Prognostic Value of Mitral Regurgitation in Patients with Primary Hypertrophic Cardiomyopathy
Abstract
:1. Background
2. Methods
2.1. Echocardiography
2.2. Assessment of Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2020, 76, 3022–3055. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Velicki, L.; Jakovljevic, D.G.; Preveden, A.; Golubovic, M.; Bjelobrk, M.; Ilic, A.; Stojsic, S.; Barlocco, F.; Tafelmeier, M.; Okwose, N.; et al. Genetic determinants of clinical phenotype in hypertrophic cardiomyopathy. BMC Cardiovasc. Disord. 2020, 20, 516. [Google Scholar] [CrossRef]
- Popa-Fotea, N.M.; Micheu, M.M.; Bataila, V.; Scafa-Udriste, A.; Dorobantu, L.; Scarlatescu, A.I.; Zamfir, D.; Stoian, M.; Onciul, S.; Dorobantu, M. Exploring the Continuum of Hypertrophic Cardiomyopathy—From DNA to Clinical Expression. Medicina 2019, 55, 299. [Google Scholar] [CrossRef]
- Maron, B.J.; Peterson, E.E.; Maron, M.S.; Peterson, J.E. Prevalence of hypertrophic cardiomyopathy in an outpatient population referred for echocardiographic study. Am. J. Cardiol. 1994, 73, 577–580. [Google Scholar] [CrossRef]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef]
- Tesic, M.; Djordjevic-Dikic, A.; Giga, V.; Stepanovic, J.; Dobric, M.; Jovanovic, I.; Petrovic, M.; Mehmedbegovic, Z.; Milasinovic, D.; Dedovic, V.; et al. Prognostic Value of Transthoracic Doppler Echocardiography Coronary Flow Velocity Reserve in Patients with Asymmetric Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021936. [Google Scholar] [CrossRef]
- Klues, H.G.; Maron, B.J.; Dollar, A.L.; Roberts, W.C. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation 1992, 85, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Molisana, M.; Selimi, A.; Gizzi, G.; D’Agostino, S.; Ianni, U.; Parato, V.M. Different mechanisms of mitral regurgitation in hypertrophic cardiomyopathy: A clinical case and literature review. Front. Cardiovasc. Med. 2022, 9, 1020054. [Google Scholar] [CrossRef] [PubMed]
- Klues, H.G.; Roberts, W.C.; Maron, B.J. Anomalous insertion of papillary muscle directly into anterior mitral leaflet in hypertrophic cardiomyopathy. Significance in producing left ventricular outflow obstruction. Circulation 1991, 84, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Peteiro, J.; Bouzas-Mosquera, A.; Fernandez, X.; Monserrat, L.; Pazos, P.; Estevez-Loureiro, R.; Castro-Beiras, A. Prognostic value of exercise echocardiography in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2012, 25, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.A.; Forrester, J.S. