Gastritis Cystica Profunda: A Rare Disease, a Challenging Diagnosis, and an Uncertain Malignant Potential: A Case Report and Review of the Literature
Abstract
1. Introduction
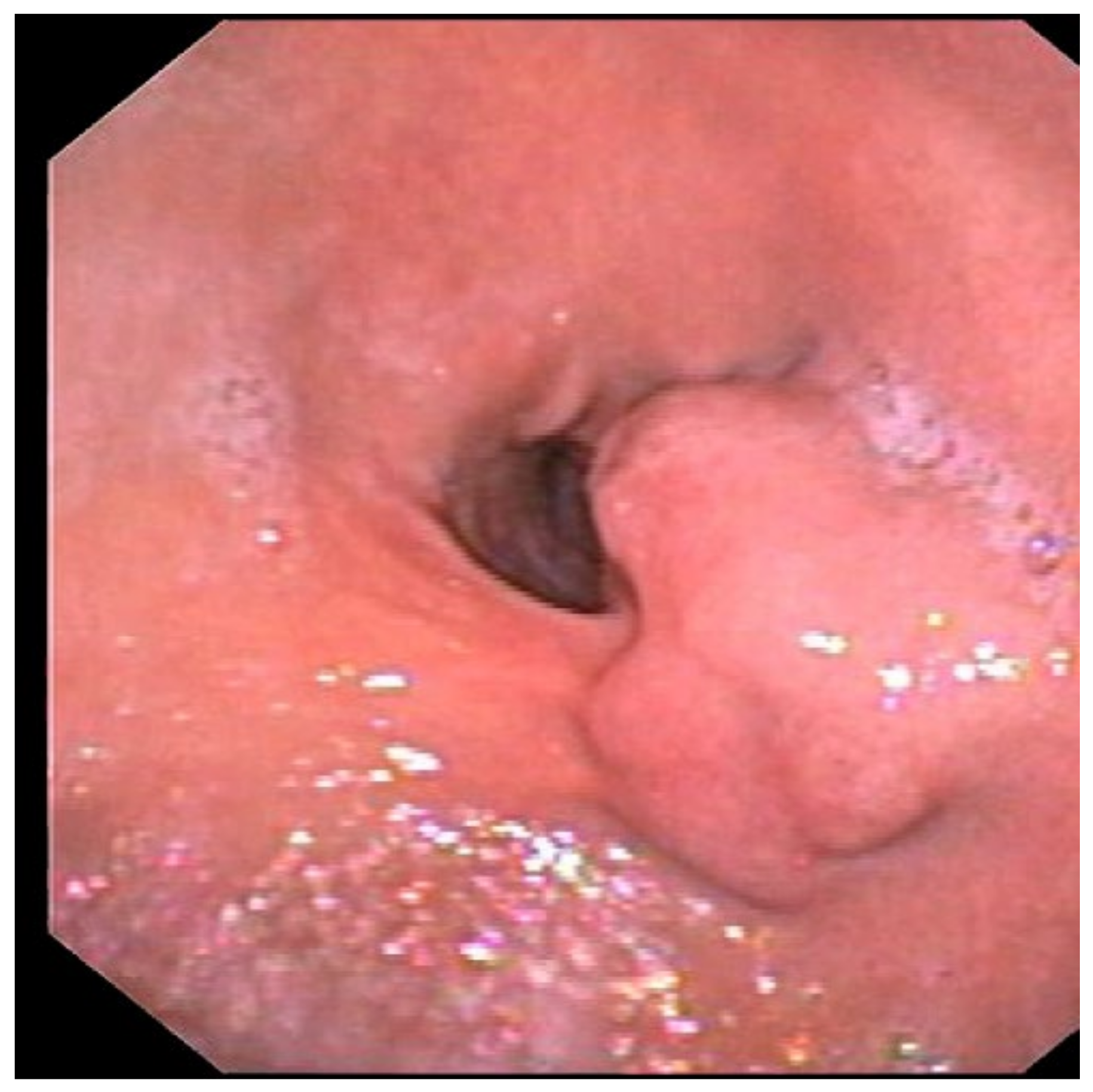
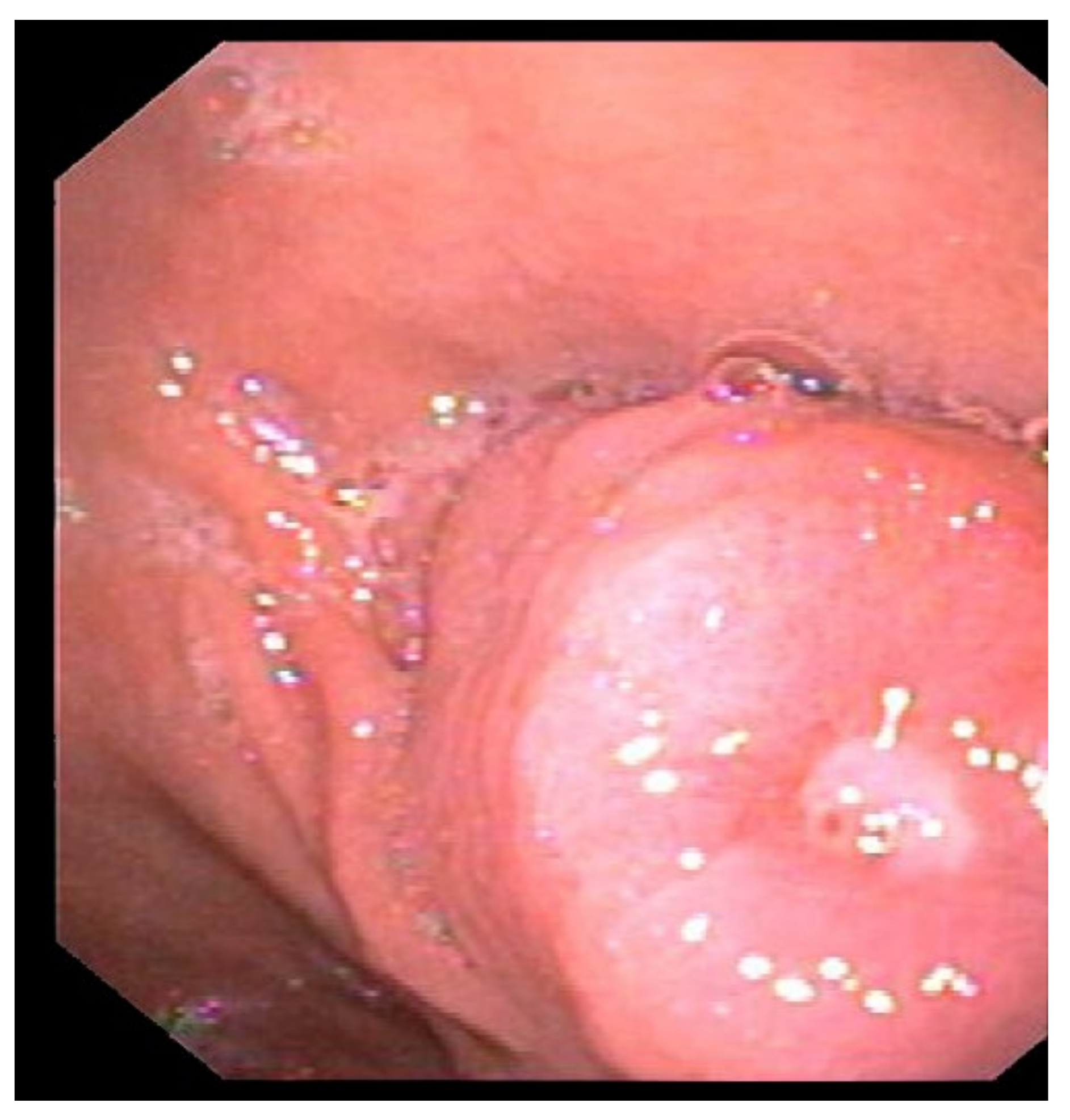
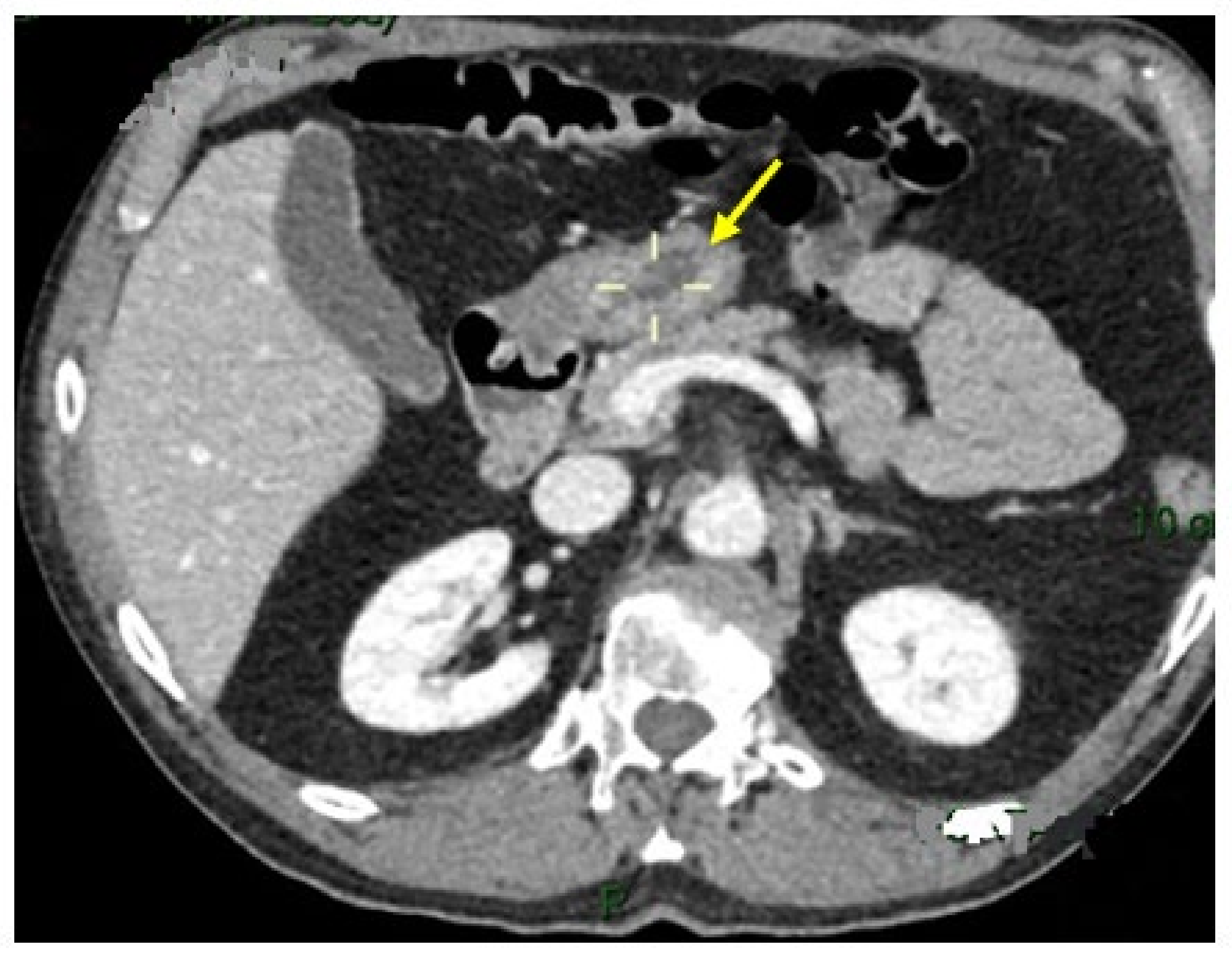
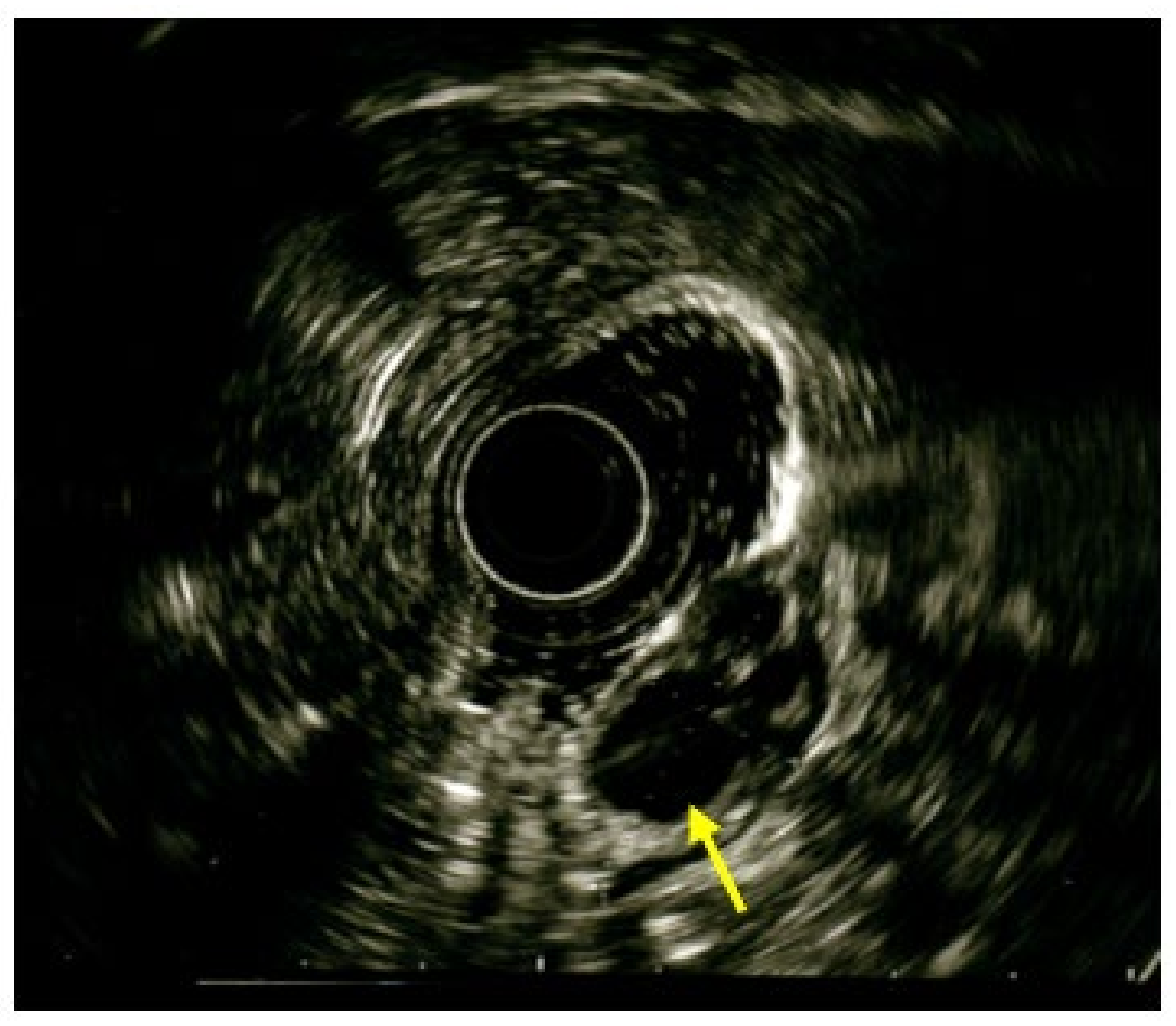
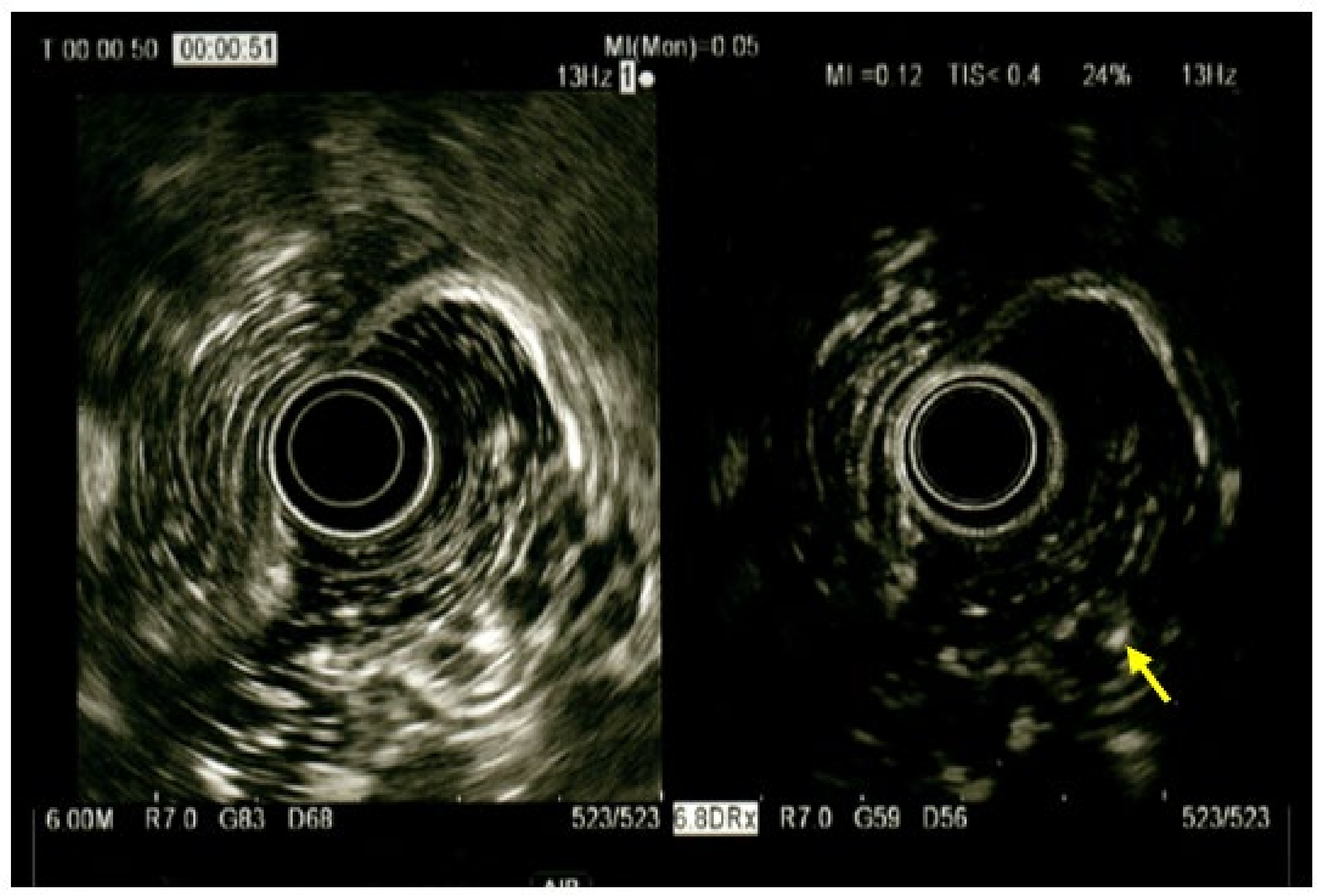
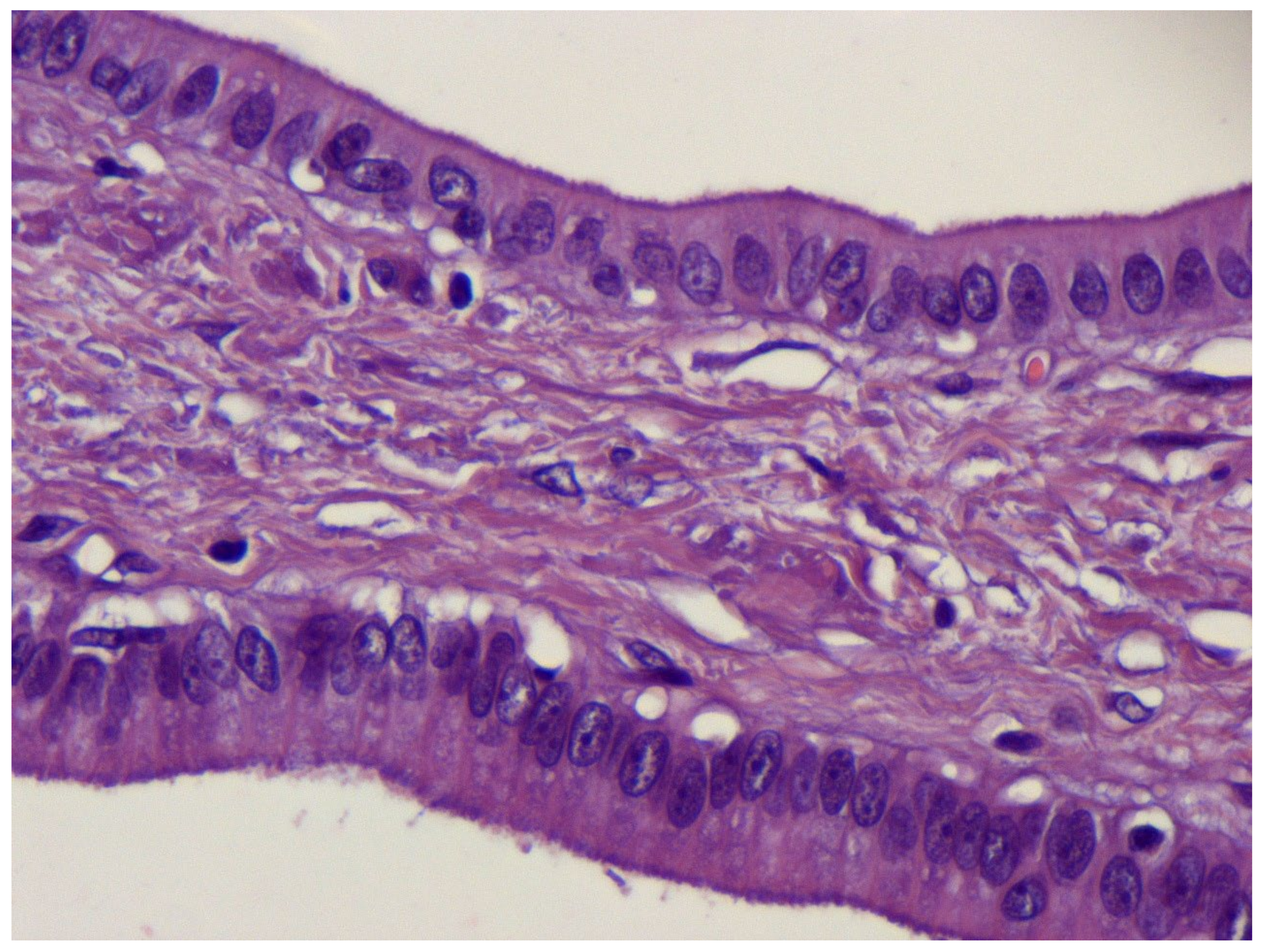
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, H.W.; Payne, T.P.B. Diffuse congenital cystic hyperplasia of stomach clinically simulating carcinoma; report of a case. Bull. Johns Hopkins Hosp. 1947, 81, 448–455. [Google Scholar] [PubMed]
- Littler, E.R.; Gleibermann, E. Gastritis cystica polyposa. (Gastric mucosal prolapse at gastroenterostomy site, with cystic and infiltrative epithelial hyperplasia). Cancer 1972, 29, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Franzin, G.; Novelli, P. Gastritis cystica profunda. Histopathology 1981, 5, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Qizilbash, A.H. Gastritis cystica and carcinoma arising in old gastrojejunostomy stoma. Can. Med Assoc. J. 1975, 112, 1432–1433. [Google Scholar] [PubMed]
- Yu, X.-F.; Guo, L.-W.; Chen, S.-T.; Teng, L.-S. Gastritis cystica profunda in a previously unoperated stomach: A case report. World J. Gastroenterol. 2015, 21, 3759–3762. [Google Scholar] [CrossRef] [PubMed]
