ventimiglia, M.; Sinagra, E. Effectiveness and safety of NER1006 versus standard bowel preparations: A meta-analysis of randomized phase-3 clinical trials. Dig. Liver Dis. 2020, 52, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Maida, M.; Ventimiglia, M.; Facciorusso, A.; Vitello, A.; Sinagra, E.; Marasco, G. Effectiveness and safety of 1-L PEG-ASC versus other bowel preparations for colonoscopy: A meta-analysis of nine randomized clinical trials. Dig. Liver Dis. 2023, 55, 1010–1018. [Google Scholar] [CrossRef]
- Lee, J.M.; Keum, B.; Yoo, I.K.; Kim, S.H.; Choi, H.S.; Kim, E.S.; Seo, Y.S.; Jeen, Y.T.; Chun, H.J.; Lee, H.S.; et al. Polyethylene glycol plus ascorbic acid for bowel preparation in chronic kidney disease. Medicine 2016, 95, e4755. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Naito, Y.; Murakami, T.; Hirose, R.; Ogiso, K.; Inada, Y.; Dohi, O.; Okayama, T.; Kamada, K.; Uchiyama, K.; et al. Safety and Efficacy of a Same-Day Low-Volume 1 L PEG Bowel Preparation in Colonoscopy for the Elderly People and People with Renal Dysfunction. Dig. Dis. Sci. 2016, 61, 3229–3235. [Google Scholar] [CrossRef]
- Turnage, R.H.; Guice, K.S.; Gannon, P.; Gross, M. The effect of polyethylene glycol gavage on plasma volume. J. Surg. Res. 1994, 57, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Halphen, M.; Heresbach, D.; Gruss, H.J.; Belsey, J. Validation of the Harefield Cleansing Scale: A tool for the evaluation of bowel cleansing quality in both research and clinical practice. Gastrointest. Endosc. 2013, 78, 121–131. [Google Scholar] [CrossRef]
- Kastenberg, D.; Bertiger, G.; Brogadir, S. Bowel preparation quality scales for colonoscopy. World J. Gastroenterol. 2018, 24, 2833–2843. [Google Scholar] [CrossRef]
- Parmar, R.; Martel, M.; Rostom, A.; Barkun, A.N. Validated Scales for Colon Cleansing: A Systematic Review. Am. J. Gastroenterol. 2016, 111, 197–204; quiz 205. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Tofani, C.; Sokach, C.; Patel, D.; Kastenberg, D.; Daskalakis, C. Patient Characteristics Associated with Quality of Colonoscopy Preparation: A Systematic, Review, and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 357–369.e310. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Cesbron-Metivier, E.; Bertrais, S.; Olivier, A.; Becq, A.; Boursier, J.; Lannes, A.; Luet, D.; Pateu, E.; Dib, N.; et al. A predictive score of inadequate bowel preparation based on a self-administered questionnaire: PREPA-CO. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101693. [Google Scholar] [CrossRef]
- Dik, V.K.; Moons, L.M.; Huyuk, M.; van der Schaar, P.; de Vos Tot Nederveen Cappel, W.H.; Ter; Borg, P.C.; Meijssen, M.A.; Ouwendijk, R.J.; Le Fevre, D.M.; et al. Predicting inadequate bowel preparation for colonoscopy in participants receiving split-dose bowel preparation: Development and validation of a prediction score. Gastrointest. Endosc. 2015, 81, 665–672. [Google Scholar] [CrossRef]
- Gimeno-Garcia, A.Z.; Baute, J.L.; Hernandez, G.; Morales, D.; Gonzalez-Perez, C.D.; Nicolas-Perez, D.; Alarcon-Fernandez, O.; Jimenez, A.; Hernandez-Guerra, M.; Romero, R.; et al. Risk factors for inadequate bowel preparation: A validated predictive score. Endoscopy 2017, 49, 536–543. [Google Scholar] [CrossRef]
- Hassan, C.; Fuccio, L.; Bruno, M.; Pagano, N.; Spada, C.; Carrara, S.; Giordanino, C.; Rondonotti, E.; Curcio, G.; Dulbecco, P.; et al. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin. Gastroenterol. Hepatol. 2012, 10, 501–506. [Google Scholar] [CrossRef]