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N. Engl. J. Med. 1979, 300, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, E.; Picano, E.; Dodi, C.; Morozzi, L.; Chiriatti, G.P.; Lu, C.; Botti, G.; Eeho-Persantine International Cooperative (EPIC) Study Group—Subproject Hypertrophic Cardiomyopathy. Dipyridamole echocardiography for diagnosis of coexistent coronary artery disease in hypertrophic cardiomyopathy. Echo-Persantine International Cooperative (EPIC) Study Group--Subproject Hypertrophic Cardiomyopathy. Am. J. Cardiol. 1995, 75, 810–813. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Kim, D.Y.; Seo, J.; Cho, I.; Hong, G.R.; Ha, J.W.; Shim, C.Y. Prognostic Implication of Mitral Valve Disease and Its Progression in East Asian Patients with Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2023, 12, e024792. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Autore, C.; Rapezzi, C.; Bernabò, P.; Badagliacca, R.; Maron, M.S.; Bongioanni, S.; Coccolo, F.; Estes, N.M.; Barillà, C.S.; et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation 2009, 119, 1703–1710. [Google Scholar] [CrossRef]
- Losi, M.-A.; Betocchi, S.; Barbati, G.; Parisi, V.; Tocchetti, C.-G.; Pastore, F.; Migliore, T.; Contaldi, C.; Caputi, A.; Romano, R.; et al. Prognostic significance of left atrial volume dilatation in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2009, 22, 76–81. [Google Scholar] [CrossRef]
- Adabag, A.S.; Casey, S.A.; Kuskowski, M.A.; Zenovich, A.G.; Maron, B.J. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 45, 697–704. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef]
- Elliott, P.M.; Gimeno Blanes, J.R.; Mahon, N.G.; Poloniecki, J.D.; McKenna, W.J. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet 2001, 357, 420–424. [Google Scholar] [CrossRef]
- Ciampi, Q.; Olivotto, I.; Gardini, C.; Mori, F.; Peteiro, J.; Monserrat, L.; Fernandez, X.; Cortigiani, L.; Rigo, F.; Lopes, L.R.; et al. Prognostic role of stress echocardiography in hypertrophic cardiomyopathy: The International Stress Echo Registry. Int. J. Cardiol. 2016, 219, 331–338. [Google Scholar] [CrossRef]