- Béchade, D.; Desramé, J.; Algayres, J.P. Gastritis cystica profunda in a patient with no history of gastric surgery. Endoscopy 2007, 39 (Suppl. S1), E80–E81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Effenberger, M.; Steinle, H.; Offner, F.A.; Vogel, W.; Millonig, G. Holes in gastric mucosa in upper gastrointestinal endoscopy. Eur. J. Gastroenterol. Hepatol. 2014, 26, 676–678. [Google Scholar] [CrossRef]
- Itte, V.; Mallick, I.H.; Moore, P.J. Massive gastrointestinal haemorrhage due to gastritis cystica profunda. Cases J. 2008, 1, 1–3. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, M.; Imai, Y.; Inokuma, T. A rare cause of gastric outlet obstruction: Gastritis cystica profunda accompanied by adenocarcinoma. Endoscopy 2012, 44 (Suppl. S2), E138–E139. [Google Scholar] [CrossRef]
- Noh, S.J.; Kim, K.M.; Jang, K.Y. Gastritis cystica profunda with predominant histiocytic reaction mimicking solid submucosal tumor. Turk. J. Gastroenterol. 2020, 31, 726–728. [Google Scholar] [CrossRef]
- Franzin, G.; Musola, R.; Zamboni, G.; Manfrini, C. Gastritis Cystica Polyposa: A Possible Precancerous Lesion. Tumori J. 1985, 71, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, J.K.; Seo, H.I.; Han, K.H.; Kim, Y.D.; Jeong, W.J.; Cheon, G.J.; Eom, D.-W. A Case of Gastric Inverted Hyperplastic Polyp Found with Gastritis Cystica Profunda and Early Gastric Cancer. Clin. Endosc. 