Synthetic Pharmacotherapy for Systemic Lupus Erythematosus: Potential Mechanisms of Action, Efficacy, and Safety
Abstract
1. Introduction
2. Antimalarial Drugs
2.1. Efficacy
2.2. Safety
2.2.1. Gastrointestinal Adverse Reactions
2.2.2. Dermatologic Adverse Events
2.2.3. Ocular Toxicity
2.2.4. Cardiotoxicity
2.2.5. Neuromuscular Adverse Events
2.3. Monitoring
- In CKD patients with a GFR < 30 mL/min, the dosage must be adjusted to a maximum of 3 mg/kg of body weight [41].
- Monitor complete blood count at the beginning and during prolonged therapy [8].
- Surveillance of muscle strength and tendon reflexes [7].
- Use CPK and LDH as a screening test for myopathy and cardiomyopathy at the beginning of treatment and 3 to 6 months later [41].
- Monitor cQT prolongation in patients at risk [7].
- During the first year of treatment, use fundoscopy with visual field and spectral-domain optical coherence tomography (SDOCT) or other objective tests as needed, according to the ophthalmologist criteria, such as multifocal electroretinogram and autofluorescence imaging (in case of maculopathy) to monitor ocular toxicity [5,41].
- Inform patients at the beginning of therapy about possible adverse events and the importance of early recognition.
- Monitoring for the presence of new cardiac conduction abnormalities, biventricular and septal hypertrophy, or elevations in troponin, BNP, and CPK, can help identify patients at risk of cardiotoxicity to facilitate a diagnosis [35]. For this reason, an electrocardiogram could be performed at the start of treatment and annually.
- Given its safety and benefits during pregnancy and lactation, treatment should continue if indicated.
3. Glucocorticoids (GCs)
3.1. Mechanism of Action
3.2. Dosage
3.3. Corticosteroid Resistance
3.4. Safety
3.4.1. Musculoskeletal Side Effects
Osteoporosis
Osteonecrosis
Myopathy
Growth Retardation
3.4.2. Metabolic Side Effects
Hyperglycemia/Diabetes Mellitus (DM)
Dyslipidemia
Weight Gain and Lipodystrophy
3.4.3. Cardiovascular Side Effects
Arterial Hypertension
Cardiovascular Risk
3.4.4. Adrenal Insufficiency
3.4.5. Skin Disorders
3.4.6. Neuropsychiatric Disorders
3.4.7. Ophthalmic Alterations
3.5. Safety in Pregnancy
3.6. Monitoring
- Determine anthropometric measurements, blood pressure, metabolic profile (glucose, glycosylated hemoglobin, LDL, HDL, TC, TG, apoB), and densitometry at the beginning of treatment [79].
- Guidelines for a healthier lifestyle, such as diet, regular physical activity, avoiding smoking, and reducing alcohol consumption [79].
- Monitor blood glucose at least 48 h after the start of therapy, then every 3–6 months during the first year, and then annually [134].
- Patients receiving prednisone doses >7.5 mg/day for more than 3 months should be prescribed calcium and vitamin D supplements [134].
- Use FRAX scores to evaluate the risk of fractures at 10 years [79].
- Determine anthropometric measurements in each consultation [134].
- Perform bone densitometry at the start of therapy and annually if there is a decrease in bone mineral density or biannually if it remains stable [134].
- X-ray of the lateral spine in patients ≥65 years for early detection of vertebral fractures [134].
- Determine if the patient requires bisphosphonate therapy according to risk factors and bone mineral density [134].
- Monitor lipid profile after 1 month of treatment, then every 6 to 12 months.
- Assess cardiovascular risk periodically.
- Perform bone densitometry and lateral column radiography in children receiving ≥3 months of GC therapy and repeat annually.
- Monitor the growth rates of children and adolescents, and refer to endocrinology, if necessary, to ascertain if growth hormone therapy is needed [134].
- Request an annual ophthalmological evaluation or earlier if there are risk factors or symptoms [134].
- Monitor blood pressure, signs of fluid overload, and heart failure at each visit [134].
- Watch for signs/symptoms of adverse reactions during therapy.
- Patients treated with a GC concurrently with a nonsteroidal anti-inflammatory drug should receive gastroprotection with proton pump inhibitors or misoprostol. Alternatively, they could switch to a selective cyclooxygenase-2 inhibitor (taking into account increased cardiovascular risk) [134].
- In patients requiring more than 10 mg prednisone/day, other less toxic immunosuppressants should be combined with GCs to accomplish quick tapering of prednisone and ultimately reduce GC-associated organ damage [58,62,135]. The immunosuppressants play an essential role in managing severe SLE manifestations, minimizing the risk of organ damage, reducing the cumulative dose of GCs, and preventing new flares of the disease [135]. Among the agents used are CYC (Cyclophosphamide), AZA (azathioprine), MMF (Mycophenolate Mofetil), Tacrolimus (TAC), and Methotrexate (MTX) [135].
4. Cyclophosphamide (CYC)
4.1. Mechanism of Action
4.2. Efficacy
4.3. Safety
4.3.1. Gastrointestinal Events
4.3.2. Gonadal Insufficiency
4.3.3. Urotoxicity
4.3.4. Infections
4.3.5. Pulmonary Toxicity
4.3.6. Cardiac Toxicity
4.4. Safety in Pregnancy
4.5. Monitoring
- Rule out pregnancy in women of childbearing age before starting therapy [165].
- Advise women of childbearing potential to use effective contraception during treatment with cyclophosphamide and for up to one year after the last dose [165].
- Recommend male patients with female partners of reproductive potential to use effective contraception during CYC treatment and for four months after the last dose.
- Inform patients about the possible risks of infertility with therapy [165].
- Perform a baseline blood count weekly for the first four weeks, every two weeks until the second month, and monthly thereafter. Do not start treatment in patients with an absolute neutrophil count of <1500/mm3 and platelets of <50,000/mm3 [166].
- Correct or exclude any type of urinary obstruction because this may increase the risk of urotoxicity [166].
- Perform urinalysis to evaluate the presence of hematuria, proteinuria, or bacterial infections. This test is initially recommended weekly for the first four weeks, then twice weekly until the second month, and monthly thereafter.
- Surveillance for signs/symptoms of infection.
- Monitor for signs and symptoms of cardiotoxicity or pulmonary toxicity [167].
