The Effectiveness of Antiplatelet Therapy and the Factors Influencing It in Patients with Acute Coronary Syndrome before and during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Aspirin Treatment
3.2. Treatment with P2Y12 Inhibitors
4. Discussion
4.1. Aspirin Treatment
4.2. Treatment with P2Y12 Inhibitors
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 13 November 2022).
- Worthley, S.G.; Osende, J.I.; Helft, G.; Badimon, J.J.; Fuster, V. Coronary artery disease: Pathogenesis and acute coronary syndromes. Mt. Sinai J. Med. N. Y. 2001, 68, 167–181. [Google Scholar]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef] [PubMed]
- Sarno, G.; Lagerqvist, B.; Fröbert, O.; Nilsson, J.; Olivecrona, G.; Omerovic, E.; Saleh, N.; Venetzanos, D.; James, S. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: A report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur. Heart J. 2012, 33, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Dendale, P.; Bhatt, D.L.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Phil, D.; Feng, Z.M.; Shamir, R.M.; Chrolavicius, S.; Tognoni, G.; Keith, K.F. Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Butler, K.; Tantry, U.S.; Gesheff, T.; Wei, C.; Teng, R.; Antonino, M.J.; Patil, S.B.; Karunakaran, A.; et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation 2009, 120, 2577–2585. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, t.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.; Ardissino, D.; de Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.; Cuisset, T.; Franchi, F.; Gross, L. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. Intv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Winter, M.P.; Schneeweiss, T.; Cremer, R.; Biesinger, B.; Hengstenberg, C.; Prüller, F.; Wallner, M.; Kolesnik, E.; von Lewinski, D.; Lang, I.M.; et al. Platelet reactivity patterns in patients treated with dual antiplatelet therapy. Eur. J. Clin. Investig. 2019, 49, e13102. [Google Scholar] [CrossRef] [PubMed]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.H.; Angiolillo, D.J.; Stone, G.W.; Curzen, N.; Geisler, T.; Berg, J.t.; et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, A.J.; Dalçóquio, T.F.; Salsoso, R.; Furtado, R.H.d.M.; Kalil-Filho, R.; Hajjar, L.A.; Siciliano, R.F.; Kallás, E.G.; Baracioli, L.M.; Lima, F.G.; et al. Platelet Reactivity and Coagulation Markers in Patients with COVID-19. Adv. Ther. 2021, 38, 3911–3923. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. Heart J. 2018, 39, 213–254. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Bhatt, D.L.; Michelson, A.D. Laboratory monitoring of antiplatelet therapy. In Platelets, 4th ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 653–682. [Google Scholar] [CrossRef]
- Le Quellec, S.; Bordet, J.C.; Negrier, C.; Dargaud, Y. Comparison of current platelet functional tests for the assessment of aspirin and clopidogrel response a review of the literature. Thromb. Haemost. 2016, 116, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, A.C.; Garcia, S.; Giri, J. Myocardial Infarction During the COVID-19 Pandemic. JAMA 2021, 326, 1916–1918. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, K.; Reinshagen, L.; Puccini, M.; Friebel, J.; Wilde, A.B.; Alsheik, A.; Rroku, A.; Landmesser, U.; Haghikia, A.; Kränkel, N.; et al. Disease Severity in Moderate-to-Severe COVID-19 Is Associated with Platelet Hyperreactivity and Innate Immune Activation. Front. Immunol. 2022, 13, 844701. [Google Scholar] [CrossRef] [PubMed]
- Heinz, C.; Miesbach, W.; Herrmann, E.; Sonntagbauer, M.; Raimann, F.J.; Zacharowski, K.; Weber, C.F.; Adam, E.H. Greater Fibrinolysis Resistance but No Greater Platelet Aggregation in Critically Ill COVID-19 Patients. Anesthesiology 2021, 134, 457–467. [Google Scholar] [CrossRef]
- Storey, R.F. More transparency for a therapeutic window in platelet P2Y12 inhibition? Eur. Heart J. 2015, 36, 1714–1717. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Castellone, D.D.; Stroobants, A.K.; Francis, J. Reference range determination for whole-blood platelet aggregation using the multiplate analyzer. Am. J. Clin. Pathol. 2014, 142, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Cho, J.R. Aspirin Treatment and Outcomes in Patients Undergoing Percutaneous Coronary Intervention: Is There a Role for Pharmacodynamic Testing? J. Am. Coll. Cardiol. 2014, 64, 872–874. [Google Scholar] [CrossRef][Green Version]
- Stone, G.W.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Mazzaferri, E.; et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013, 382, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.C.J.; Leadbeater, P.D.; Chan, M.V.; Kirkby, N.S.; Jakubowski, J.A.; Mitchell, J.A.; Warner, T.D. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J. Thromb. Haemost. 2011, 9, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: Executive Summary. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.S.; Mulder, H.; Wruck, L.M.; Pencina, M.J.; Kripalani, S.; Muñoz, D.; Crenshaw, D.L.; Effron, M.B.; Re, R.N.; Gupta, K.; et al. Comparative Effectiveness of Aspirin Dosing in Cardiovascular Disease. N. Engl. J. Med. 2021, 384, 1981–1990. [Google Scholar] [CrossRef]
- Anm.ro. Available online: https://www.anm.ro/_/_PRO/PRO_8517_14.01.16.pdf (accessed on 22 March 2022).
