Therapeutic Challenges for Gastric Neuroendocrine Neoplasms: Take It or Leave It?
Abstract
:1. Introduction
2. Materials and Methods
3. Therapeutic Approaches
3.1. Active Endoscopic Observation
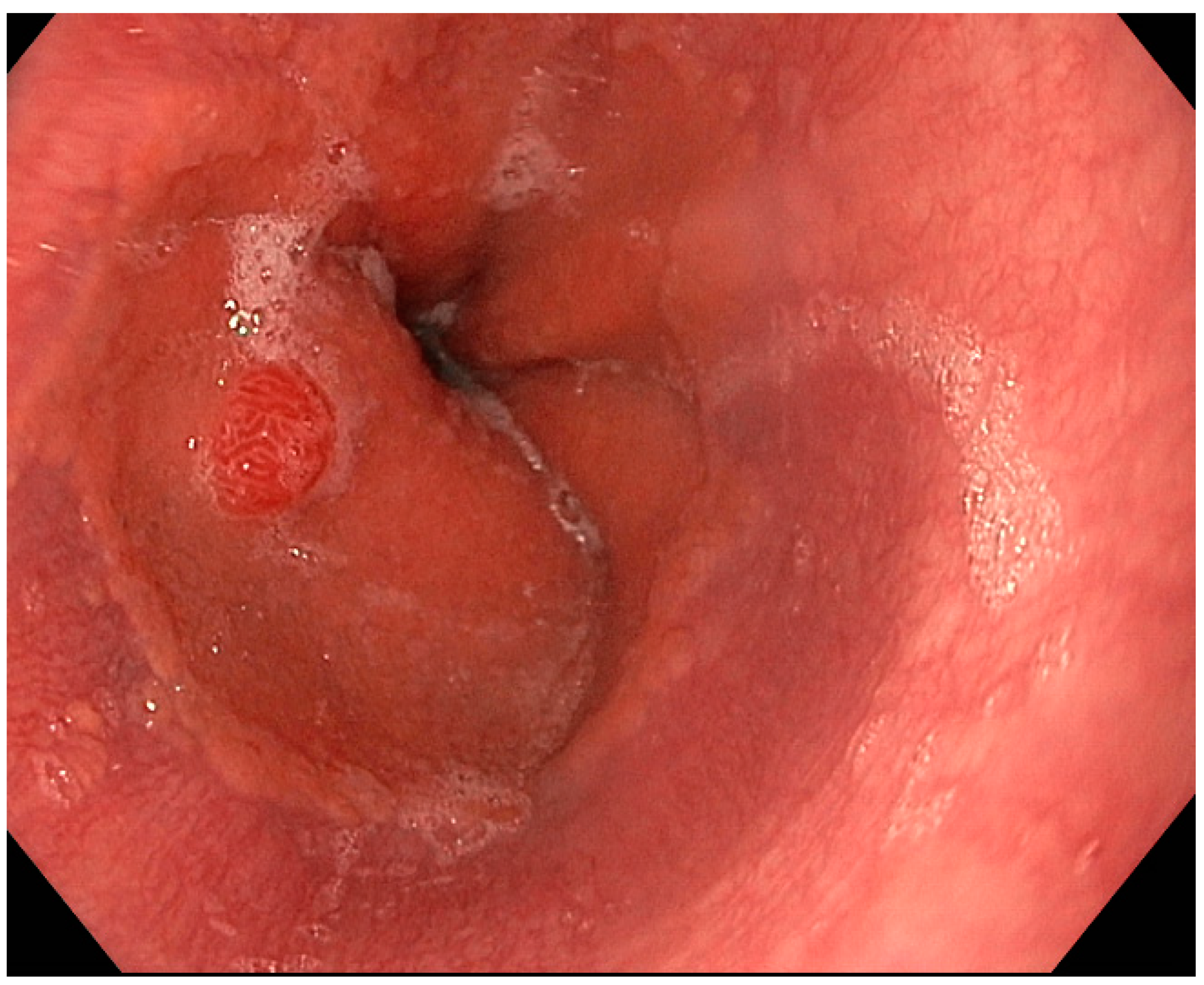
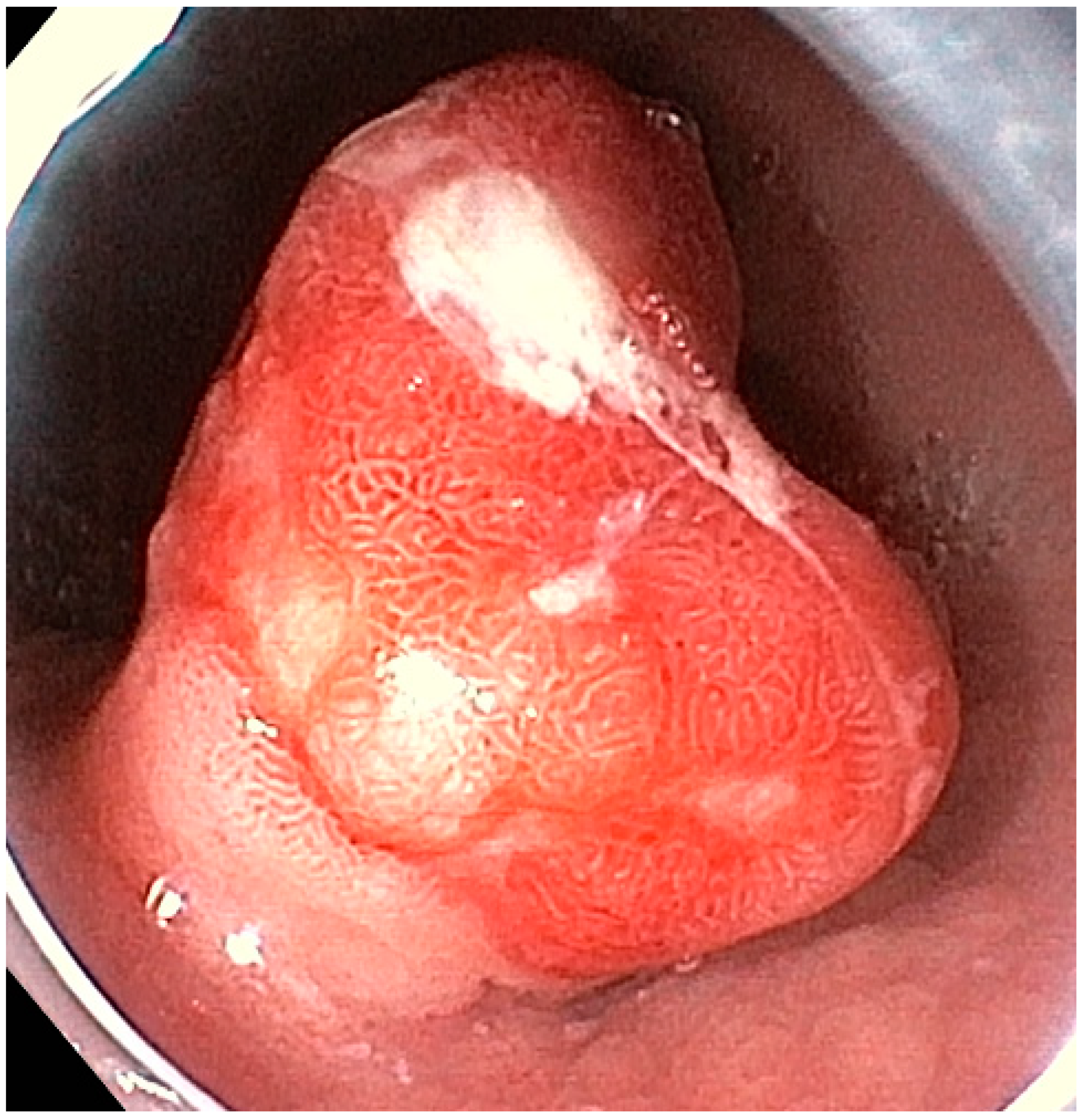
3.2. Endoscopic Resection
3.2.1. Excisional Biopsy and Polypectomy
3.2.2. Endoscopic Mucosal Resection
3.2.3. Endoscopic Submucosal Dissection
3.2.4. Endoscopic Full-Thickness Resection
3.2.5. Endoscopic Resection in Type-3 gNENs
3.3. Surgery
3.4. Systemic Therapy
3.5. Other Therapeutic Options for Advanced, Metastatic, or Progressive gNENs
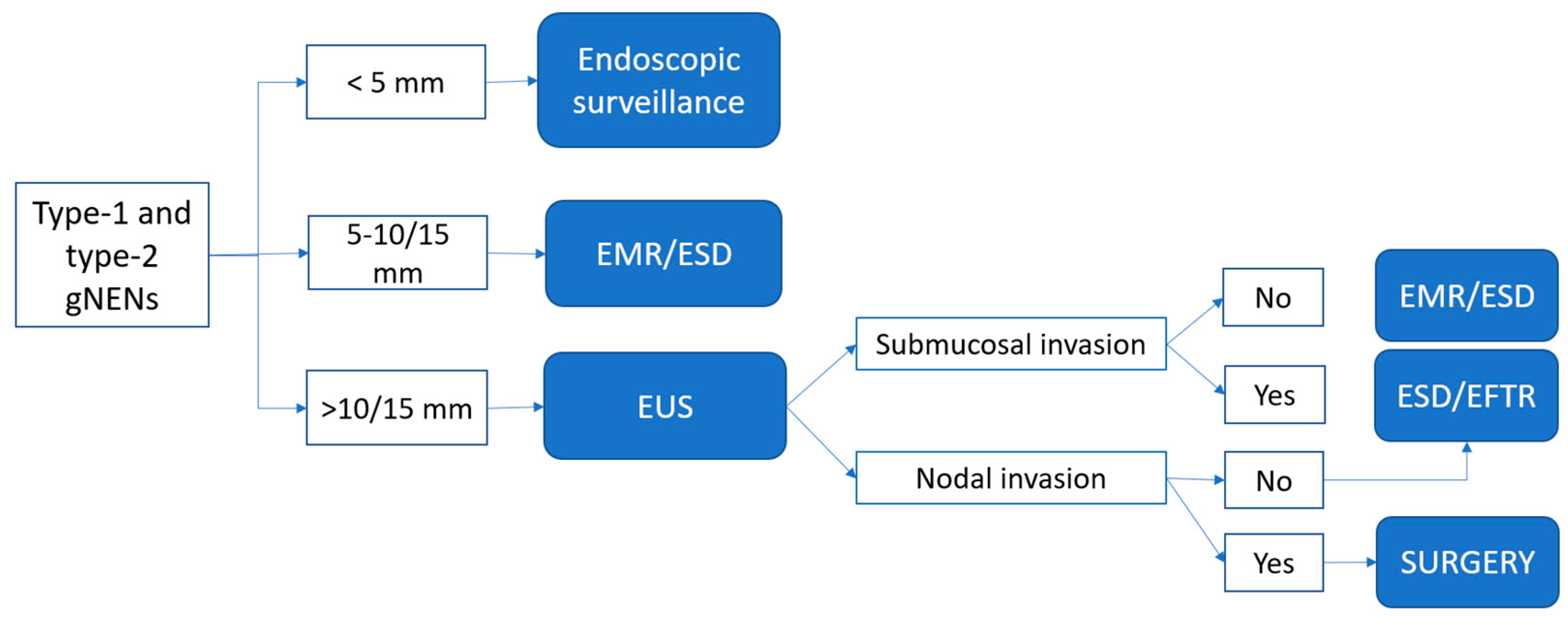
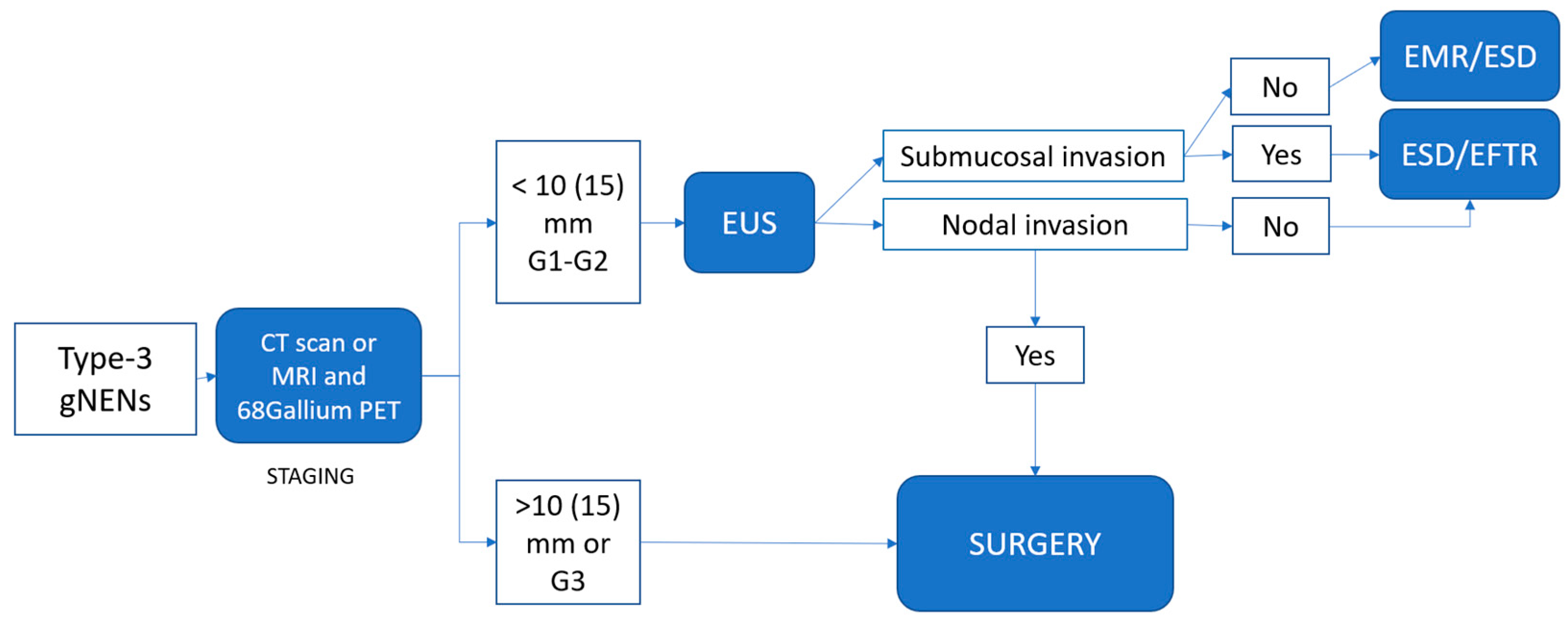
4. Proposed Practical Management of Well-Differentiated gNENs
4.1. Type-1 and Type-2 gNENs
4.2. Type-3 gNENs
5. Follow-Up
5.1. Type-1 and Type-2 gNENs
5.2. Type-3 gNENs
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Exarchou, K.; Hu, H.; Stephens, N.A.; Moore, A.R.; Kelly, M.; Lamarca, A.; Mansoor, W.; Hubner, R.; McNamara, M.G.; Smart, H.; et al. Endoscopic Surveillance Alone Is Feasible and Safe in Type I Gastric Neuroendocrine Neoplasms Less than 10 Mm in Diameter. Endocrine 2022, 78, 186–196. [Google Scholar] [CrossRef]
- Niederle, M.B.; Hackl, M.; Kaserer, K.; Niederle, B. Gastroenteropancreatic Neuroendocrine Tumours: The Current Incidence and Staging Based on the WHO and European Neuroendocrine Tumour Society Classification: An Analysis Based on Prospectively Collected Parameters. Endocr. Relat. Cancer 2010, 17, 909–918. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One Hundred Years after “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Li, A.F.-Y.; Hsu, C.-Y.; Li, A.; Tai, L.-C.; Liang, W.-Y.; Li, W.-Y.; Tsay, S.-H.; Chen, J.-Y. A 35-Year Retrospective Study of Carcinoid Tumors in Taiwan: Differences in Distribution with a High Probability of Associated Second Primary Malignancies. Cancer 2008, 112, 274–283. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. WHO Classification of Tumours Editorial Board The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Li, T.-T.; Qiu, F.; Qian, Z.R.; Wan, J.; Qi, X.-K.; Wu, B.-Y. Classification, Clinicopathologic Features and Treatment of Gastric Neuroendocrine Tumors. World J. Gastroenterol. 2014, 20, 118–125. [Google Scholar] [CrossRef]
- Panzuto, F.; Ramage, J.; Pritchard, D.M.; van Velthuysen, M.-L.F.; Schrader, J.; Begum, N.; Sundin, A.; Falconi, M.; O’Toole, D. European Neuroendocrine Tumor Society (ENETS) 2023 Guidance Paper for Gastroduodenal Neuroendocrine Tumours (NETs) G1-G3. J. Neuroendocrinol. 2023, 35, e13306. [Google Scholar] [CrossRef]
- Ahmed, M. Gastrointestinal Neuroendocrine Tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef]
- Gleeson, P.A.; Toh, B.-H.; Alderuccio, F.; van Driel, I.R. The Gastric H/K-ATPase: The Principle Target in Autoimmune Gastritis. In Proceedings of the Molecular and Cellular Mechanisms of H+ Transport; Hirst, B.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 119–126. [Google Scholar]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.-H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune Gastritis. Nat. Rev. Dis. Primer 2020, 6, 56. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Ciafardini, C.; Massironi, S. Gastric Neuroendocrine Neoplasms and Proton Pump Inhibitors: Fact or Coincidence? Scand. J. Gastroenterol. 2015, 50, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Gallo, C.; Laffusa, A.; Ciuffini, C.; Conti, C.B.; Barbaro, F.; Boskoski, I.; Dinelli, M.E.; Invernizzi, P. Endoscopic Techniques for Gastric Neuroendocrine Tumors: An Update. World J. Gastrointest. Endosc. 2023, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Roberto, G.A.; Rodrigues, C.M.B.; Peixoto, R.D.; Younes, R.N. Gastric Neuroendocrine Tumor: A Practical Literature Review. World J. Gastrointest. Oncol. 2020, 12, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Elvevi, A.; Quatrini, M.; Invernizzi, P. Somatostatin Analogs in Patients with Zollinger Ellison Syndrome (ZES): An Observational Study. Endocrine 2022, 75, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Elvevi, A.; Citterio, D.; Coppa, J.; Invernizzi, P.; Mazzaferro, V.; Massironi, S. Gastrinoma and Zollinger Ellison Syndrome: A Roadmap for the Management between New and Old Therapies. World J. Gastroenterol. 2021, 27, 5890–5907. [Google Scholar] [CrossRef]
- Lehy, T.; Cadiot, G.; Mignon, M.; Ruszniewski, P.; Bonfils, S. Influence of Multiple Endocrine Neoplasia Type 1 on Gastric Endocrine Cells in Patients with the Zollinger-Ellison Syndrome. Gut 1992, 33, 1275–1279. [Google Scholar] [CrossRef]