5. Azathioprine (AZA)
5.1. Mechanism of Action
5.2. Efficacy
5.3. Safety
5.3.1. Genetic Predispositions
5.3.2. Hematological Effects
5.3.3. Increased Risk of Infections
5.3.4. Gastrointestinal Effects
5.4. Safety in Pregnancy and Lactation
5.5. Considerations in Renal Insufficiency
5.6. Monitoring
- Consider genotyping or phenotyping patients for TPMT deficiency and genotyping for NUDT15 deficiency in patients who develop severe myelosuppression [195].
- Monitor hemogram, including platelet counts weekly during the first month, twice monthly for the second and third months of treatment, then monthly or more frequently if dosage alterations or other therapy changes are necessary [196].
- Liver function tests should be monitored periodically during therapy for early detection of hepatotoxicity https://www-micromedexsolutions-com.roseman.idm.oclc.org/micromedex2/librarian/CS/7F44AE/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/217C6E/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=Azathioprine&fromInterSaltBase=true&UserMdxSearchTerm=%24userMdxSearchTerm&false=null&=null-cite97_de, every two weeks for the first four weeks and monthly thereafter [196].
- Surveillance for signs/symptoms of infection.
6. Mycophenolate
6.1. Mechanism of Action
6.2. Efficacy
6.3. Safety
6.4. Safety in Pregnancy
6.5. Monitoring
- There is pharmacokinetic variability with MPA metabolism, and side effects are more probable with higher plasma concentrations in SLE patients. Hence, ascertaining the MPA concentration per patient can help reduce the risk of adverse reactions and improve effectiveness. Plasma MPA levels can be requested before and after any modification in MPA therapy, or when initiating or stopping concomitant medications [212].
- Monitor patients with previous hepatitis B virus (HBV) or hepatitis C virus (HCV) infection for signs of reactivation [216].
- Complete Blood Count weekly for the first month, twice a month for the second and third months, and then monthly for the first year of therapy [216].
- Watch for signs/symptoms of infection [216].
- Perform a pregnancy test 8 to 10 days before starting MMF, another immediately before starting the drug, and periodically with controls [217].
- Women must use two effective contraception methods simultaneously before starting and during treatment and for six weeks after treatment [217].
- Sexually active men (including vasectomized ones) taking MMF are advised to use condoms for intercourse during treatment and for 90 days after cessation. Their partners of childbearing potential should also use contraception during the same period [217].
- Patients should be advised not to donate blood during therapy or within six weeks of stopping treatment [217].
- Men should not donate sperm during therapy or for 90 days after discontinuation [217].
7. Calcineurin Inhibitors
7.1. Tacrolimus
7.1.1. Mechanism of Action
7.1.2. Efficacy
7.1.3. Safety
Drug–Drug Interactions
Renal Effects
Neurological Effects
Metabolic Abnormalities
Infections
7.1.4. Safety in Pregnancy and Lactation
7.1.5. Recommendations in Drug Administration and Monitoring
- Monitor ECGs periodically during treatment, especially in patients at risk for QT prolongation (concomitant use of other QT-prolonging drugs or CYP3A inhibitors, electrolyte disturbances, congestive heart failure, or bradyarrhythmia) [253].
- Surveillance for signs/symptoms of opportunistic infections.
- Surveillance for signs/symptoms of Neurologic abnormalities.
7.2. Cyclosporine
7.2.1. Safety
Nephrotoxicity
Metabolic
8. Methotrexate (MTX)
8.1. Mechanism of Action
8.2. Efficacy
8.3. Safety
8.3.1. Gastrointestinal Side Effects
8.3.2. Hepatotoxicity
8.3.3. Hematological Side Effects
8.3.4. Pulmonary Side Effects
8.3.5. Renal Side Effects
8.3.6. Neurotoxicity
8.4. Safety in Pregnancy
8.5. Monitoring
- If liver function tests are elevated less than three times the upper normal value, a dose reduction is recommended. If they are persistently elevated more than three times the upper normal value despite dose reduction, it will be necessary to suspend the drug [299] and carry out complementary studies with evaluation by hepatology if the elevation of transaminases persists despite suspension [299].
- It is recommended to take hepatitis B and C serology and measure serum albumin before starting treatment and repeat it in those patients who persist with altered liver function tests despite suspension of treatment [285].
- Perform a pregnancy test before the start of treatment and periodically during treatment. Discuss with the patient the importance of contraception during treatment and the need to discontinue treatment with MTX 3 months before conception [299].
- Determine the glomerular filtration rate before starting treatment, every month for the first 3 months, and then every 4–12 weeks during treatment [285]. The dose of MTX should be adjusted to renal function: Glomerular filtration rates (GFR) between 30 and 60 mL/min, require a reduction of the MTX dose of 30–50%, and perform renal function tests during therapy, initially twice a week and then every 4 weeks. The administration of MTX with a GFR <30 mL/min is not recommended [294].
- Evaluation of respiratory symptoms and history in patients with suspected parenchymal lung disease, perform pulmonary function tests and chest radiography. Consider more frequent monitoring of respiratory symptoms and pulmonary function tests during therapy in this type of patient [299].
9. Dapsone
9.1. Mechanism of Action
9.2. Efficacy
9.3. Safety
9.3.1. Hematological Alterations
9.3.2. Cutaneous and Immunological Alterations
9.3.3. Neuropsychiatric
9.3.4. Gastrointestinal
9.3.5. Safety in Pregnancy and Lactation
9.4. Monitoring
- Avoid use in patients with a history of allergy to sulfas and in patients with severe liver disease [302].
- Perform baseline CBC, then weekly for the first month, monthly for 6 months, and then semi-annually thereafter [305].
- Request reticulocyte count at the beginning of treatment, and then periodically every 3–4 months [305].
- Perform liver and renal function tests at the start of treatment, and then every 3–4 months thereafter [305].
- Consider determining the methemoglobin level at the beginning of treatment and according to symptoms [302].