- Grosser, T.; Fries, S.; Lawson, J.A.; Kapoor, S.C.; Grant, G.R.; FitzGerald, G.A. Drug resistance and pseudoresistance: An unintended consequence of enteric coating aspirin. Circulation 2013, 127, 377–385. [Google Scholar] [CrossRef]
- Le Blanc, J.; Lordkipanidzé, M. Platelet Function in Aging. Front. Cardiovasc. Med. 2019, 6, 109. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Cook, N.R.; Gaziano, J.M.; Price, J.F.; Belch, J.F.F.; Roncaglioni, M.C.; Morimoto, T.; Mehta, Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet 2018, 392, 387–399. [Google Scholar] [CrossRef]
- Paven, E.; Dillinger, J.G.; Bal Dit Sollier, C.; Vidal-Trecan, T.; Berge, N.; Dautry, R.; Gautier, J.F.; Drouet, L.; Riveline, J.P.; Henry, P. Determinants of aspirin resistance in patients with type 2 diabetes. Diabetes Metab. 2020, 46, 370–376. [Google Scholar] [CrossRef]
- Tasdemir, E.; Toptas, T.; Demir, C.; Esen, R.; Atmaca, M. Aspirin resistance in patients with type II diabetes mellitus. Upsala J. Med. Sci. 2014, 119, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Morrow, D.A.; Cannon, C.P.; Murphy, S.A.; Demopoulos, L.A.; DiBattiste, P.M.; McCabe, C.H.; Braunwald, E.; Gibson, C.M. Relationship between Baseline White Blood Cell Count and Degree of Coronary Artery Disease and Mortality in Patients with Acute Coronary Syndromes A TACTICS-TIMI 18 Substudy. J. Am. Coll. Cardiol. 2002, 40, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Alkhalfan, F.; Nafee, T.; Yee, M.K.; Chi, G.; Kalayci, A.; Plotnikov, A.; Braunwald, E.; Gibson, C.M. Relation of White Blood Cell Count to Bleeding and Ischemic Events in Patients with Acute Coronary Syndrome (from the ATLAS ACS 2-TIMI 51 Trial). Am. J. Cardiol. 2020, 125, 661–669. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Recovery Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (Recovery): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 143–151. [Google Scholar] [CrossRef]
- Jakl, M.; Sevcik, R.; Ceral, J.; Fatorova, I.; Horacek, J.M.; Vojacek, J. Mean platelet volume and platelet count: Overlooked markers of high on-treatment platelet reactivity and worse outcome in patients with acute coronary syndrome. Anadolu Kardiyol. Derg. 2014, 14, 85–86. [Google Scholar] [CrossRef]
- Lordkipanidzé, M.; Diodati, J.G.; Turgeon, J.; Schampaert, E.; Palisaitis, D.A.; Pharand, C. Platelet count, not oxidative stress, may contribute to inadequate platelet inhibition by aspirin. Int. J. Cardiol. 2010, 143, 43–50. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef]
- Deharo, P.; Quilici, J.; Camoin-Jau, L.; Johnson, T.W.; Bassez, C.; Bonnet, G.; Fernandez, M.; Ibrahim, M.; Suchon, P.; Verdier, V.; et al. Benefit of Switching Dual Antiplatelet Therapy After Acute Coronary Syndrome According to On-Treatment Platelet Reactivity: The TOPIC-VASP Pre-Specified Analysis of the TOPIC Randomized Study. JACC Cardiovasc. Interv. 2017, 10, 2560–2570. [Google Scholar] [CrossRef]