- Massironi, S.; Rossi, R.E.; Laffusa, A.; Eller-Vainicher, C.; Cavalcoli, F.; Zilli, A.; Ciafardini, C.; Sciola, V.; Invernizzi, P.; Peracchi, M. Sporadic and MEN1-Related Gastrinoma and Zollinger-Ellison Syndrome: Differences in Clinical Characteristics and Survival Outcomes. J. Endocrinol. Investig. 2023, 46, 957–965. [Google Scholar] [CrossRef]
- Kaltsas, G.; Caplin, M.; Davies, P.; Ferone, D.; Garcia-Carbonero, R.; Grozinsky-Glasberg, S.; Hörsch, D.; Tiensuu Janson, E.; Kianmanesh, R.; Kos-Kudla, B.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology 2017, 105, 245–254. [Google Scholar] [CrossRef]
- Rindi, G.; Azzoni, C.; La Rosa, S.; Klersy, C.; Paolotti, D.; Rappel, S.; Stolte, M.; Capella, C.; Bordi, C.; Solcia, E. ECL Cell Tumor and Poorly Differentiated Endocrine Carcinoma of the Stomach: Prognostic Evaluation by Pathological Analysis. Gastroenterology 1999, 116, 532–542. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. Carcinoid Tumors of the Stomach. Surg. Oncol. 2003, 12, 153–172. [Google Scholar] [CrossRef]
- Rugge, M.; Bricca, L.; Guzzinati, S.; Sacchi, D.; Pizzi, M.; Savarino, E.; Farinati, F.; Zorzi, M.; Fassan, M.; Dei Tos, A.P.; et al. Autoimmune Gastritis: Long-Term Natural History in Naïve Helicobacter Pylori-Negative Patients. Gut 2022, 72, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Thakker, R.V.; Newey, P.J.; Walls, G.V.; Bilezikian, J.; Dralle, H.; Ebeling, P.R.; Melmed, S.; Sakurai, A.; Tonelli, F.; Brandi, M.L.; et al. Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1). J. Clin. Endocrinol. Metab. 2012, 97, 2990–3011. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Patients. Available online: https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients (accessed on 15 August 2023).