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basta, F.; Fasola, F.; Triantafyllias, K.; Schwarting, A. Systemic Lupus Erythematosus (SLE) Therapy: The Old and the New. Rheumatol. Ther. 2020, 7, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Velo-García, A.; Ntatsaki, E.; Isenberg, D. The Safety of Pharmacological Treatment Options for Lupus Nephritis. Expert. Opin. Drug. Saf. 2016, 15, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; Molto, A.; Romand, X.; Puyraimond-Zemmour, D.; Lavielle, M.; Beauvais, C.; Senbel, E.; Flipo, R.M.; Pouplin, S.; Richez, C.; et al. Recommandations Pour l’évaluation et l’optimisation de l’adhésion Aux Traitements de Fond Médicamenteux Des Rhumatismes Inflammatoires Chroniques: Un Processus Basé Sur Des Revues de La Littérature et Un Consensus d’experts. Rev. Du Rhum. 2019, 86, 555–562. [Google Scholar] [CrossRef]
- Costedoat-Chalumeau, N.; Pouchot, J.; Guettrot-Imbert, G.; le Guern, V.; Leroux, G.; Marra, D.; Morel, N.; Piette, J.-C. Adherence to Treatment in Systemic Lupus Erythematosus Patients. Best. Pract. Res. Clin. Rheumatol. 2013, 27, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, S.; Samson, C.M. Are the Current Recommendations for Chloroquine and Hydroxychloroquine Screening Appropriate? Rheum. Dis. Clin. North Am. 2019, 45, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of Action of Hydroxychloroquine and Chloroquine: Implications for Rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- dos Reis Neto, E.T.; Kakehasi, A.M.; de Medeiros Pinheiro, M.; Ferreira, G.A.; Lopes Marques, C.D.; da Mota, L.M.H.; dos Santos Paiva, E.; Salviato Pileggi, G.C.; Sato, E.I.; Gomides Reis, A.P.M.; et al. Revisiting Hydroxychloroquine and Chloroquine for Patients with Chronic Immunity-Mediated Inflammatory Rheumatic Diseases. Adv. Rheumatol. 2020, 60, 32. [Google Scholar] [CrossRef]
- Ponticelli, C.; Moroni, G. Hydroxychloroquine in Systemic Lupus Erythematosus (SLE). Expert. Opin. Drug. Saf. 2017, 16, 411–419. [Google Scholar] [CrossRef]
- Nosál’, R.; Jančinová, V.; Danihelová, E. Chloroquine: A Multipotent IInhibitor of Human Platelets in Vitro. Thromb. Res. 2000, 98, 411–421. [Google Scholar] [CrossRef]
- Jancínová, V.; Nosál, R.; Petriková, M. On the Inhibitory Effect of Chloroquine on Blood Platelet Aggregation. Thromb. Res. 1994, 74, 495–504. [Google Scholar] [CrossRef]
- Rand, J.H.; Wu, X.X.; Quinn, A.S.; Chen, P.P.; Hathcock, J.J.; Taatjes, D.J. Hydroxychloroquine Directly Reduces the Bindin of Antiphospholipid Antibody-Β2-Glycoprotein I Complexes to Phospholipid Bilayers. Blood 2008, 112, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, N.; Eto, H.; Tanikawa, A.; Ikeda, T.; Yamamoto, K.; Takahashi, T.; Mizukami, H.; Sato, T.; Yokota, N.; Furukawa, F. Effects of Hydroxychloroquine in Patients with Cutaneous Lupus Erythematosus: A Multicenter, Double-Blind, Randomized, Parallel-Group Trial. Arthritis Rheumatol. 2017, 69, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Singh, J.A.; Khaleel, M.S.; Shrestha, S. Efficacy and Toxicity of Antimalarials in Systematic Lupus Erythematosus: A Systematic Review—ACR Meeting Abstracts. Available online: https://acrabstracts.org/abstract/efficacy-and-toxicity-of-antimalarials-in-systematic-lupus-erythematosus-a-systematic-review/ (accessed on 15 December 2020).
- Shinjo, S.K.; Bonfá, E.; Wojdyla, D.; Borba, E.F.; Ramirez, L.A.; Scherbarth, H.R.; Brenol, J.C.T.; Chacón-Diaz, R.; Neira, O.J.; Berbotto, G.A.; et al. Antimalarial Treatment May Have a Time-Dependent Effect on Lupus Survival: Data from a Multinational Latin American Inception Cohort. Arthritis. Rheum. 2010, 62, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Aouhab, Z.; Hong, H.; Felicelli, C.; Tarplin, S.; Ostrowski, R.A. Outcomes of Systemic Lupus Erythematosus in Patients Who Discontinue Hydroxychloroquine. ACR Open. Rheumatol. 2019, 1, 593–599. [Google Scholar] [CrossRef]
- Meinao, I.M.; Sato, E.I.; Andrade, L.E.C.; Ferraz, M.B.; Atra, E. Controlled Trial with Chloroquine Diphosphate in Systemic Lupus Erythematosus. Lupus 1996, 5, 237–241. [Google Scholar] [CrossRef]
- Parikh, S.V.; Almaani, S.; Brodsky, S.; Rovin, B.H. Update on Lupus Nephritis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 76, 265–281. [Google Scholar] [CrossRef]
- Shirley, E.; Chakravarty, E.F. Treatment of Systemic Lupus Erythematosus (SLE) in Pregnancy. Curr. Treatm. Opt. Rheumatol. 2018, 4, 110–118. [Google Scholar] [CrossRef]
- Seo, M.R.; Chae, J.; Kim, Y.M.; Cha, H.S.; Choi, S.J.; Oh, S.; Roh, C.R. Hydroxychloroquine Treatment during Pregnancy in Lupus Patients Is Associated with Lower Risk of Preeclampsia. Lupus 2019, 28, 722–730. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Kim, M.Y.; Llanos, C.; Le, P.U.; Guerra, M.M.; Askanase, A.D.; Salmon, J.E.; Buyon, J.P. Evaluation of the Risk of Anti-SSA/Ro-SSB/La Antibody-Associated Cardiac Manifestations of Neonatal Lupus in Fetuses of Mothers with Systemic Lupus Erythematosus Exposed to Hydroxychloroquine. Ann. Rheum. Dis. 2010, 69, 1827–1830. [Google Scholar] [CrossRef]
- Kumar, D.M.; Kamath, L.; Reddy, N. Efficacy of Hydroxychloroquine as a Potential Antidiabetic Drug. Int. J. Basic. Clin. Pharmacol. 2017, 6, 895. [Google Scholar] [CrossRef][Green Version]