- Généreux, P.; Giustino, G.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Rinaldi, M.J.; Neumann, F.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; et al. Incidence, Predictors, and Impact of Post-Discharge Bleeding after Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2015, 66, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Sinnaeve, P.R.; Van de Werf, F. Clopidogrel instead of prasugrel or ticagrelor after 1 month in stabilized ACS patients: Back to square one for DAPT? Eur. Heart J. 2017, 38, 3079–3081. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Benenati, S.; Franchi, F.; Rollini, F.; Capodanno, D.; Biondi-Zoccai, G.; Vescovo, G.M.; Cavallari, L.H.; Bikdeli, B.; Berg, J.t.; et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: A network meta-analysis of 61 898 patients from 15 randomized trials. Eur. Heart J. 2021, 43, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Husted, S.; James, S.; Becker, R.C.; Horrow, J.; Katus, H.; Storey, R.F.; Cannon, C.P.; Heras, M.; Lopes, R.D.; Morais, J.; et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: A substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc. Qual. Outcomes 2012, 5, 680–688. [Google Scholar] [CrossRef]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Nardin, M.; Barbieri, L.; Schaffer, A.; Marino, P.; Suryapranata, H.; de Luca, G. Mean platelet volume and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Expert Opin. Pharmacother. 2015, 16, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, T.; Vats, R.; Bennewitz, M.F.; Tutuncuoglu, E.; Watkins, S.C.; Ragni, M.V.; Neal, M.D.; Gladwin, M.T.; Sundd, P. Intravascular hemolysis triggers ADP-mediated generation of platelet-rich thrombi in precapillary pulmonary arterioles. JCI Insight 2020, 5, 14. [Google Scholar] [CrossRef]
- Berger, J.S.; Kornblith, L.Z.; Gong, M.N.; Reynolds, H.R.; Cushman, M.; Cheng, Y.; McVerry, B.J.; Kim, K.S.; Lopes, R.D.; Atassi, B.; et al. Effect of P2Y12 Inhibitors on Survival Free of Organ Support Among Non–Critically Ill Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA 2022, 327, 227–236. [Google Scholar] [CrossRef]
- Nelson, T.A.; Parker, W.A.E.; Ghukasyan Lakic, T.; Westerbergh, J.; James, S.K.; Siegbahn, A.; Becker, R.C.; Himmelmann, A.; Wallentin, L.; Storey, R.F. Differential effect of clopidogrel and ticagrelor on leukocyte count in relation to patient characteristics, biomarkers and genotype: A PLATO substudy. Platelets 2022, 33, 425–431. [Google Scholar] [CrossRef]
- McAlister, F.A.; Ezekowitz, J.; Tonelli, M.; Armstrong, P.W. Renal Insufficiency and Heart Failure: Prognostic and Therapeutic Implications from a Prospective Cohort Study. Circulation 2004, 109, 1004–1009. [Google Scholar] [CrossRef]
- Ema.europa.eu. Available online: https://www.ema.europa.eu/en/documents/product-information/brilique-epar-product-information_en.pdf (accessed on 22 March 2022).