- Chin, J.L.; O’Connell, J.; Muldoon, C.; Swan, N.; Reynolds, J.V.; Ravi, N.; Geoghegan, J.; Conlon, K.C.; O’Shea, D.; O’Toole, D. Selective Resection of Type 1 Gastric Neuroendocrine Neoplasms and the Risk of Progression in an Endoscopic Surveillance Programme. Dig. Surg. 2021, 38, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Deprez, P.H.; Moons, L.M.G.; O’Toole, D.; Gincul, R.; Seicean, A.; Pimentel-Nunes, P.; Fernández-Esparrach, G.; Polkowski, M.; Vieth, M.; Borbath, I.; et al. Endoscopic Management of Subepithelial Lesions Including Neuroendocrine Neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 412–429. [Google Scholar] [CrossRef]
- Tsolakis, A.V.; Ragkousi, A.; Vujasinovic, M.; Kaltsas, G.; Daskalakis, K. Gastric Neuroendocrine Neoplasms Type 1: A Systematic Review and Meta-Analysis. World J. Gastroenterol. 2019, 25, 5376–5387. [Google Scholar] [CrossRef]
- Zilli, A.; Arcidiacono, P.G.; Conte, D.; Massironi, S. Clinical Impact of Endoscopic Ultrasonography on the Management of Neuroendocrine Tumors: Lights and Shadows. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2018, 50, 6–14. [Google Scholar] [CrossRef]
- Karaca, C.; Turner, B.G.; Cizginer, S.; Forcione, D.; Brugge, W. Accuracy of EUS in the Evaluation of Small Gastric Subepithelial Lesions. Gastrointest. Endosc. 2010, 71, 722–727. [Google Scholar] [CrossRef]
- Panzuto, F.; Magi, L.; Esposito, G.; Rinzivillo, M.; Annibale, B. Comparison of Endoscopic Techniques in the Management of Type I Gastric Neuroendocrine Neoplasia: A Systematic Review. Gastroenterol. Res. Pract. 2021, 2021, 6679397. [Google Scholar] [CrossRef]
- Scherübl, H.; Cadiot, G. Early Gastroenteropancreatic Neuroendocrine Tumors: Endoscopic Therapy and Surveillance. Visc. Med. 2017, 33, 332–338. [Google Scholar] [CrossRef]
- Carvão, J.; Dinis-Ribeiro, M.; Pimentel-Nunes, P.; Libânio, D. Neuroendocrine Tumors of the Gastrointestinal Tract: A Focused Review and Practical Approach for Gastroenterologists. GE Port. J. Gastroenterol. 2021, 28, 336–348. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, D.H.; Yoon, H.; Hsing, L.-C.; Na, H.K.; Ahn, J.Y.; Lee, J.H.; Jung, K.W.; Choi, K.D.; Song, H.J.; et al. Clinical Outcomes of Endoscopic Treatment for Type 1 Gastric Neuroendocrine Tumor. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2021, 25, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Merola, E.; Sbrozzi-Vanni, A.; Panzuto, F.; D’Ambra, G.; Di Giulio, E.; Pilozzi, E.; Capurso, G.; Lahner, E.; Bordi, C.; Annibale, B.; et al. Type I Gastric Carcinoids: A Prospective Study on Endoscopic Management and Recurrence Rate. Neuroendocrinology 2012, 95, 207–213. [Google Scholar] [CrossRef] [PubMed]
- de Benito Sanz, M.; Hernández, L.; Garcia Martinez, M.I.; Diez-Redondo, P.; Joao Matias, D.; Gonzalez-Santiago, J.M.; Ibáñez, M.; Núñez Rodríguez, M.H.; Cimavilla, M.; Tafur, C.; et al. Efficacy and Safety of Cold versus Hot Snare Polypectomy for Small (5–9 Mm) Colorectal Polyps: A Multicenter Randomized Controlled Trial. Endoscopy 2022, 54, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Yoshikane, H.; Tsukamoto, Y.; Niwa, Y.; Goto, H.; Hase, S.; Mizutani, K.; Nakamura, T. Carcinoid Tumors of the Gastrointestinal Tract: Evaluation with Endoscopic Ultrasonography. Gastrointest. Endosc. 1993, 39, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.K.; De Herder, W.W.; Delle Fave, G.; Ferolla, P.; Ferone, D.; Ito, T.; Ruszniewski, P.; Sundin, A.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 139–143. [Google Scholar] [CrossRef]
- Thiruvengadam, S.S.; Fung, B.M.; Barakat, M.T.; Tabibian, J.H. Endoscopic Mucosal Resection: Best Practices for Gastrointestinal Endoscopists. Gastroenterol. Hepatol. 2022, 18, 133–144. [Google Scholar]