- Fatima, H.; Johnson, C.S.; Rex, D.K. Patients’ description of rectal effluent and quality of bowel preparation at colonoscopy. Gastrointest. Endosc. 2010, 71, 1244–1252.e1242. [Google Scholar] [CrossRef]
- Gimeno-Garcia, A.Z.; Benitez-Zafra, F.; Hernandez, A.; Hernandez-Negrin, D.; Nicolas-Perez, D.; Hernandez, G.; Baute-Dorta, J.L.; Cedres, Y.; Del-Castillo, R.; Mon, J.; et al. Agreement between the perception of colon cleansing reported by patients and colon cleansing assessed by a validated colon cleansing scale. Gastroenterol. Hepatol. 2023. [CrossRef]
- Fang, J.; Wang, S.L.; Fu, H.Y.; Li, Z.S.; Bai, Y. Impact of gum chewing on the quality of bowel preparation for colonoscopy: An endoscopist-blinded, randomized controlled trial. Gastrointest. Endosc. 2017, 86, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, E.; Kim, Y.; Kim, E.; Lee, Y. Effects of gum chewing on abdominal discomfort, nausea, vomiting and intake adherence to polyethylene glycol solution of patients in colonoscopy preparation. J. Clin. Nurs. 2016, 25, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Shim, C.S.; Kim, G.W.; Kim, J.S.; Lee, S.Y.; Sung, I.K.; Park, H.S.; Kim, J.H. Orange juice intake reduces patient discomfort and is effective for bowel cleansing with polyethylene glycol during bowel preparation. Dis. Colon. Rectum 2014, 57, 1220–1227. [Google Scholar] [CrossRef]
- Hao, Z.; Gong, L.; Shen, Q.; Wang, H.; Feng, S.; Wang, X.; Cai, Y.; Chen, J. Effectiveness of concomitant use of green tea and polyethylene glycol in bowel preparation for colonoscopy: A randomized controlled study. BMC Gastroenterol. 2020, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Seow-En, I.; Seow-Choen, F. A prospective randomized trial on the use of Coca-Cola Zero((R)) vs water for polyethylene glycol bowel preparation before colonoscopy. Colorectal Dis. 2016, 18, 717–723. [Google Scholar] [CrossRef]
- Kamran, U.; Abbasi, A.; Tahir, I.; Hodson, J.; Siau, K. Can adjuncts to bowel preparation for colonoscopy improve patient experience and result in superior bowel cleanliness? A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2020, 8, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Menees, S.B.; Elliott, E.; Govani, S.; Anastassiades, C.; Judd, S.; Urganus, A.; Boyce, S.; Schoenfeld, P. The impact of bowel cleansing on follow-up recommendations in average-risk patients with a normal colonoscopy. Am. J. Gastroenterol. 2014, 109, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Shih, S.C.; Wang, H.Y.; Chu, C.H.; Wang, T.E.; Hung, C.Y.; Shieh, T.Y.; Lin, Y.S.; Chen, M.J. Meta-analysis: The effect of patient education on bowel preparation for colonoscopy. Endosc. Int. Open 2015, 3, E646–E652. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Wang, Z.; Zhai, J.; Liu, Q.; Ding, K.; Pan, Y. Reinforced education improves the quality of bowel preparation for colonoscopy: An updated meta-analysis of randomized controlled trials. PLoS ONE 2020, 15, e0231888. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Zhao, L.; Leung, F.; Luo, H.; Kang, X.; Li, X.; Jia, H.; Yang, S.; Tao, Q.; et al. Enhanced instructions improve the quality of bowel preparation for colonoscopy: A meta-analysis of randomized controlled trials. Gastrointest. Endosc. 2017, 85, 90–97.e96. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, S.; Lei, J.; Ren, W.; Xiao, L.; Liu, X.; Lu, M.; Zhou, K. Supplementary education can improve the rate of adequate bowel preparation in outpatients: A systematic review and meta-analysis based on randomized controlled trials. PLoS ONE 2022, 17, e0266780. [Google Scholar] [CrossRef] [PubMed]