- James, S.; Budaj, A.; Aylward, P.; Buck, K.K.; Cannon, C.P.; Cornel, J.H.; Harrington, R.A.; Horrow, J.; Katus, H.; Keltai, M.; et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the platelet inhibition and patient outcomes (PLATO) trial. Circulation 2010, 122, 1056–1067. [Google Scholar] [CrossRef]

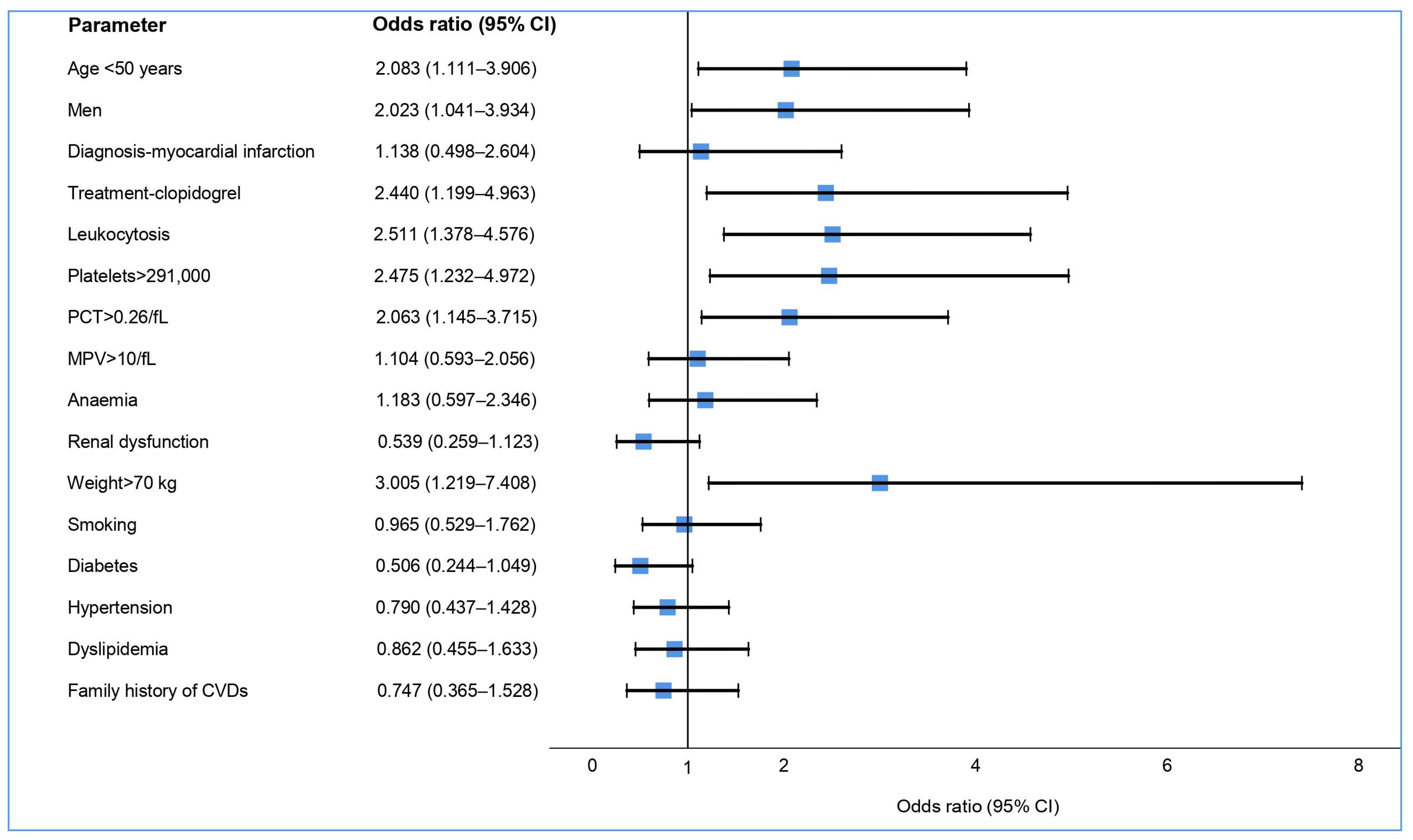
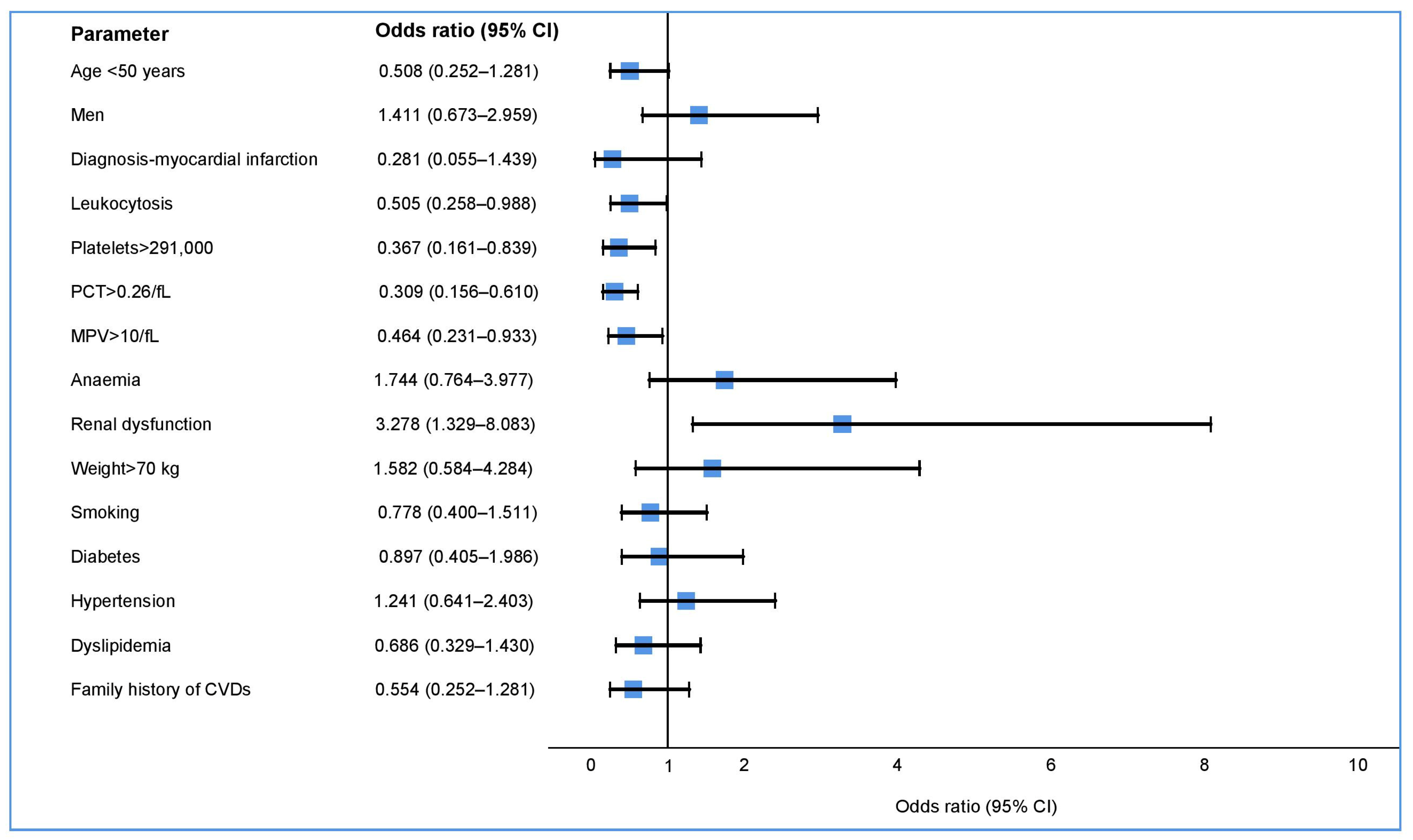
| Parameter | Sample | Aspirin | Clopidogrel | Ticagrelor | p Value |
|---|---|---|---|---|---|
| n (%) | 193 (100) | 191 (98.96) | 41 (21.24) | 148 (76.68) | NA |
| Age mean ± SD | 58.54 ± 13.34 | 58.40 ± 13.34 | 65.29 ± 13.84 | 56.74 ± 12.81 | 0.001 |
| Age < 50 years n (%) | 57 (29.53) | 57 (29.84) | 5 (12.19) | 52 (35.13) | 0.005 |
| Men n (%) | 138 (71.50) | 137 (71.72) | 28 (68.39) | 109 (73.64) | 0.497 |
| Weight mean ± SD | 84.14 ± 16.19 | 84.19 ± 16.22 | 83.02 ± 17.20 | 84.79 ± 15.91 | 0.541 |
| Weight > 70 kg n (%) | 158 (84.04) | 157 (83.95) | 31 (77.50) | 126 (86.89) | 0.142 |
| Diagnosis | |||||
| Myocardial infarction n (%) | 166 (86.01) | 164 (85.86) | 26 (63.41) | 140 (94.59) | <0.001 |
| STEMI n (%) | 127 (65.80) | 126 (65.96) | 18 (43.90) | 109 (73.64) | <0.001 |
| Laboratory parameters | |||||
| Renal dysfunction n (%) | 41 (21.57) | 41 (21.80) | 13 (32.50) | 27 (18.49) | 0.056 |
| Platelets > 291,000 n (%) | 43 (22.75) | 43 (22.99) | 8 (20.00) | 34 (23.44) | 0.645 |
| PDW median (IQR) | 12.00 (11.00–13.35) | 12.00 (11.00–13.40) | 12.20 (11.05–13.37) | 11.90 (10.90–13.45) | 0.350 |
| MPV median (IQR) | 10.40 (9.90–11.00) | 10.40 (9.90–11.00) | 10.40 (10.02–11.17) | 10.40 (9.80–11.00) | 0.341 |
| MPV > 10/fL | 128 (67.72) | 127 (67.91) | 30 (75.00) | 95 (65.51) | 0.257 |
| P-LCR median (IQR) | 28.40 (23.85–33.40) | 28.40 (24.00–33.40) | 29.15 (24.97–33.80) | 28.30 (23.30–33.40) | 0.342 |
| PCT median (IQR) | 0.26 (0.22–0.29) | 0.26 (0.22–0.29) | 0.24 (0.21–0.29) | 0.26 (0.22–0.29) | 0.231 |
| PCT > 0.26/fL | 86 (45.50) | 85 (45.45) | 13 (32.50) | 70 (48.27) | 0.076 |