- Pimentel-Nunes, P.; Ortigão, R.; Afonso, L.P.; Bastos, R.P.; Libânio, D.; Dinis-Ribeiro, M. Endoscopic Resection of Gastrointestinal Neuroendocrine Tumors: Long-Term Outcomes and Comparison of Endoscopic Techniques. GE Port. J. Gastroenterol. 2023, 30, 98–106. [Google Scholar] [CrossRef]
- Uygun, A.; Kadayifci, A.; Polat, Z.; Yilmaz, K.; Gunal, A.; Demir, H.; Bagci, S. Long-Term Results of Endoscopic Resection for Type I Gastric Neuroendocrine Tumors. J. Surg. Oncol. 2014, 109, 71–74. [Google Scholar] [CrossRef]
- Sivandzadeh, G.R.; Ejtehadi, F.; Shoaee, S.; Aminlari, L.; Niknam, R.; Taghavi, A.R.; Geramizadeh, B.; Hormati, A.; Safarpour, A.R.; Bagheri Lankarani, K. Endoscopic Mucosal Resection: Still a Reliable Therapeutic Option for Gastrointestinal Neuroendocrine Tumors. BMC Gastroenterol. 2021, 21, 238. [Google Scholar] [CrossRef]
- Alzoubaidi, D.; Graham, D.; Bassett, P.; Magee, C.; Everson, M.; Banks, M.; Novelli, M.; Jansen, M.; Lovat, L.B.; Haidry, R. Comparison of Two Multiband Mucosectomy Devices for Endoscopic Resection of Barrett’s Esophagus-Related Neoplasia. Surg. Endosc. 2019, 33, 3665–3672. [Google Scholar] [CrossRef]
- Duette® Multi-Band Mucosectomy Device | Cook Medical. Available online: https://www.cookmedical.com/products/esc_duette_webds/ (accessed on 15 August 2023).
- Schölvinck, D.W.; Belghazi, K.; Pouw, R.E.; Curvers, W.L.; Weusten, B.L.a.M.; Bergman, J.J.G.H.M. In Vitro Assessment of the Performance of a New Multiband Mucosectomy Device for Endoscopic Resection of Early Upper Gastrointestinal Neoplasia. Surg. Endosc. 2016, 30, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Karaca, C.; Daglilar, E.S.; Soyer, O.M.; Gulluoglu, M.; Brugge, W.R. Endoscopic Submucosal Resection of Gastric Subepithelial Lesions Smaller than 20 Mm: A Comparison of Saline Solution-Assisted Snare and Cap Band Mucosectomy Techniques. Gastrointest. Endosc. 2017, 85, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Spadaccini, M.; Fuccio, L.; Lamonaca, L.; Frazzoni, L.; Maselli, R.; Di Leo, M.; Galtieri, P.A.; Craviotto, V.; D’Amico, F.; Hassan, C.; et al. Underwater EMR for Colorectal Lesions: A Systematic Review with Meta-Analysis (with Video). Gastrointest. Endosc. 2019, 89, 1109–1116.e4. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, G.H.; Kim, J.H.; Choi, M.-G.; Song, G.A.; Kim, S.E. The Efficacy of Endoscopic Submucosal Dissection of Type I Gastric Carcinoid Tumors Compared with Conventional Endoscopic Mucosal Resection. Gastroenterol. Res. Pract. 2014, 2014, 253860. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ono, H.; Hasuike, N.; Takizawa, K. Endoscopic Submucosal Dissection of Early Gastric Cancer. Digestion 2008, 77 (Suppl. S1), 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kakushima, N.; Fujishiro, M. Endoscopic Submucosal Dissection for Gastrointestinal Neoplasms. World J. Gastroenterol. 2008, 14, 2962–2967. [Google Scholar] [CrossRef]
- Chen, W.-F.; Zhou, P.-H.; Li, Q.-L.; Xu, M.-D.; Yao, L.-Q. Clinical Impact of Endoscopic Submucosal Dissection for Gastric Neuroendocrine Tumors: A Retrospective Study from Mainland China. Sci. World J. 2012, 2012, 869769. [Google Scholar] [CrossRef]
- Si, Y.; Huang, C.; Yuan, J.; Zhang, X.; He, Q.; Lin, Z.; He, L.; Liu, Z. Analysis of Prognostic Risk Factors of Endoscopic Submucosal Dissection (ESD) and Curative Resection of Gastrointestinal Neuroendocrine Neoplasms. Contrast Media Mol. Imaging 2022, 2022, 5248256. [Google Scholar] [CrossRef]
- Schmidt, A.; Meier, B.; Caca, K. Endoscopic Full-Thickness Resection: Current Status. World J. Gastroenterol. 2015, 21, 9273–9285. [Google Scholar] [CrossRef]