- Preveden, A.; Golubovic, M.; Bjelobrk, M.; Miljkovic, T.; Ilic, A.; Stojsic, S.; Gajic, D.; Glavaski, M.; Maier, L.S.; Okwose, N.; et al. Gender Related Differences in the Clinical Presentation of Hypertrophic Cardiomyopathy—An Analysis from the SILICOFCM Database. Medicina 2022, 58, 314. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.H.; Omran, A.S.; Wigle, E.D.; Williams, W.G.; Siu, S.C.; Rakowski, H. Mitral regurgitation in hypertrophic obstructive cardiomyopathy: Relationship to obstruction and relief with myectomy. J. Am. Coll. Cardiol. 2000, 36, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, Q.; Olivotto, I.; Peteiro, J.; D’alfonso, M.G.; Mori, F.; Tassetti, L.; Milazzo, A.; Monserrat, L.; Fernandez, X.; Pálinkás, A.; et al. Prognostic Value of Reduced Heart Rate Reserve during Exercise in Hypertrophic Cardiomyopathy. J. Clin. Med. 2021, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Link, M.S.; Udelson, J.E.; Kuvin, J.T.; Pandian, N.G.; Olivotto, I.; Nistri, S.; Cecchi, F.; Maron, B.J.; Zenovich, A.G.; et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006, 114, 2232–2239. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Balaram, S.; Kim, B.; Axel, L.; Swistel, D.G. The Mitral Valve in Obstructive Hypertrophic Cardiomyopathy: A Test in Context. J. Am. Coll. Cardiol. 2016, 67, 1846–1858. [Google Scholar] [CrossRef]
- Levine, R.A.; Vlahakes, G.J.; Lefebvre, X.; Guerrero, J.L.; Cape, E.G.; Yoganathan, A.P.; Weyman, A.E. Papillary muscle displacement causes systolic anterior motion of the mitral valve. Experimental validation and insights into the mechanism of subaortic obstruction. Circulation 1995, 91, 1189–1195. [Google Scholar] [CrossRef]
- Palinkas, E.D.; Re, F.; Peteiro, J.; Tesic, M.; Palinkas, A.; Torres, M.A.R.; Dikic, A.D.; Beleslin, B.; Van De Heyning, C.M.; D’Alfonso, M.G.; et al. Pulmonary congestion during Exercise stress Echocardiography in Hypertrophic Cardiomyopathy. Int. J. Cardiovasc. Imaging 2022, 38, 2593–2604. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Bhonsale, A.; Patel, P.; Naji, P.; Smedira, N.G.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M. Exercise echocardiography in asymptomatic HCM: Exercise capacity, and not LV outflow tract gradient predicts long-term outcomes. JACC Cardiovasc. Imaging 2014, 7, 26–36. [Google Scholar] [CrossRef]