| Leukocytosis n (%) | 102 (53.96) | 101 (54.01) | 16 (40.00) | 86 (59.31) | 0.030 |
| Anaemia n (%) | 43 (22.75) | 43 (22.99) | 13 (32.50) | 29 (20.00) | 0.095 |
| Risk factors | |||||
| Smoking n (%) | 70 (37.83) | 69 (37.50) | 5 (13.15) | 64 (45.07) | <0.001 |
| Diabetes n (%) | 42 (21.76) | 42 (21.98) | 8 (19.51) | 31 (20.94) | 0.841 |
| Hypertension n (%) | 123 (63.73) | 121 (63.35) | 30 (73.17) | 89 (60.13) | 0.126 |
| Dyslipidemia n (%) | 131 (70.81) | 129 (70.49) | 25 (64.10) | 102 (71.83) | 0.350 |
| Hypercholesterolaemia n (%) | 50 (27.17) | 49 (26.92) | 9 (23.07) | 40 (28.36) | 0.511 |
| Hypertriglyceridaemia n (%) | 94 (51.08) | 92 (50.54) | 15 (38.46) | 76 (53.90) | 0.088 |
| HipoHDL n (%) | 69 (37.29) | 67 (36.61) | 14 (35.89) | 53 (37.32) | 0.870 |
| LDL > 70 mg/dL n (%) | 135 (73.77) | 134 (74.03) | 25 (64.10) | 107 (76.42) | 0.122 |
| Family history of CVDs n (%) | 41 (23.69) | 41 (23.97) | 5 (13.51) | 36 (27.06) | 0.088 |
| Overweight/obesity n (%) | 139 (78.08) | 138 (77.97) | 29 (78.37) | 109 (78.98) | 0.936 |
| ADP test median (IQR) | NA | NA | 25.00 (18.00–33.00) | 19.00 (14.25–24.00) | <0.001 |
| LTPR n (%) | NA | NA | 12 (29.27) | 70 (47.30) | 0.039 |
| MTPR n (%) | NA | NA | 27 (65.85) | 76 (51.35) | 0.098 |
| HTPR n (%) | NA | NA | 2 (4.88) | 2 (1.35) | NA |
| ASPI test median (IQR) | NA | 17.00 (11.00–26.00) | 24.00 (11.00–38.00) | 15.50 (11.00–24.00) | 0.023 |
| HTPR n (%) | NA | 83 (43.46) | 25 (60.97) | 57 (39.04) | 0.012 |
| T2 n (%) | 85 (44.04) | 85 (44.50) | 14 (34.14) | 70 (47.30) | 0.134 |
| Parameter | Total n = 191 | Aspirin-Resistant n = 83 (43.46%) | Aspirin-Sensitive n = 108 (56.54%) | p Value |
|---|---|---|---|---|
| Age < 50 years n (%) | 57 (29.84) | 32 (56.14) | 25 (43.86) | 0.021 |
| Age mean ± SD | 58.40 ± 13.34 | 56.41 ± 13.59 | 59.94 ± 13.007 | 0.042 |
| Men n (%) | 137 (71.73) | 66 (48.18) | 71 (51.82) | 0.036 |
| Women n (%) | 54 (28.27) | 17 (31.48) | 37 (68.52) | |