- Bisogni, D.; Manetti, R.; Talamucci, L.; Staderini, F.; Coratti, F.; Rossi, M.; Naspetti, R. Efficacy and Safety of Full-Thickness Resection Device Based on over-the-Scope Clip System for Resecting of Gastric Lesions in Selected Patients. Case Series from a Referral Center for Gastrointestinal Diseases Treatment and Literature Overview. Il G. Chir. 2019, 40, 569–577. [Google Scholar]
- Meier, B.; Schmidt, A.; Glaser, N.; Meining, A.; Walter, B.; Wannhoff, A.; Riecken, B.; Caca, K. Endoscopic Full-Thickness Resection of Gastric Subepithelial Tumors with the GFTRD-System: A Prospective Pilot Study (RESET Trial). Surg. Endosc. 2020, 34, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Jeon, S.W.; Kim, G.H.; Kim, J.I.; Chung, I.-K.; Jee, S.R.; Kim, H.U.; Seo, G.S.; Baik, G.H.; Choi, K.D.; et al. Long-Term Follow up of Endoscopic Resection for Type 3 Gastric NET. World J. Gastroenterol. 2013, 19, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-H.; Hong, M.; Lee, J.H.; Rhee, P.-L.; Sohn, T.S.; Kim, S.; Kim, K.-M.; Kim, J.J. Clinicopathological Features and Outcome of Type 3 Gastric Neuroendocrine Tumours. Br. J. Surg. 2018, 105, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Yamamoto, N.; Sano, T. Is Endoscopic Resection Appropriate for Type 3 Gastric Neuroendocrine Tumors? Retrospective Multicenter Study. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2021, 33, 408–417. [Google Scholar] [CrossRef]
- Laffi, A.; Lania, A.G.A.; Ragni, A.; Di Vito, V.; Liccardi, A.; Rubino, M.; Sesti, F.; Colao, A.; Faggiano, A.; on behalf of the Nike Group. Gastric Neuroendocrine Tumors (g-NETs): A Systematic Review of the Management and Outcomes of Type 3 g-NETs. Cancers 2023, 15, 2202. [Google Scholar] [CrossRef]
- Vanoli, A.; La Rosa, S.; Miceli, E.; Klersy, C.; Maragliano, R.; Capuano, F.; Persichella, A.; Martino, M.; Inzani, F.; Luinetti, O.; et al. Prognostic Evaluations Tailored to Specific Gastric Neuroendocrine Neoplasms: Analysis Of 200 Cases with Extended Follow-Up. Neuroendocrinology 2018, 107, 114–126. [Google Scholar] [CrossRef]
- Chung, C.-S.; Tsai, C.-L.; Chu, Y.-Y.; Chen, K.-C.; Lin, J.-C.; Chen, B.-C.; Sun, W.-C.; Yen, H.-H.; Chen, C.-Y.; Wu, I.-C.; et al. Clinical Features and Outcomes of Gastric Neuroendocrine Tumors after Endoscopic Diagnosis and Treatment: A Digestive Endoscopy Society of Tawian (DEST). Medicine 2018, 97, e12101. [Google Scholar] [CrossRef]
- Ye, H.; Yuan, Y.; Chen, P.; Zheng, Q. Risk Factors for Metastasis and Survival of Patients with T1 Gastric Neuroendocrine Carcinoma Treated with Endoscopic Therapy versus Surgical Resection. Surg. Endosc. 2022, 36, 6162–6169. [Google Scholar] [CrossRef]
- Exarchou, K.; Kamieniarz, L.; Tsoli, M.; Victor, A.; Oleinikov, K.; Khan, M.S.; Srirajaskanthan, R.; Mandair, D.; Grozinsky-Glasberg, S.; Kaltsas, G.; et al. Is Local Excision Sufficient in Selected Grade 1 or 2 Type III Gastric Neuroendocrine Neoplasms? Endocrine 2021, 74, 421–429. [Google Scholar] [CrossRef]
- Hanna, A.; Kim-Kiselak, C.; Tang, R.; Metz, D.C.; Yang, Z.; DeMatteo, R.; Fraker, D.L.; Roses, R.E. Gastric Neuroendocrine Tumors: Reappraisal of Type in Predicting Outcome. Ann. Surg. Oncol. 2021, 28, 8838–8846. [Google Scholar] [CrossRef]
- Rossi, R.E.; Invernizzi, P.; Mazzaferro, V.; Massironi, S. Response and Relapse Rates after Treatment with Long-Acting Somatostatin Analogs in Multifocal or Recurrent Type-1 Gastric Carcinoids: A Systematic Review and Meta-Analysis. United Eur. Gastroenterol. J. 2020, 8, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida Neoplasie Neuroendocrine. Available online: https://www.aiom.it/linee-guida-aiom-2021-neoplasie-neuroendocrine/ (accessed on 15 August 2023).