- Hughes, S.E. The pathology of hypertrophic cardiomyopathy. Histopathology 2004, 44, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Cecchi, F.; Casey, S.A.; Dolara, A.; Traverse, J.H.; Maron, B.J. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001, 104, 2517–2524. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Udelson, J.E.; Maron, M.S. Clinical Spectrum and Management of Heart Failure in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2018, 6, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Lakkis, N.M.; Middleton, K.J.; Spencer, W.H., 3rd; Zoghbi, W.A.; Quinones, M.A. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 1999, 99, 254–261. [Google Scholar] [CrossRef]
- Tesic, M.; Seferovic, J.; Trifunovic, D.; Djordjevic-Dikic, A.; Giga, V.; Jovanovic, I.; Petrovic, O.; Marinkovic, J.; Stankovic, S.; Stepanovic, J.; et al. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J. Cardiol. 2017, 70, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.K.; Frenneaux, M.P.; Steeds, R.P. Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management. Eur. J. Echocardiogr. 2009, 10, iii9–iii14. [Google Scholar] [CrossRef]
- Tesic, M.; Djordjevic-Dikic, A.; Beleslin, B.; Trifunovic, D.; Giga, V.; Marinkovic, J.; Petrovic, O.; Petrovic, M.; Stepanovic, J.; Dobric, M.; et al. Regional difference of microcirculation in patients with asymmetric hypertrophic cardiomyopathy: Transthoracic Doppler coronary flow velocity reserve analysis. J. Am. Soc. Echocardiogr. 2013, 26, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef]
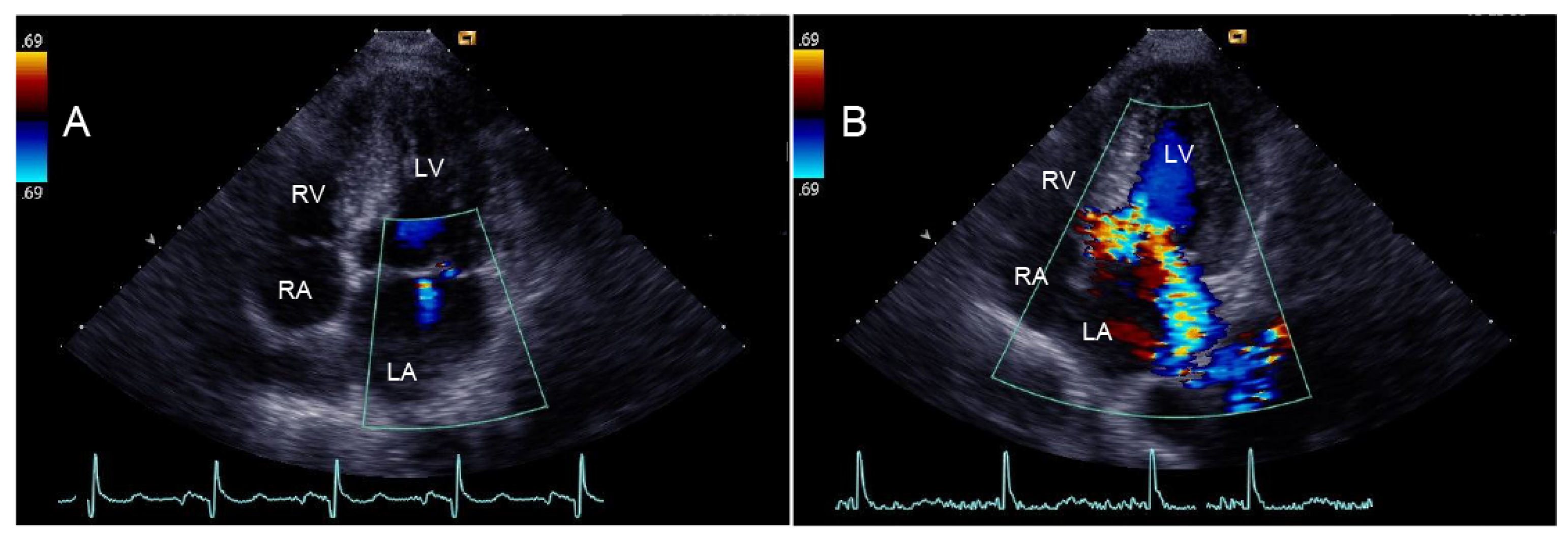
| Variables | Total (n = 176) | Group 1 (n = 116) | Group 2 (n = 60) | p Value Group 1 vs. Group 2 |
|---|---|---|---|---|
| Age—years | 48 ± 15 | 45 ± 14 | 54 ± 15 | <0.001 |
| BSA—m2 | 1.85 ± 0.2 | 1.87 ± 0.2 | 1.82 ± 0.17 | 0.06 |
| Female sex—no. (%) | 94 (53) | 53 (46) | 41 (68) | 0.004 |
| Hypertension—no. (%) | 61 (35) | 33 (28) | 28 (47) | 0.016 |
| Syncope—no. (%) | 22 (12) | 10 (9) | 12 (20) | 0.03 |
| Family history of HCM—no. (%) | 67 (38) | 48 (41) | 19 (32) | 0.208 |
| Family history of SCD—no. (%) | 25 (14) | 17 (15) | 8 (13.3) | 0.812 |
| NYHA functional class—no. (%) | <0.001 | |||
| I | 110 (62) | 91 (78) | 19 (32) | |
| II | 66 (37) | 25 (22) | 41 (68) | |
| Unsustained ventricular tachycardia on Holter ECG—no. (%) | 36 (21) | 28 (25) | 8 (14) | 0.091 |
| Atrial fibrillation on Holter ECG—no. (%) | 31 (18) | 15 (13) | 16 (27) | 0.023 |
| Diastolic blood pressure—mmHg | 77 ± 8 | 78 ± 8 | 78 ± 9 | 0.811 |
| Systolic blood pressure—mmHg | 120 ± 15 | 119 ± 15 | 120 ± 15 | 0.658 |