| Weight > 70 kg n (%) | 157 (83.95) | 75 (47.77) | 82 (52.23) | 0.013 |
| Diagnosis | ||||
| Myocardial infarction n (%) | 164 (85.86) | 72 (43.90) | 92 (56.10) | 0.759 |
| STEMI n (%) | 126 (65.96) | 60 (47.62) | 66 (52.38) | 0.106 |
| Laboratory parameters | ||||
| Renal dysfunction n (%) | 41 (21.80) | 13 (31.71) | 28 (68.29) | 0.096 |
| Platelets > 291,000 n (%) | 43 (22.99) | 26 (60.47) | 17 (39.53) | 0.010 |
| PDW median (IQR) | 12.00 (11.00–13.40) | 12.00 (10.95–13.35) | 12.00 (11.00–13.42) | 0.998 |
| MPV median (IQR) | 10.40 (9.90–11.00) | 10.40 (9.85–11.15) | 10.40 (9.90–11.00) | 0.789 |
| MPV > 10/fL | 127 (67.91) | 56 (44.09) | 71 (55.91) | 0.754 |
| P-LCR median (IQR) | 28.40 (24.00–33.40) | 28.70 (23.40–33.70) | 28.05 (24.37–33.40) | 0.808 |
| PCT median (IQR) | 0.26 (0.22–0.29) | 0.27 (0.22–0.31) | 0.25 (0.21–0.29) | 0.011 |
| PCT > 0.26/fL | 85 (45.45) | 45 (52.94) | 40 (47.06) | 0.015 |
| Anaemia n (%) | 43 (22.99) | 20 (46.51) | 23 (53.49) | 0.630 |
| Leukocytosis n (%) | 101 (54.01) | 54 (53.47) | 47 (46.53) | 0.002 |
| Risk factors | ||||
| Smoking n (%) | 69 (37.50) | 30 (43.48) | 39 (56.52) | 0.908 |
| Diabetes n (%) | 42 (21.98) | 13 (30.95) | 29 (69.05) | 0.064 |
| Hypertension n (%) | 121 (63.35) | 50 (41.32) | 71 (58.68) | 0.434 |
| Dyslipidemia n (%) | 129 (70.49) | 55 (42.64) | 74 (57.36) | 0.649 |
| Hypercholesterolaemia n (%) | 49 (26.92) | 22 (44.90) | 27 (55.10) | 0.877 |
| Hypertriglyceridaemia n (%) | 92 (50.54) | 38 (41.30) | 54 (58.70) | 0.466 |
| HipoHDL n (%) | 67 (36.61) | 29 (43.28) | 38 (56.71) | 0.929 |
| LDL > 70 mg/dL n (%) | 134 (74.03) | 57 (42.54) | 77 (57.46) | 0.447 |
| Family history of CVDs n (%) | 41 (23.97) | 16 (39.02) | 25 (60.98) | 0.423 |
| T1 n (%) | 106 (55.50) | 44 (41.51) | 62 (58.49) | 0.545 |
| T2 n (%) | 85 (44.50) | 39 (45.88) | 46 (54.12) |
| Parameter | Total n = 148 | Hemorrhagic Risk n = 70 (47.30%) | Without Hemorrhagic Risk n = 78 (52.70%) | p Value |