2013, 46, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kim, J.M.; Hur, Y.S.; Shin, Y.W.; Park, I.S.; Choi, S.J.; Han, J.Y.; Chu, Y.C.; Kim, K.H. Extended gastritis cystica profunda associated with Epstein-Barr virus-positive dysplasia and carcinoma with lymphoid stroma. Pathol. Int. 2012, 62, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, X.; Zhang, W.; Lv, Y.; Ling, T.; Zhou, Z.; Zhuge, Y.; Wang, L.; Zou, X.; Zhang, X.; et al. Endoscopic resection of gastritis cystica profunda: Preliminary experience with 34 patients from a single center in China. Gastrointest. Endosc. 2015, 81, 1493–1498. [Google Scholar] [CrossRef]
- Li, C.; Song, S.; Wu, G.; Zhang, Z. Gastritis cystica profunda: Clinical and pathologic study of seven cases and review of literature. Int. J. Clin. Exp. Pathol. 2021, 14, 261–266. [Google Scholar] [PubMed]
- Park, J.S.; Myung, S.-J.; Jung, H.-Y.; Yang, S.-K.; Hong, W.-S.; Kim, J.-H.; Kang, G.H.; Ha, H.K.; Min, Y.I. Endoscopic treatment of gastritis cystica polyposa found in an unoperated stomach. Gastrointest. Endosc. 2001, 54, 101–103. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, J.S.; Jin, S.Y. Gastritis cystica profunda with a long stalk. Gastrointest. Endosc. 2013, 77, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, T.; Loell, E.; Mueller, S.; Stoeckelhuber, M.; Stolte, M.; Haas, R.; Rieder, G. Helicobacter pylori cag-Pathogenicity Island-Dependent Early Immunological Response Triggers Later Precancerous Gastric Changes in Mongolian Gerbils. PLoS ONE 2009, 4, e4754. [Google Scholar] [CrossRef]