| Baseline heart rate—beats/min | 69 ± 14 | 70 ± 14 | 68 ± 14 | 0.482 |
| Medical therapy—no. (%) | ||||
| Beta blockers | 150 (85) | 95 (82) | 55 (92) | 0.083 |
| Ca antagonists | 32 (18) | 17 (15) | 15 (25) | 0.092 |
| ACEI/ARB | 47 (27) | 27 (23) | 20 (33) | 0.153 |
| Diuretic | 33 (19) | 15 (13) | 18 (30) | 0.006 |
| Amiodarone | 24 (14) | 12 (10) | 12 (20) | 0.077 |
| Variables | Total (n = 176) | Group 1 (n = 116) | Group 2 (n = 60) | p Value Group 1 vs. Group 2 |
|---|---|---|---|---|
| LV end-diastolic dimension—mm | 46 ± 5 | 45 ± 5 | 46 ± 5 | 0.099 |
| LV end-systolic dimension—mm | 27 ± 5 | 28 ± 5 | 27 ± 4 | 0.089 |
| IVS thickness—mm | 19 ± 4 | 19 ± 4 | 20 ± 4 | 0.339 |
| PW thickness—mm | 10 ± 2 | 9.8 ± 2 | 11 ± 3 | <0.001 |
| IVS/PW ratio | 1.96 ± 0.57 | 2.0 ± 0.53 | 1.8 ± 0.62 | 0.039 |
| Maximal wall thickness—mm | 21 ± 5 | 21 ± 5 | 22 ± 4 | 0.099 |
| LV wall thickness ≥ 30 mm—no. (%) | 8 (5) | 4 (3) | 4 (7) | 0.331 |
| LV ejection fraction—% | 70 ± 8 | 69 ± 8 | 70 ± 8 | 0.222 |
| LVOTG at rest—median (IQR)—mmHg | 10 (6–30) | 7 (6–12) | 36 (12–63) | <0.001 |
| LVOTG at rest ≥ 30 mmHg—no. (%) | 47 (27) | 13 (11) | 34 (57) | <0.001 |
| Maximal induced LVOTG ≥ 50 mmHg—no. (%) | 44 (25) | 9 (8) | 35 (58) | <0.001 |
| Left atrial dimension—mm | 43 ± 6 | 41 ± 6 | 45 ± 6 | <0.001 |
| LAVI—mL/m2 | 38 ± 14 | 34 ± 12 | 45 ± 16 | <0.001 |
| LAVI > 34 mL/m2—br. (%) | 94 (53) | 46 (40) | 48 (80) | <0.001 |
| RVSP—mmHg | 34 ± 9 | 32 ± 7 | 38 ± 10 | <0.001 |
| E-wave—m/s | 0.73 ± 0.20 | 0.68 ± 0.17 | 0.81 ± 0.25 | <0.001 |
| A-wave—m/s | 0.67 ± 0.26 | 0.59 ± 0.18 | 0.82 ± 0.31 | <0.001 |
| E/A | 1.26 ± 0.69 | 1.31 ± 0.69 | 1.16 ± 0.69 | 0.192 |
| Mitral lateral annular e′—m/s | 0.103 ± 0.033 | 0.110 ± 0.033 | 0.088 ± 0.026 | <0.001 |
| Mitral lateral annular a′—m/s | 0.112 ± 0.036 | 0.110 ± 0.034 | 0.088 ± 0.026 | 0.286 |
| E/e′ | 7.640 ± 3.036 | 6.58 ± 2.27 | 9.68 ± 3.29 | <0.001 |
| Ln NT-pro-BNP—pg/mL | 6.88 ± 0.99 | 6.63 ± 0.95 | 7.37 ± 0.86 | <0.001 |
| Eccentric jet of MR—no. (%) | 52 (30) | 12 (10) | 40 (67) | <0.001 |
| Systolic anterior motion—no. (%) | 74 (42) | 29 (25) | 45 (75) | <0.001 |
| Mitral annular calcification—no. (%) | 29 (17) | 9 (8) | 20 (33) | <0.001 |
| Variables | Univariable Analysis | ||
|---|---|---|---|
| HR | p Value | 95% CI | |
| Female sex | 2.494 | 0.007 | 1.284–4.845 |
| Age—years | 1.027 | 0.018 | 1.005–1.050 |
| Family history of SCD | 1.833 | 0.122 | 0.850–3.952 |