|---|---|---|---|---|
| Age < 50 years n (%) | 52 (35.13) | 19 (36.54) | 33 (63.46) | 0.054 |
| Age mean ± SD | 56.74 ± 12.81 | 59.09 ± 12.15 | 54.64 ± 13.09 | 0.020 |
| Men n (%) | 109 (73.65) | 54 (49.54) | 55 (50.46) | 0.361 |
| Women n (%) | 39 (26.35) | 16 (41.03) | 23 (58.97) | |
| Weight > 70 kg n (%) | 126 (86.89) | 60 (47.62) | 66 (52.38) | 0.380 |
| Diagnosis | ||||
| Myocardial infarction n (%) | 140 (94.59) | 64 (45.71) | 76 (54.28) | 0.107 |
| STEMI n (%) | 109 (73.64) | 47 (43.12) | 62 (56.88) | 0.089 |
| Laboratory parameters | ||||
| Renal dysfunction n (%) | 27 (18.49) | 19 (70.37) | 8 (29.63) | 0.008 |
| Platelets > 291,000 n (%) | 34 (23.44) | 10 (29.41) | 24 (70.59) | 0.015 |
| PDW median (IQR) | 11.90 (10.90–13.45) | 11.80 (10.80–13.15) | 11.90 (10.92–13.95) | 0.516 |
| MPV median (IQR) | 10.40 (9.80–11.00) | 10.20 (9.70–10.90) | 10.50 (9.92–11.17) | 0.118 |
| MPV > 10/fL n (%) | 95 (65.51) | 39 (41.05) | 56 (58.95) | 0.030 |
| P-LCR median (IQR) | 28.30 (23.30–33.40) | 26.90 (22.65–32.30) | 29.05 (24.02–34.15) | 0.159 |
| PCT median (IQR) | 0.26 (0.22–0.29) | 0.24 (0.21–0.28) | 0.28 (0.24–0.30) | <0.001 |
| PCT > 0.26/fL n (%) | 70 (48.27) | 23 (32.86) | 47 (67.14) | 0.001 |
| Anaemia n (%) | 29 (20.0) | 17 (58.62) | 12 (41.38) | 0.183 |
| Leukocytosis n (%) | 86 (59.31) | 35 (40.70) | 51 (59.30) | 0.045 |
| Risk factors | ||||
| Smoking n (%) | 64 (45.07) | 28 (43.75) | 36 (56.25) | 0.458 |
| Diabetes n (%) | 31 (20.94) | 14 (45.16) | 17 (54.83) | 0.789 |
| Hypertension n (%) | 89 (60.13) | 44 (49.44) | 45 (50.56) | 0.522 |
| Dyslipidemia n (%) | 102 (71.83) | 44 (43.14) | 58 (56.86) | 0.314 |
| Hypercholesterolaemia n (%) | 40 (28.36) | 20 (50.00) | 20 (50.00) | 0.489 |
| Hypertriglyceridaemia n (%) | 76 (53.90) | 32 (42.11) | 44 (57.89) | 0.397 |
| HipoHDL n (%) | 53 (37.32) | 24 (45.28) | 29 (54.72) | 0.928 |
| LDL > 70 mg/dL n (%) | 107 (76.42) | 51 (47.66) | 56 (52.34) | 0.254 |