- Matsushita, M.; Mori, S.; Tahashi, Y.; Uchida, K.; Nishio, A.; Okazaki, K. Gastritis cystica polyposa in the operated stomach and heterotopic submucosal cysts in the unoperated stomach. Gastrointest. Endosc. 2010, 71, 1100–1101. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, W.; Ma, Y.; Qiu, Z. Gastritis cystica profunda: A case report and literature review. Ann. Palliat. Med. 2020, 9, 3668–3677. [Google Scholar] [CrossRef]
- Okada, M.; Iizuka, Y.; Oh, K.; Murayama, H.; Maekawa, T. Gastritis cystica profunda presenting as giant gastric mucosal folds: The role of endoscopic ultrasonography and mucosectomy in the diagnostic work-up. Gastrointest. Endosc. 1994, 40, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.N.; Wang, X.W.; Chen, Y.Q.; Cui, Z.; Bin Tian, Z.; Zhao, Q.X.; Mao, T.; Xie, M.; Yin, X.Y. A retrospective analysis of 13 cases of gastritis cystica profunda treated by endoscopic resection and surgery. J. Dig. Dis. 2022, 23, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, H.; Yu, J.; Huang, W.; Li, J.; Cheng, M.; Liang, P.; Li, L.; Zhao, H.; Gao, J. Computed tomography features and clinical characteristics of gastritis cystica profunda. Insights Into Imaging 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.K.; Kim, E.J.; Kim, D.J.; Kim, H.S.; Park, S.H.; Lee, M.H.; Kim, S.J. 4541 Clinical significance of gastritis cystica polyposa and role of endoscopic ultrasonography. Gastrointest. Endosc. 