- Hage, M.P.; Al-Badri, M.R.; Azar, S.T. A Favorable Effect of Hydroxychloroquine on Glucose and Lipid Metabolism beyond Its Anti-Inflammatory Role. Ther. Adv. Endocrinol. Metab. 2014, 5, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Capell, H.A. Effect of Antimalarial Agents on Fasting Lipid Profile in Systemic Lupus Erythematosus. J. Rheumatol. 2001, 28, 1742. [Google Scholar] [PubMed]
- Stapley, L. Bone Loss Prevention by an Antimalarial Drug. Trends Endocrinol. Metab. 2001, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Ugarte, A.; Egurbide, M.V.; Garmendia, M.; Pijoan, J.I.; Martinez-Berriotxoa, A.; Aguirre, C. Antimalarials May Influence the Risk of Malignancy in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2007, 66, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Abdel Galil, S.M. Hydroxychloroquine-Induced Toxic Hepatitis in a Patient with Systemic Lupus Erythematosus: A Case Report. Lupus 2015, 24, 638–640. [Google Scholar] [CrossRef]
- Jimenez-Alonso, J.; Tercedor, J.; Jaimez, L.; Garcia-Lora, E. Antimalarial Drug-Induced Aquagenic-Type Pruritus in Patients with Lupus. Arthritis. Rheum. 1998, 41, 744–745. [Google Scholar] [CrossRef]
- Marriott, P.; Borrie, P.F. Pigmentary Changes Following Chloroquine. J. R. Soc. Med. 1975, 68, 535–536. [Google Scholar] [CrossRef]
- Jallouli, M.; Francès, C.; Piette, J.C.; Huong, D.L.T.; Moguelet, P.; Factor, C.; Zahr, N.; Miyara, M.; Saadoun, D.; Mathian, A.; et al. Hydroxychloroquine-Induced Pigmentation in Patients with Systemic Lupus Erythematosus a Case-Control Study. JAMA Dermatol. 2013, 149, 935–940. [Google Scholar] [CrossRef]
- Bernstein, H.; Zvaifler, N.; Rubin, M.; Agnes Mary Mansour, A.M. The Ocular Deposition of Chloroquine. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2160156 (accessed on 16 December 2020).
- Garza-Leon, M.; Flores-Alvarado, D.E.; Muñoz-Bravo, J.M. Retinal Toxicity Induced by Antimalarial Drugs: Literature Review and Case Report. Medwave 2016, 16, e6471. [Google Scholar] [CrossRef]
- Jorge, A.; Ung, C.; Young, L.H.; Melles, R.B.; Choi, H.K. Hydroxychloroquine Retinopathy—Implications of Research Advances for Rheumatology Care. Nat. Rev. Rheumatol. 2018, 14, 693–703. [Google Scholar] [CrossRef]
- Costedoat-Chalumeau, N.; Dunogué, B.; Morel, N.; le Guern, V.; Guettrot-Imbert, G. Hydroxychloroquine: A Multifaceted Treatment in Lupus. Presse. Med. 2014, 43, e167–e180. [Google Scholar] [CrossRef] [PubMed]
- Mubagwa, K. Cardiac Effects and Toxicity of Chloroquine: A Short Update. Int. J. Antimicrob. Agents. 2020, 56, 106057. [Google Scholar] [CrossRef] [PubMed]
- Tselios, K.; Deeb, M.; Gladman, D.D.; Harvey, P.; Urowitz, M.B. Antimalarial-Induced Cardiomyopathy: A Systematic Review of the Literature. Lupus 2018, 27, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Hamm, B.S.; Rosenthal, L.J. Psychiatric Aspects of Chloroquine and Hydroxychloroquine Treatment in the Wake of Coronavirus Disease-2019: Psychopharmacological Interactions and Neuropsychiatric Sequelae. Psychosomatics 2020, 61, 597–606. [Google Scholar] [CrossRef]
- Siddiqui, A.K.; Huberfeld, S.I.; Weidenheim, K.M.; Einberg, K.R.; Efferen, L.S. Hydroxychloroquine-Induced Toxic Myopathy Causing Respiratory Failure. Chest 2007, 131, 588–590. [Google Scholar] [CrossRef]
- Khosa, S.; Khanlou, N.; Khosa, G.S.; Mishra, S.K. Hydroxychloroquine-Induced Autophagic Vacuolar Myopathy with Mitochondrial Abnormalities. Neuropathology 2018, 38, 646–652. [Google Scholar] [CrossRef]
- Casado, E.; Graticós, J.; Tolosa, C.; Martínez, J.M.; Ojanguren, I.; Ariza, A.; Real, J.; Sanjuan, A.; Larrosa, M. Antimalarial Myopathy: An Underdiagnosed Complication? Prospective Longitudinal Study of 119 Patients. Ann. Rheum. Dis. 2006, 65, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Pillittere, J.; Mian, S.; Richardson, T.E.; Perl, A. Hydroxychloroquine-Induced Toxic Myopathy Causing Diaphragmatic Weakness and Lung Collapse Requiring Prolonged Mechanical Ventilation. J. Investig. Med. High Impact. Case Rep. 2020, 8, 1–5. [Google Scholar] [CrossRef]
- Fiehn, C.; Ness, T.; Weseloh, C.; Specker, C.; Hadjiski, D.; Detert, J.; Krüger, K. Safety Management in Treatment with Antimalarials in Rheumatology. Interdisciplinary Recommendations on the Basis of a Systematic Literature Review. Z. Rheumatol. 2020, 80, 1–9. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids-New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- Luijten, R.K.M.A.C.; Fritsch-Stork, R.D.; Bijlsma, J.W.J.; Derksen, R.H.W.M. The Use of Glucocorticoids in Systemic Lupus Erythematosus. After 60years Still More an Art than Science. Autoimmun. Rev. 2013, 12, 617–628. [Google Scholar] [CrossRef]
- Chatham, W.W.; Kimberly, R.P. Treatment of Lupus with Corticosteroids. Lupus 2001, 10, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Exploring the Molecular Mechanisms of Glucocorticoid Receptor Action from Sensitivity to Resistance. Endocr. Dev. 2013, 24, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Hafezi-Moghadam, A.; Simoncini, T.; Yang, Z.; Limbourg, F.P.; Plumier, J.C.; Rebsamen, M.C.; Hsieh, C.M.; Chui, D.S.; Thomas, K.L.; Prorock, A.J.; et al. Acute Cardiovascular Protective Effects of Corticosteroids Are Mediated by Non-Transcriptional Activation of Endothelial Nitric Oxide Synthase. Nat. Med. 2002, 8, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.M. The History of Cortisone Discovery and Development. Rheum. Dis. Clin. North Am. 2016, 42, 1–14. [Google Scholar] [CrossRef]