- Elvas, L.; Brito, D.; Areia, M.; Carvalho, R.; Alves, S.; Saraiva, S.; Cadime, A.T. Impact of Personalised Patient Education on Bowel Preparation for Colonoscopy: Prospective Randomised Controlled Trial. GE Port. J. Gastroenterol. 2017, 24, 22–30. [Google Scholar] [CrossRef]
- Modi, C.; Depasquale, J.R.; Digiacomo, W.S.; Malinowski, J.E.; Engelhardt, K.; Shaikh, S.N.; Kothari, S.T.; Kottam, R.; Shakov, R.; Maksoud, C.; et al. Impact of patient education on quality of bowel preparation in outpatient colonoscopies. Qual. Prim. Care 2009, 17, 397–404. [Google Scholar] [PubMed]
- Calderwood, A.H.; Lai, E.J.; Fix, O.K.; Jacobson, B.C. An endoscopist-blinded, randomized, controlled trial of a simple visual aid to improve bowel preparation for screening colonoscopy. Gastrointest. Endosc. 2011, 73, 307–314. [Google Scholar] [CrossRef]
- Spiegel, B.M.; Talley, J.; Shekelle, P.; Agarwal, N.; Snyder, B.; Bolus, R.; Kurzbard, N.; Chan, M.; Ho, A.; Kaneshiro, M.; et al. Development and validation of a novel patient educational booklet to enhance colonoscopy preparation. Am. J. Gastroenterol. 2011, 106, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, M.S.; Kim, H.; Kim, S.I.; Shin, C.H.; Lee, H.J.; Lee, W.S.; Moon, S. A randomized controlled trial of an educational video to improve quality of bowel preparation for colonoscopy. BMC Gastroenterol. 2016, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, H.; Zhang, L.; Leung, F.W.; Liu, Z.; Wang, X.; Huang, R.; Hui, N.; Wu, K.; Fan, D.; et al. Telephone-based re-education on the day before colonoscopy improves the quality of bowel preparation and the polyp detection rate: A prospective colonoscopist-blinded randomised controlled study. Gut 2014, 63, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Back, S.Y.; Kim, H.G.; Ahn, E.M.; Park, S.; Jeon, S.R.; Im, H.H.; Kim, J.O.; Ko, B.M.; Lee, J.S.; Lee, T.H.; et al. Impact of patient audiovisual re-education via a smartphone on the quality of bowel preparation before colonoscopy: A single-blinded randomized study. Gastrointest. Endosc. 2018, 87, 789–799.e784. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, L.; Leung, F.; Luo, H.; Wang, L.; Wu, J.; Guo, X.; Wang, X.; Zhang, L.; Hui, N.; et al. Delivery of Instructions via Mobile Social Media App Increases Quality of Bowel Preparation. Clin. Gastroenterol. Hepatol. 2016, 14, 429–435.e423. [Google Scholar] [CrossRef]
- Gimeno-Garcia, A.Z.; Hernandez, G.; Aldea, A.; Nicolas-Perez, D.; Jimenez, A.; Carrillo, M.; Felipe, V.; Alarcon-Fernandez, O.; Hernandez-Guerra, M.; Romero, R.; et al. Comparison of Two Intensive Bowel Cleansing Regimens in Patients with Previous Poor Bowel Preparation: A Randomized Controlled Study. Am. J. Gastroenterol. 2017, 112, 951–958. [Google Scholar] [CrossRef]
- Cadoni, S.; Falt, P.; Rondonotti, E.; Radaelli, F.; Fojtik, P.; Gallittu, P.; Liggi, M.; Amato, A.; Paggi, S.; Smajstrla, V.; et al. Water exchange for screening colonoscopy increases adenoma detection rate: A multicenter double-blinded randomized controlled trial. Endoscopy 2017, 49, 456–467. [Google Scholar] [CrossRef]