- Guarnotta, V.; Martini, C.; Davì, M.V.; Pizza, G.; Colao, A.; Faggiano, A.; NIKE group. The Zollinger-Ellison Syndrome: Is There a Role for Somatostatin Analogues in the Treatment of the Gastrinoma? Endocrine 2018, 60, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Igarashi, H.; Uehara, H.; Jensen, R.T. Pharmacotherapy of Zollinger-Ellison Syndrome. Expert Opin. Pharmacother. 2013, 14, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Carnaghi, C.; Buzzoni, R.; Pommier, R.F.; Raderer, M.; Tomasek, J.; Lahner, H.; Valle, J.W.; Voi, M.; Bubuteishvili-Pacaud, L.; et al. Everolimus in Neuroendocrine Tumors of the Gastrointestinal Tract and Unknown Primary. Neuroendocrinology 2018, 106, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Moore, A.R.; Sagatun, L.; Parsons, B.N.; Varro, A.; Campbell, F.; Fossmark, R.; Waldum, H.L.; Pritchard, D.M. Netazepide, a Gastrin/Cholecystokinin-2 Receptor Antagonist, Can Eradicate Gastric Neuroendocrine Tumours in Patients with Autoimmune Chronic Atrophic Gastritis. Br. J. Clin. Pharmacol. 2017, 83, 466–475. [Google Scholar] [CrossRef]
- Wang, R.; Zheng-Pywell, R.; Chen, H.A.; Bibb, J.A.; Chen, H.; Rose, J.B. Management of Gastrointestinal Neuroendocrine Tumors. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419884058. [Google Scholar] [CrossRef] [PubMed]
- Puliani, G.; Chiefari, A.; Mormando, M.; Bianchini, M.; Lauretta, R.; Appetecchia, M. New Insights in PRRT: Lessons From 2021. Front. Endocrinol. 2022, 13, 861434. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Hubelé, F.; De Marini, P.; Ouvrard, E.; Salvadori, J.; Addeo, P.; Garnon, J.; Kurtz, J.-E.; Greget, M.; Mertz, L.; et al. Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers 2021, 13, 6368. [Google Scholar] [CrossRef]
- Clift, A.K.; Frilling, A. Liver-Directed Therapies for Neuroendocrine Neoplasms. Curr. Oncol. Rep. 2021, 23, 44. [Google Scholar] [CrossRef]
- Suzuki, K.; Yoneda, M.; Kobayashi, N.; Nakajima, A. Neuroendocrine Tumor Treated with Arterial Chemoembolization Using DEB-TACE. Intern. Med. Tokyo Jpn. 2019, 58, 2895–2896. [Google Scholar] [CrossRef] [PubMed]
- Desmaison, C.; Niccoli, P.; Oziel Taieb, S.; Faure, M.; Ewald, J.; Izaaryene, J.; Piana, G. Transarterial Chemoembolization (TACE) for Neuroendocrine Liver Metastasis (NELM): Predictive Value of Volumetric Arterial Enhancement (VAE) on Baseline MRI. Bull. Cancer 2023, 110, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Scoggins, C.R. TACE or TARE for Unresectable Neuroendocrine Liver Metastases: Can We Finally Start to Focus on Value? Ann. Surg. Oncol. 2021, 28, 1876–1877. [Google Scholar] [CrossRef]
- Gallo, C.; Rossi, R.E.; Cavalcoli, F.; Barbaro, F.; Boškoski, I.; Invernizzi, P.; Massironi, S. Rectal Neuroendocrine Tumors: Current Advances in Management, Treatment, and Surveillance. World J. Gastroenterol. 2022, 28, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcoli, F.; Gallo, C.; Coltro, L.A.; Rausa, E.; Cantù, P.; Invernizzi, P.; Massironi, S. Therapeutic Challenges for Gastric Neuroendocrine Neoplasms: Take It or Leave It? Medicina 2023, 59, 1757. https://doi.org/10.3390/medicina59101757
Cavalcoli F, Gallo C, Coltro LA, Rausa E, Cantù P, Invernizzi P, Massironi S. Therapeutic Challenges for Gastric Neuroendocrine Neoplasms: Take It or Leave It? Medicina. 2023; 59(10):1757. https://doi.org/10.3390/medicina59101757
Chicago/Turabian StyleCavalcoli, Federica, Camilla Gallo, Lorenzo Andrea Coltro, Emanuele Rausa, Paolo Cantù, Pietro Invernizzi, and Sara Massironi. 2023. "Therapeutic Challenges for Gastric Neuroendocrine Neoplasms: Take It or Leave It?" Medicina 59, no. 10: 1757. https://doi.org/10.3390/medicina59101757
APA StyleCavalcoli, F., Gallo, C., Coltro, L. A., Rausa, E., Cantù, P., Invernizzi, P., & Massironi, S. (2023). Therapeutic Challenges for Gastric Neuroendocrine Neoplasms: Take It or Leave It? Medicina, 59(10), 1757. https://doi.org/10.3390/medicina59101757