2000, 51, AB163. [Google Scholar] [CrossRef]
- Thosani, N.; Machicado, J.; Shroff, J.; Quesada, A.; Jelinek, K.; Spinn, M.; Scott, L. Gastritis cystica profunda: Endoscopic ultrasound findings and review of the literature. Endosc. Ultrasound 2014, 3, 131–134. [Google Scholar] [CrossRef]
- Seven, G.; Arici, D.S.; Senturk, H. Correlation of Endoscopic Ultrasonography Features with the Mitotic Index in 2- to 5-cm Gastric Gastrointestinal Stromal Tumors. Dig. Dis. 2022, 40, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Y.; Guo, T. Gastritis profunda cystica presenting as a tumor-like lesion under endoscopy. Asian J. Surg. 2023, 46, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, N.; Noda, H.; Kondo, Y.; Yoshimine, T.; Sugiyama, T.; Kimura, M.; Inoue, S.; Takahashi, E.; Sasaki, M.; Kasugai, K. A Case of Early Gastric Cancer Arising from Gastritis Cystica Profunda Treated by Endoscopic Submucosal Dissection. Case Rep. Gastroenterol. 2014, 8, 270–275. [Google Scholar] [CrossRef]
- Tsuji, T.; Iwahashi, M.; Nakamori, M.; Ueda, K.; Ishida, K.; Naka, T.; Ojima, T.; Akamatsu, H.; Yamaue, H. Multiple early gastric cancer with gastritis cystica profunda showing various histological types. Hepatogastroenterology 2008, 55, 1150–1152. [Google Scholar]