- Moshkowitz, M.; Fokra, A.; Itzhak, Y.; Arber, N.; Santo, E. Feasibility study of minimal prepared hydroflush screening colonoscopy. United Eur. Gastroenterol. J. 2016, 4, 105–109. [Google Scholar] [CrossRef]
- van Keulen, K.E.; Neumann, H.; Schattenberg, J.M.; van Esch, A.A.J.; Kievit, W.; Spaander, M.C.W.; Siersema, P.D. A novel device for intracolonoscopy cleansing of inadequately prepared colonoscopy patients: A feasibility study. Endoscopy 2019, 51, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Berzin, T.M.; Parasa, S.; Wallace, M.B.; Gross, S.A.; Repici, A.; Sharma, P. Position statement on priorities for artificial intelligence in GI endoscopy: A report by the ASGE Task Force. Gastrointest. Endosc. 2020, 92, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Calderwood, A.H.; Karnes, W.; Requa, J.; Jacobson, B.C.; Wallace, M.B. Artificial intelligence for the assessment of bowel preparation. Gastrointest. Endosc. 2022, 95, 512–518e511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, L.; Wan, X.; Shen, L.; Liu, J.; Zhang, J.; Jiang, X.; Wang, Z.; Yu, S.; Kang, J.; et al. A novel artificial intelligence system for the assessment of bowel preparation (with video). Gastrointest. Endosc. 2020, 91, 428–435.e422. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.B.; Lu, S.C.; Huang, Y.N.; Cai, S.T.; Le, P.H.; Hsu, F.Y.; Hu, Y.X.; Hsieh, H.S.; Chen, W.T.; Xia, G.L.; et al. A Novel Convolutional Neural Network Model as an Alternative Approach to Bowel Preparation Evaluation Before Colonoscopy in the COVID-19 Era: A Multicenter, Single-Blinded, Randomized Study. Am. J. Gastroenterol. 2022, 117, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, D.F.; Wu, H.L.; Fu, P.Y.; Feng, L.; Zhuang, K.; Geng, Z.H.; Li, K.K.; Zhang, X.H.; Zhu, B.Q.; et al. Improving, bowel preparation for colonoscopy with a smartphone application driven by artificial intelligence. NPJ Digit. Med. 2023, 6, 41. [Google Scholar] [CrossRef]
| Hassan et al. | Dik et al. | Gimeno et al. | Berger et al. | |
|---|---|---|---|---|
| Variables |
|
|
|
|
| AUC †, 95% CI | 0.63 | 0.72–0.77 | 0.72–0.70 | 0.622–0.621 |
| Sensitivity (%) | 60 | 66 | 50 | 46 |
| Specificity (%) | 59 | 79 | 80 | 76 |
| NPV ‡ (%) | 41 | 29 | 36 | 39 |
| PPV # (%) | 76 | 95 | 88 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimeno-García, A.Z.; Benítez-Zafra, F.; Nicolás-Pérez, D.; Hernández-Guerra, M. Colon Bowel Preparation in the Era of Artificial Intelligence: Is There Potential for Enhancing Colon Bowel Cleansing? Medicina 2023, 59, 1834. https://doi.org/10.3390/medicina59101834
Gimeno-García AZ, Benítez-Zafra F, Nicolás-Pérez D, Hernández-Guerra M. Colon Bowel Preparation in the Era of Artificial Intelligence: Is There Potential for Enhancing Colon Bowel Cleansing? Medicina. 2023; 59(10):1834. https://doi.org/10.3390/medicina59101834
Chicago/Turabian StyleGimeno-García, Antonio Z, Federica Benítez-Zafra, David Nicolás-Pérez, and Manuel Hernández-Guerra. 2023. "Colon Bowel Preparation in the Era of Artificial Intelligence: Is There Potential for Enhancing Colon Bowel Cleansing?" Medicina 59, no. 10: 1834. https://doi.org/10.3390/medicina59101834
APA StyleGimeno-García, A. Z., Benítez-Zafra, F., Nicolás-Pérez, D., & Hernández-Guerra, M. (2023). Colon Bowel Preparation in the Era of Artificial Intelligence: Is There Potential for Enhancing Colon Bowel Cleansing? Medicina, 59(10), 1834. https://doi.org/10.3390/medicina59101834