- Pasero, G.; Marson, P. Short History of Anti-Rheumatic Therapy. IV. Corticosteroids. Reumatismo 2011, 62, 292–299. [Google Scholar] [CrossRef][Green Version]
- Benedek, T.G. History of the Development of Corticosteroid Therapy. Clin. Exp. Rheumatol. 2011, 29, 5–12. [Google Scholar]
- Compston, J. Glucocorticoid-Induced Osteoporosis: An Update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Stojan, G.; Petri, M. The Risk Benefit Ratio of Glucocorticoids in SLE: Have Things Changed over the Past 40 Years? Curr. Treatm. Opt. Rheumatol. 2017, 3, 164–172. [Google Scholar] [CrossRef]
- Biddie, S.C.; Conway-campbell, B.L.; Lightman, S.L. Dynamic Regulation of Glucocorticoid Signalling in Health and Disease. Rheumatology 2012, 51, 403–412. [Google Scholar] [CrossRef]
- Surjit, M.; Ganti, K.P.; Mukherji, A.; Ye, T.; Hua, G.; Metzger, D.; Li, M.; Chambon, P. Widespread Negative Response Elements Mediate Direct Repression by Agonist-Liganded Glucocorticoid Receptor. Cell 2011, 145, 224–241. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Saag, K.G.; Cutolo, M.; da Silva, J.A.P.; Bijlsma, J.W.J. The Molecular Basis for the Effectiveness, Toxicity, and Resistance to Glucocorticoids: Focus on the Treatment of Rheumatoid Arthritis. Scand J. Rheumatol. 2005, 34, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids-All-Rounders Tackling the Versatile Players of the Immune System. Front. Immunol. 2019, 10, 1744. [Google Scholar] [CrossRef]
- Kasturi, S.; Sammaritano, L.R. Corticosteroids in Lupus. Rheum. Dis. Clin. North Am. 2016, 42, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.C.; Budziñski, M.L.; Sokn, C.; Gobbini, R.P.; Steininger, A.; Arzt, E. Regulatory and Mechanistic Actions of Glucocorticoids on T and Inflammatory Cells. Front. Endocrinol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Porta, S.; Danza, A.; Arias Saavedra, M.; Carlomagno, A.; Goizueta, M.C.; Vivero, F.; Ruiz-Irastorza, G. Glucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some Issues. J. Clin. Med. 2020, 9, 2709. [Google Scholar] [CrossRef]
- Zhou, W.J.; Yang, C. de The Causes and Clinical Significance of Fever in Systemic Lupus Erythematosus: A Retrospective Study of 487 Hospitalised Patients. Lupus 2009, 18, 807–812. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, L.; Gao, S.; Chen, L.; Wang, L.; Zhu, Z.; Lu, W.; Zhu, H. Gastrointestinal Symptom Due to Lupus Peritonitis: A Rare Form of Onset of SLE. Int. J. Clin. Exp. Med. 2014, 7, 5917–5920. [Google Scholar]
- Parker, B.J.; Bruce, I.N. High Dose Methylprednisolone Therapy for the Treatment of Severe Systemic Lupus Erythematosus. Lupus 2007, 16, 387–393. [Google Scholar] [CrossRef]
- Amissah Arthur, M.B.; Gordon, C. Contemporary Treatment of Systemic Lupus Erythematosus: An Update for Clinicians. Ther. Adv. Chronic. Dis. 2010, 1, 163–175. [Google Scholar] [CrossRef]
- Chan, T.M. Lupus Nephritis: Induction Therapy. Lupus 2005, 14, s27–s32. [Google Scholar] [CrossRef] [PubMed]
- Barile-Fabris, L.; Ariza-Andraca, R.; Olguín-Ortega, L.; Jara, L.J.; Fraga-Mouret, A.; Miranda-Limón, J.M.; Fuentes De La Mata, J.; Clark, P.; Vargas, F.; Alcocer-Varela, J. Controlled Clinical Trial of IV Cyclophosphamide versus IV Methylprednisolone in Severe Neurological Manifestations in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2005, 64, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Afzal, W.; Arab, T.; Ullah, T.; Teller, K.; Doshi, K.J. Generalized Lymphadenopathy as Presenting Feature of Systemic Lupus Erythematosus: Case Report and Review of the Literature. J. Clin. Med. Res. 2016, 8, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Weinberger, A.; Wysenbeek, A.J. Lymphadenopathy in Systemic Lupus Erythematosus. Prevalence and Relation to Disease Manifestations. Clin. Rheumatol. 1996, 15, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Tsang-A-Sjoe, M.W.P.; Bultink, I.E.M. Systemic Lupus Erythematosus: Review of Synthetic Drugs. Expert. Opin. Pharmacother. 2015, 16, 2793–2806. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Danza, A.; Perales, I.; Villar, I.; Garcia, M.; Delgado, S.; Khamashta, M. Prednisone in Lupus Nephritis: How Much Is Enough? Autoimmun. Rev. 2014, 13, 206–214. [Google Scholar] [CrossRef]
- Ruiz-Arruza, I.; Barbosa, C.; Ugarte, A.; Ruiz-Irastorza, G. Comparison of High versus Low-Medium Prednisone Doses for the Treatment of Systemic Lupus Erythematosus Patients with High Activity at Diagnosis. Autoimmun. Rev. 2015, 14, 875–879. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 Update of the EULAR Recommendations for the Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- Zahr, Z.A.; Fang, H.; Magder, L.S.; Petri, M. Predictors of Corticosteroid Tapering in SLE Patients: The Hopkins Lupus Cohort. Lupus 2013, 22, 697–701. [Google Scholar] [CrossRef]
- Vitellius, G.; Trabado, S.; Bouligand, J.; Delemer, B.; Lombès, M. Pathophysiology of Glucocorticoid Signaling. Ann. Endocrinol. 2018, 79, 98–106. [Google Scholar] [CrossRef]