- Park, C.-H.; Park, J.M.; Jung, C.K.; Kim, D.-B.; Kang, S.H.; Lee, S.W.; Cho, Y.K.; Kim, S.W.; Choi, M.-G.; Chung, I.-S. Early gastric cancer associated with gastritis cystica polyposa in the unoperated stomach treated by endoscopic submucosal dissection. Gastrointest. Endosc. 2009, 69, e47–e50. [Google Scholar] [CrossRef]
- Teng, T.Z.J.; Ishraq, F.; Chay, A.F.T.; Tay, K.V. Lap-Endo cooperative surgery (LECS) in gastric GIST: Updates and future advances. Surg. Endosc. 2023, 37, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Laratta, J.L.; Buhtoiarova, T.N.; Sparber, L.S.; Chamberlain, R.S. Gastritis Cystica Profunda: A Rare Gastric Tumor Masquerading as a Malignancy. Surg. Sci. 2012, 3, 158–164. [Google Scholar] [CrossRef]
- Wang, L.; Yan, H.; Cao, D.-C.; Huo, L.; Huo, H.-Z.; Wang, B.; Chen, Y.; Liu, H.-L. Gastritis cystica profunda recurrence after surgical resection: 2-year follow-up. World J. Surg. Oncol. 2014, 12, 133. [Google Scholar] [CrossRef]
- Huang, D.; Zhan, Q.; Yang, S.; Sun, Q.; Zhou, Z. Synchronous double superficial mixed gastrointestinal mucus phenotype gastric cancer with gastritis cystica profunda and submucosal lipoma: A case report. Medicine 2018, 97, e10825. [Google Scholar] [CrossRef] [PubMed]