| Atrial fibrillation on Holter ECG | 2.269 | 0.011 | 1.211–4.252 |
| NSVT on Holter ECG | 1.409 | 0.329 | 0.708–2.804 |
| Syncope | 0.756 | 0.556 | 0.298–1.920 |
| Maximal wall thickness—mm | 1.056 | 0.066 | 0.996–1.119 |
| LV wall thickness ≥ 30 mm | 1.365 | 0.603 | 0.422–4.420 |
| Maximal induced LVOTG ≥ 50 mmHg | 1.949 | 0.031 | 1.061–3.580 |
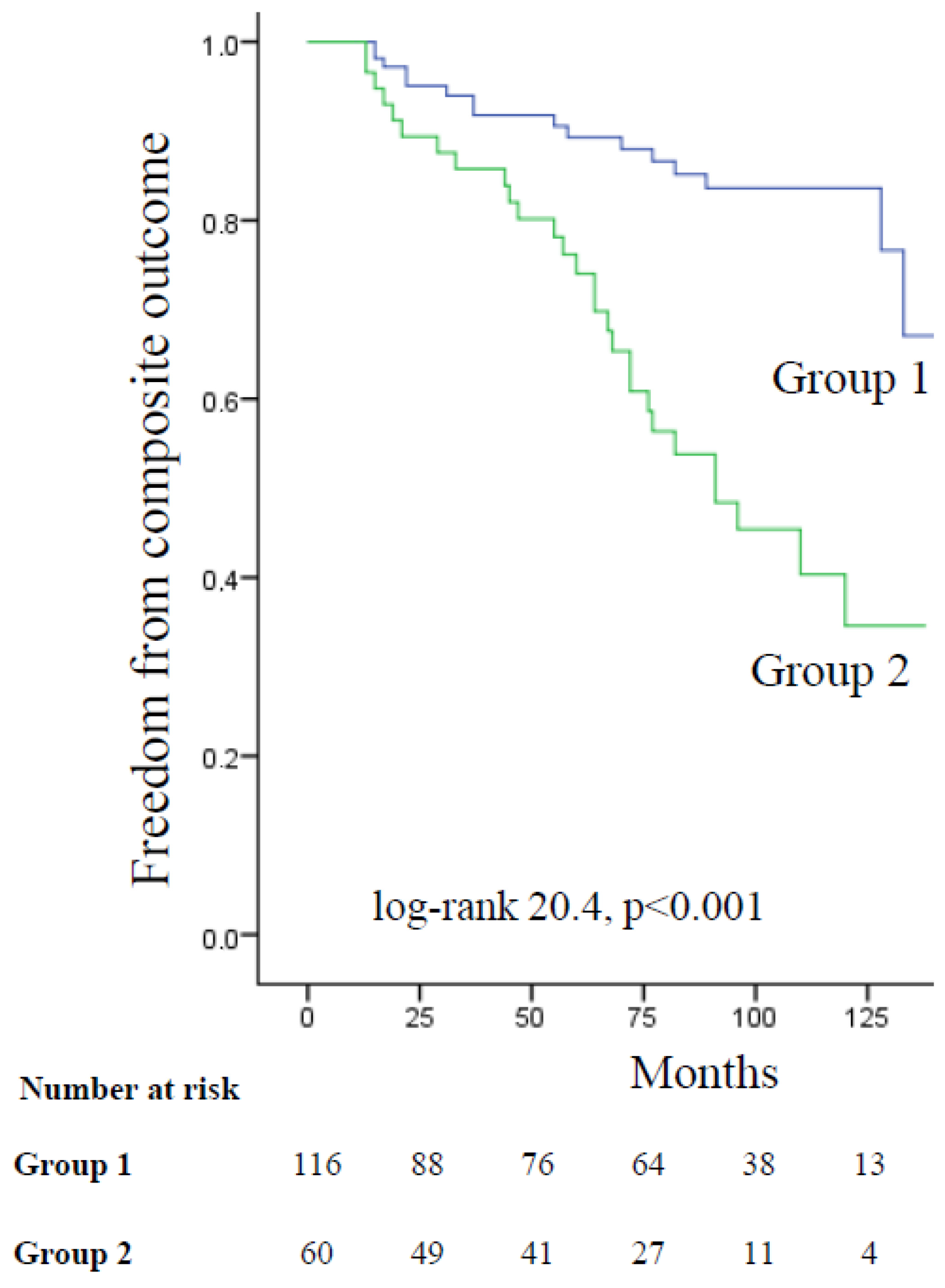
| Moderate or moderately severe MR | 3.758 | <0.001 | 2.028–6.964 |
| LAVI > 34 mL/m2 | 2.578 | 0.005 | 1.341–4.954 |
| Variables | Multivariable Analysis | ||
|---|---|---|---|
| HR | p Value | 95% CI | |
| Female sex | 1.940 | 0.057 | 0.981–3.836 |
| Age—years | 1.000 | 0.987 | 0.976–1.025 |
| Atrial fibrillation on Holter ECG | 1.640 | 0.157 | 0.827–3.253 |
| LAVI > 34 mL/m2 | 1.546 | 0.248 | 0.738–3.239 |
| Maximal induced LVOTG ≥ 50 mmHg | 0.889 | 0.759 | 0.421–1.878 |
| Moderate or moderately severe MR | 2.788 | 0.015 | 1.221–6.364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesic, M.; Travica, L.; Giga, V.; Jovanovic, I.; Trifunovic Zamaklar, D.; Popovic, D.; Mladenovic, D.; Radomirovic, M.; Vratonjic, J.; Boskovic, N.; et al. Prognostic Value of Mitral Regurgitation in Patients with Primary Hypertrophic Cardiomyopathy. Medicina 2023, 59, 1798. https://doi.org/10.3390/medicina59101798
Tesic M, Travica L, Giga V, Jovanovic I, Trifunovic Zamaklar D, Popovic D, Mladenovic D, Radomirovic M, Vratonjic J, Boskovic N, et al. Prognostic Value of Mitral Regurgitation in Patients with Primary Hypertrophic Cardiomyopathy. Medicina. 2023; 59(10):1798. https://doi.org/10.3390/medicina59101798
Chicago/Turabian StyleTesic, Milorad, Lazar Travica, Vojislav Giga, Ivana Jovanovic, Danijela Trifunovic Zamaklar, Dejana Popovic, Djordje Mladenovic, Marija Radomirovic, Jelena Vratonjic, Nikola Boskovic, and et al. 2023. "Prognostic Value of Mitral Regurgitation in Patients with Primary Hypertrophic Cardiomyopathy" Medicina 59, no. 10: 1798. https://doi.org/10.3390/medicina59101798
APA StyleTesic, M., Travica, L., Giga, V., Jovanovic, I., Trifunovic Zamaklar, D., Popovic, D., Mladenovic, D., Radomirovic, M., Vratonjic, J., Boskovic, N., Dedic, S., Nedeljkovic Arsenovic, O., Aleksandric, S., Juricic, S., Beleslin, B., & Djordjevic Dikic, A. (2023). Prognostic Value of Mitral Regurgitation in Patients with Primary Hypertrophic Cardiomyopathy. Medicina, 59(10), 1798. https://doi.org/10.3390/medicina59101798