- Melo, A.; Melo, M.; Saramago, A.; Demartino, G.; Souza, B.; Longui, C. Persistent Glucocorticoid Resistance in Systemic Lupus Erythematosus Patients during Clinical Remission. Genet. Mol. Res. 2013, 12, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Zhu, L.A.; Zou, Y.Q.; Zheng, H.; Wilson, A.; de Yang, C.; Shen, N.; Wallace, D.J.; Weisman, M.H.; Chen, S.-L.; et al. New Insights into the Role and Mechanism of Macrophage Migration Inhibitory Factor in Steroid-Resistant Patients with Systemic Lupus Erythematosus. Arthritis. Res. Ther. 2012, 14, R103. [Google Scholar] [CrossRef] [PubMed]
- Nataraja, C.; Morand, E. Systemic Glucocorticoid Therapy for SLE. In Dubois’ Lupus Erythematosus and Related Syndromes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 661–672. ISBN 9780323479271. [Google Scholar]
- Apostolopoulos, D.; Kandane-Rathnayake, R.; Raghunath, S.; Hoi, A.; Nikpour, M.; Morand, E.F. Independent Association of Glucocorticoids with Damage Accrual in SLE. Lupus. Sci. Med. 2016, 3, e000157. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arruza, I.; Ugarte, A.; Cabezas-Rodriguez, I.; Medina, J.A.; Moran, M.A.; Ruiz-Irastorza, G. Glucocorticoids and Irreversible Damage in Patients with Systemic Lupus Erythematosus. Rheumatology 2014, 53, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Zonana-Nacach, A.; Barr, S.G.; Magder, L.S.; Petri, M. Damage in Systemic Lupus Erythematosus and Its Association with Corticosteroids. Arthritis. Rheum. 2000, 43, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A Practical Guide to the Monitoring and Management of the Complications of Systemic Corticosteroid Therapy. Allergy Asthma Clin. Immunol. 2013, 9, 1–25. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Michael Parfitt, A.; Manolagas, S.C. Inhibition of Osteoblastogenesis and Promotion of Apoptosis of Osteoblasts End Osteocytes by Glucocorticoids Potential Mechanisms of Their Deleterious Effects on Bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, G.; Angeli, A.; Bilezikian, J.P.; Canalis, E.; Giustina, A. Glucocorticoid-Induced Osteoporosis: An Update. Trends Endocrinol. Metab. 2006, 17, 144–149. [Google Scholar] [CrossRef]
- van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Zhang, B.; Cooper, C. Use of Oral Corticosteroids and Risk of Fractures. J. Bone Miner. Res. 2000, 15, 993–1000. [Google Scholar] [CrossRef]
- Jagpal, A.; Saag, K.G. Glucocorticoid-Induced Osteoporosis: Update on Management. Curr. Treatm. Opt. Rheumatol. 2018, 4, 279–287. [Google Scholar] [CrossRef]
- Mirzai, R.; Chang, C.; Greenspan, A.; Gershwin, M.E. The Pathogenesis of Osteonecrosis and the Relationships to Corticosteroids. J. Asthma 1999, 36, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Sayarlioglu, M.; Yuzbasioglu, N.; Inanc, M.; Kamali, S.; Cefle, A.; Karaman, O.; Onat, A.M.; Avan, R.; Cetin, G.Y.; Gul, A.; et al. Risk Factors for Avascular Bone Necrosis in Patients with Systemic Lupus Erythematosus. Rheumatol. Int. 2012, 32, 177–182. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cui, Q.; Koo, K.H. Is There a Role of Pharmacological Treatments in the Prevention or Treatment of Osteonecrosis of the Femoral Head?: A Systematic Review. J. Bone Metab. 2019, 26, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.; Gupta, A. Glucocorticoid-Induced Myopathy: Pathophysiology, Diagnosis, and Treatment. Indian J. Endocrinol. Metab. 2013, 17, 913. [Google Scholar] [CrossRef]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic Glucocorticoids: Mechanisms of Actions in Rheumatic Diseases. Nat. Rev. Rheumatol. 2020, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Poetker, D.M.; Reh, D.D. A Comprehensive Review of the Adverse Effects of Systemic Corticosteroids. Otolaryngol. Clin. North Am. 2010, 43, 753–768. [Google Scholar] [CrossRef]
- Silver, E.M.; Ochoa, W. Glucocorticoid-Induced Myopathy in a Patient with Systemic Lupus Erythematosus (SLE): A Case Report and Review of the Literature. Am. J. Case Rep. 2018, 19, 277–283. [Google Scholar] [CrossRef]
- Nagpal, S.; Tierney, M. Corticosteroid-Induced Myopathy. Can. J. Hosp. Pharm. 1995, 48, 242–243. [Google Scholar] [CrossRef]
- Allen, D.B.; Julius, J.R.; Breen, T.J.; Attie, K.M. Treatment of Glucocorticoid-Induced Growth Suppression with Growth Hormone. J. Clin. Endocrinol. Metab. 1998, 83, 2824–2829. [Google Scholar] [CrossRef]
- Mushtaq, T.; Ahmed, S.F. The Impact of Corticosteroids on Growth and Bone Health. Arch. Dis. Child. 2002, 87, 93–96. [Google Scholar] [CrossRef]
- Abdalla, E.; Jeyaseelan, L.; Ullah, I.; Abdwani, R. Growth Pattern in Children with Systemic Lupus Erythematosus. Oman. Med. J. 2017, 32, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Oeser, A.; Solus, J.F.; Gebretsadik, T.; Shintani, A.; Avalos, I.; Sokka, T.; Raggi, P.; Pincus, T.; Stein, C.M. Inflammation-Associated Insulin Resistance: Differential Effects in Rheumatoid Arthritis and Systemic Lupus Erythematosus Define Potential Mechanisms. Arthritis Rheum. 2008, 58, 2105–2112. [Google Scholar] [CrossRef]
- Angelopoulos, T.P.; Tentolouris, N.K.; Bertsias, G.K.; Boumpas, D.T. Steroid-Induced Diabetes in Rheumatologic Patients. Clin. Exp. Rheumatol. 2014, 32, 126–130. [Google Scholar] [PubMed]
- Gurwitz, J.H. Glucocorticoids and the Risk for Initiation of Hypoglycemic Therapy. Arch. Intern. Med. 1994, 154, 97. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L. Effets Indésirables Métaboliques et Cardiovasculaires Des Corticothérapies Systémiques. Rev. Med. Interne 2013, 34, 303–309. [Google Scholar] [CrossRef]