- Vauhkonen, M.; Vauhkonen, H.; Sipponen, P. Pathology and molecular biology of gastric cancer. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Kim, K.-O.; Park, S.H.; Yoo, K.-S.; Park, C.H.; Kim, J.H.; Park, C.K.; Jun, S.-Y. Gastritis Cystica Profunda Accompanied by Multiple Early Gastric Cancers. Korean J. Gastroenterol. 2010, 55, 325–330. [Google Scholar] [CrossRef]
- Mitomi, H.; Iwabuchi, K.; Amemiya, A.; Kaneda, G.; Adac, I.H.K.; Asao, T. Immunohistochemical Analysis of a Case of Gastritis Cystica Profunda Associated with Carcinoma Development. Scand. J. Gastroenterol. 1998, 33, 1226–1229. [Google Scholar] [CrossRef]
- Choi, M.-G.; Jeong, J.Y.; Kim, K.-M.; Bae, J.M.; Noh, J.H.; Sohn, T.S.; Kim, S. Clinical significance of gastritis cystica profunda and its association with Epstein-Barr virus in gastric cancer. Cancer 2012, 118, 5227–5233. [Google Scholar] [CrossRef]
- Roepke, T.K.; Purtell, K.; King, E.C.; La Perle, K.M.D.; Lerner, D.J.; Abbott, G.W. Targeted Deletion of Kcne2 Causes Gastritis Cystica Profunda and Gastric Neoplasia. PLoS ONE 2010, 5, e11451. [Google Scholar] [CrossRef]
- Kuwahara, N.; Kitazawa, R.; Fujiishi, K.; Nagai, Y.; Haraguchi, R.; Kitazawa, S. Gastric adenocarcinoma arising in gastritis cystica profunda presenting with selective loss of KCNE2 expression. World J. Gastroenterol. 2013, 19, 1314–1317. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Stefano, F.; Graziano, G.M.P.; Viganò, J.; Mauro, A.; Peloso, A.; Peverada, J.; Fellegara, R.; Vanoli, A.; Faillace, G.G.; Ansaloni, L. Gastritis Cystica Profunda: A Rare Disease, a Challenging Diagnosis, and an Uncertain Malignant Potential: A Case Report and Review of the Literature. Medicina 2023, 59, 1770. https://doi.org/10.3390/medicina59101770
De Stefano F, Graziano GMP, Viganò J, Mauro A, Peloso A, Peverada J, Fellegara R, Vanoli A, Faillace GG, Ansaloni L. Gastritis Cystica Profunda: A Rare Disease, a Challenging Diagnosis, and an Uncertain Malignant Potential: A Case Report and Review of the Literature. Medicina. 2023; 59(10):1770. https://doi.org/10.3390/medicina59101770
Chicago/Turabian StyleDe Stefano, Francesca, Giorgio M. P. Graziano, Jacopo Viganò, Aurelio Mauro, Andrea Peloso, Jacopo Peverada, Raffaele Fellegara, Alessandro Vanoli, Giuseppe G. Faillace, and Luca Ansaloni. 2023. "Gastritis Cystica Profunda: A Rare Disease, a Challenging Diagnosis, and an Uncertain Malignant Potential: A Case Report and Review of the Literature" Medicina 59, no. 10: 1770. https://doi.org/10.3390/medicina59101770
APA StyleDe Stefano, F., Graziano, G. M. P., Viganò, J., Mauro, A., Peloso, A., Peverada, J., Fellegara, R., Vanoli, A., Faillace, G. G., & Ansaloni, L. (2023). Gastritis Cystica Profunda: A Rare Disease, a Challenging Diagnosis, and an Uncertain Malignant Potential: A Case Report and Review of the Literature. Medicina, 59(10), 1770. https://doi.org/10.3390/medicina59101770