| Family history of CVDs n (%) | 36 (27.06) | 13 (36.11) | 23 (63.89) | 0.139 |
| T1 n (%) | 78 (52.70) | 39 (50.00) | 39 (50.00) | 0.487 |
| T2 n (%) | 70 (47.30) | 31 (44.29) | 39 (55.71) |
| Before the COVID-19 Pandemic | During the COVID-19 Pandemic | p Value | |
|---|---|---|---|
| Aspirin | |||
| ASPI test median (IQR) | 17.00 (10.00–27.00) | 16.00 (12.00–25.00) | 0.745 |
| Ticagrelor | |||
| ADP test median (IQR) | 18.50 (15.00–24.00) | 20.00 (14.00–23.00) | 0.881 |
| Clopidogrel | |||
| ADP test median (IQR) | 22.00 (15.50–27.00) | 31.00 (24.00–36.00) | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anchidin, O.-I.; Rosianu, S.H.; Nemes, A.; Aldica, M.; Blendea, D.; Molnar, A.; Moldovan, H.; Pop, D. The Effectiveness of Antiplatelet Therapy and the Factors Influencing It in Patients with Acute Coronary Syndrome before and during the COVID-19 Pandemic. Medicina 2023, 59, 84. https://doi.org/10.3390/medicina59010084
Anchidin O-I, Rosianu SH, Nemes A, Aldica M, Blendea D, Molnar A, Moldovan H, Pop D. The Effectiveness of Antiplatelet Therapy and the Factors Influencing It in Patients with Acute Coronary Syndrome before and during the COVID-19 Pandemic. Medicina. 2023; 59(1):84. https://doi.org/10.3390/medicina59010084
Chicago/Turabian StyleAnchidin, Ovidiu-Ionut, Stefan Horia Rosianu, Ancuta Nemes, Mihai Aldica, Dan Blendea, Adrian Molnar, Horatiu Moldovan, and Dana Pop. 2023. "The Effectiveness of Antiplatelet Therapy and the Factors Influencing It in Patients with Acute Coronary Syndrome before and during the COVID-19 Pandemic" Medicina 59, no. 1: 84. https://doi.org/10.3390/medicina59010084
APA StyleAnchidin, O.-I., Rosianu, S. H., Nemes, A., Aldica, M., Blendea, D., Molnar, A., Moldovan, H., & Pop, D. (2023). The Effectiveness of Antiplatelet Therapy and the Factors Influencing It in Patients with Acute Coronary Syndrome before and during the COVID-19 Pandemic. Medicina, 59(1), 84. https://doi.org/10.3390/medicina59010084