- Suh, S.; Park, M.K. Glucocorticoid-Induced Diabetes Mellitus: An Important but Overlooked Problem. Endocrinol. Metab. 2017, 32, 180–189. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-Term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Leighton, B.; Parry-Billings, M.; Sasson, S.; Young, M.; Krause, U.; Bevan, S.; Piva, T.; Wegener, G.; Newsholme, E.A. Effects of Glucocorticoid Excess on the Sensitivity of Glucose Transport and Metabolism to Insulin in Rat Skeletal Muscle. Biochem. J. 1997, 321, 707–712. [Google Scholar] [CrossRef]
- Tamez-Pérez, H.E. Steroid Hyperglycemia: Prevalence, Early Detection and Therapeutic Recommendations: A Narrative Review. World J. Diabetes 2015, 6, 1073. [Google Scholar] [CrossRef]
- Tselios, K.; Koumaras, C.; Gladman, D.D.; Urowitz, M.B. Dyslipidemia in Systemic Lupus Erythematosus: Just Another Comorbidity? Semin. Arthritis. Rheum. 2016, 45, 604–610. [Google Scholar] [CrossRef]
- Wijaya, L.K.; Kasjmir, Y.I.; Sukmana, N.; Subekti, I. The Proportion of Dyslipidemia in Systemic Lupus Erythematosus Patient and Distribution of Correlated Factors—PubMed. Acta. Med. Indones. 2005, 37, 132–144. [Google Scholar] [PubMed]
- Sajjad, S.; Farman, S.; Saeed, M.A.; Ahmad, N.M.; Butt, B.A. Frequency of Dyslipidemia in Patients with Lupus Nephritis. Pak. J. Med. Sci. 2017, 33, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.M.; Hunter, A.L.; Ray, D.W.; Dixon, W.G. Systemic Glucocorticoid Therapy and Adrenal Insufficiency in Adults: A Systematic Review. Semin. Arthritis Rheum. 2016, 46, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Strohmayer, E.A.; Krakoff, L.R. Glucocorticoids and Cardiovascular Risk Factors. Endocrinol. Metab. Clin. North Am. 2011, 40, 409–417. [Google Scholar] [CrossRef]
- Atik, N.; Hayati, R.U.; Hamijoyo, L. Correlation Between Steroid Therapy and Lipid Profile in Systemic Lupus Erythematosus Patients. Open Access Rheumatol. 2020, 12, 41–46. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Dhillon, V.B.; Binder, A.; Forte, C.A.; Knight, B.C.; Betteridge, D.J.; Isenberg, D.A. Fasting Lipids and Anticardiolipin Antibodies as Risk Factors for Vascular Disease in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 1992, 51, 152–155. [Google Scholar] [CrossRef]
- Fardet, L.; Cabane, J.; Lebbé, C.; Morel, P.; Flahault, A. Incidence and Risk Factors for Corticosteroid-Induced Lipodystrophy: A Prospective Study. J. Am. Acad. Dermatol. 2007, 57, 604–609. [Google Scholar] [CrossRef]
- Manaboriboon, B.; Silverman, E.D.; Homsanit, M.; Chui, H.; Kaufman, M. Weight Change Associated with Corticosteroid Therapy in Adolescents with Systemic Lupus Erythematosus. Lupus 2013, 22, 164–170. [Google Scholar] [CrossRef]
- Arnaldi, G.; Scandali, V.M.; Trementino, L.; Cardinaletti, M.; Appolloni, G.; Boscaro, M. Pathophysiology of Dyslipidemia in Cushing’s Syndrome. Neuroendocrinology 2010, 92, 86–90. [Google Scholar] [CrossRef]
- Mantero, F.; Boscaro, M. Glucocorticoid-Dependent Hypertension. J. Steroid Biochem. Mol. Biol. 1992, 43, 409–413. [Google Scholar] [CrossRef]
- Mebrahtu, T.F.; Morgan, A.W.; West, R.M.; Stewart, P.M.; Pujades-Rodriguez, M. Oral Glucocorticoids and Incidence of Hypertension in People with Chronic Inflammatory Diseases: A Population-Based Cohort Study. CMAJ 2020, 192, E295–E301. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.L.H.; Zhang, Y.; Whitworth, J.A. Reactive Oxygen Species and Glucocorticoid-Induced Hypertension. In Proceedings of the Clinical and Experimental Pharmacology and Physiology. Clin. Exp. Pharmacol. Physiol. 2008, 35, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.E.; Geller, D.S. Glucocorticoid-Induced Hypertension. Pediatr. Nephrol. 2012, 27, 1059–1066. [Google Scholar] [CrossRef]
- Magder, L.S.; Petri, M. Incidence of and Risk Factors for Adverse Cardiovascular Events among Patients with Systemic Lupus Erythematosus. Am. J. Epidemiol. 2012, 176, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Souverein, P.C.; Berard, A.; van Staa, T.P.; Cooper, C.; Egberts, A.C.G.; Leufkens, H.G.M.; Walker, B.R. Use of Oral Glucocorticoids and Risk of Cardiovascular and Cerebrovascular Disease in a Population Based Case-Control Study. Heart 2004, 90, 859–865. [Google Scholar] [CrossRef]
- Hattori, T.; Murase, T.; Iwase, E.; Takahashi, K.; Ohtake, M.; Tsuboi, K.; Ohtake, M.; Miyachi, M.; Murohara, T.; Nagata, K. Glucocorticoid-Induced Hypertension and Cardiac Injury: Effects of Mineralocorticoid and Glucocorticoid Receptor Antagonism. Nagoya J. Med. Sci. 2013, 75, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.K.; Younes, N.K. Recovery of Steroid Induced Adrenal Insufficiency. Transl. Pediatr. 2017, 6, 269–273. [Google Scholar] [CrossRef]
- Borresen, S.W.; Klose, M.; Baslund, B.; Rasmussen, Å.K.; Hilsted, L.; Friis-Hansen, L.; Locht, H.; Hansen, A.; Hetland, M.L.; Lydolph, M.C.; et al. Adrenal Insufficiency Is Seen in More than One-Third of Patients during Ongoing Low-Dose Prednisolone Treatment for Rheumatoid Arthritis. Eur. J. Endocrinol. 2017, 177, 287–295. [Google Scholar] [CrossRef]
- Guerrero Pérez, F.; Marengo, A.P.; Villabona Artero, C. The Unresolved Riddle of Glucocorticoid Withdrawal. J. Endocrinol. Investig. 2017, 40, 1175–1181. [Google Scholar] [CrossRef]
- Karangizi, A.H.K.; Al-Shaghana, M.; Logan, S.; Criseno, S.; Webster, R.; Boelaert, K.; Hewins, P.; Harper, L. Glucocorticoid Induced Adrenal Insufficiency Is Common in Steroid Treated Glomerular Diseases—Proposed Strategy for Screening and Management. BMC Nephrol. 2019, 20, 154. [Google Scholar] [CrossRef]
- Bhangle, S.D.; Kramer, N.; Rosenstein, E.D. Corticosteroid-Induced Neuropsychiatric Disorders: Review and Contrast with Neuropsychiatric Lupus. Rheumatol. Int. 2013, 33, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Smith, R.E. Steroid-Induced Psychiatric Syndromes. A Report of 14 Cases and a Review of the Literature. J. Affect. Disord. 1983, 5, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, J.M.; Warrington, T.P. Psychiatric Adverse Effects of Corticosteroids; Elsevier: Amsterdam, The Netherlands, 2006; Volume 81. [Google Scholar]
- Moore, E.; Huang, M.W.; Putterman, C. Advances in the Diagnosis, Pathogenesis and Treatment of Neuropsychiatric Systemic Lupus Erythematosus. Curr. Opin. Rheumatol. 2020, 32, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Fel, A.; Aslangul, E.; le Jeunne, C. Indications et Complications Des Corticoïdes En Ophtalmologie. Presse Med. 2012, 41, 414–421. [Google Scholar] [CrossRef]
- Sundmark, E. The Occurrence of Posterior Subcapsular Cataracts in Patients on Long-Term Systemic Cortcisteroid Therapy. Acta Ophthalmol. 2009, 41, 515–523. [Google Scholar] [CrossRef]
- Cunningham, J.; Alfred, P.R.; Irvine, A.R. Central Serous Chorioretinopathy in Patients with Systemic Lupus Erythematosus. Ophthalmology 1996, 103, 2081–2090. [Google Scholar] [CrossRef]
- Haimovici, R.; Koh, S.; Gagnon, D.R.; Lehrfeld, T.; Wellik, S. Risk Factors for Central Serous Chorioretinopathy: A Case-Control Study. Ophthalmology 2004, 111, 244–249. [Google Scholar] [CrossRef]
- Øtensen, M.; Khamashta, M.; Lockshin, M.; Parke, A.; Brucato, A.; Carp, H.; Doria, A.; Rai, R.; Meroni, P.; Cetin, I.; et al. Anti-Inflammatory and Immunosuppressive Drugs and Reproduction. Arthritis. Res. Ther. 2006, 8, 209. [Google Scholar] [CrossRef]
- Lateef, A.; Petri, M. Management of Pregnancy in Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2012, 8, 710–718. [Google Scholar] [CrossRef]
- Hoes, J.N.; Jacobs, J.W.G.; Boers, M.; Boumpas, D.; Buttgereit, F.; Caeyers, N.; Choy, E.H.; Cutolo, M.; da Silva, J.A.P.; Esselens, G.; et al. EULAR Evidence-Based Recommendations on the Management of Systemic Glucocorticoid Therapy in Rheumatic Diseases. Ann. Rheum. Dis. 2007, 66, 1560–1567. [Google Scholar] [CrossRef]
- Litvin, I.; Dvorkina, O.; Ginzler, E.M. Immunosuppressive Drug Therapy. In Dubois’ Lupus Erythematosus and Related Syndromes.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 673–688. ISBN 9780323479271. [Google Scholar]
- Veal, G.J.; Cole, M.; Chinnaswamy, G.; Sludden, J.; Jamieson, D.; Errington, J.; Malik, G.; Hill, C.R.; Chamberlain, T.; Boddy, A.V. Cyclophosphamide Pharmacokinetics and Pharmacogenetics in Children with B-Cell Non-Hodgkin’s Lymphoma. Eur. J. Cancer 2016, 55, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Houssiau, F. Thirty Years of Cyclophosphamide: Assessing the Evidence. Lupus 2007, 16, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Helsby, N.A.; Yong, M.; van Kan, M.; de Zoysa, J.R.; Burns, K. E The Importance of Both CYP2C19 and CYP2B6 Germline Variations in Cyclophosphamide Pharmacokinetics and Clinical Outcomes. Br. J. Clin. Pharmacol. 2019, 85, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Ponticelli, C. Synthetic Pharmacotherapy for Lupus Nephritis. Expert. Opin. Pharmacother. 2017, 18, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.Y.H.; Chan, T.M. B Cell Abnormalities in Systemic Lupus Erythematosus and Lupus Nephritis—Role in Pathogenesis and Effect of Immunosuppressive Treatments. Int. J. Mol. Sci. 2019, 20, 6231. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Pamfil, C.; Sidiropoulos, P.; Damian, L.; Flestea, A.; Gusetu, G.; Rednic, S.; Bertsias, G.; Boumpas, D.T. Cyclophosphamide in Combination with Glucocorticoids for Severe Neuropsychiatric Systemic Lupus Erythematosus: A Retrospective, Observational Two-Centre Study. Lupus 2016, 25, 627–636. [Google Scholar] [CrossRef]
- Fernandes Moça Trevisani, V.; Castro, A.A.; Ferreira Neves Neto, J.; Atallah, Á.N. Cyclophosphamide versus Methylprednisolone for Treating Neuropsychiatric Involvement in Systemic Lupus Erythematosus. Cochrane Database Syst. Rev. 2013, 2013. [Google Scholar] [CrossRef]
- Carmier, D.; Diot, E.; Diot, P. Shrinking Lung Syndrome: Recognition, Pathophysiology and Therapeutic Strategy. Expert. Rev. Respir. Med. 2011, 5, 33–39. [Google Scholar] [CrossRef]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus Nephritis. Nat. Rev. Dis. Primers 2020, 6, 1–25. [Google Scholar] [CrossRef]
- Houssiau, F.A.; Vasconcelos, C.; D’Cruz, D.; Sebastiani, G.D.; de Ramon Garrido, E.; Danieli, M.G.; Abramovicz, D.; Blockmans, D.; Mathieu, A.; Direskeneli, H.; et al. Immunosuppressive Therapy in Lupus Nephritis: The Euro-Lupus Nephritis Trial, a Randomized Trial of Low-Dose versus High-Dose Intravenous Cyclophosphamide. Arthritis Rheum. 2002, 46, 2121–2131. [Google Scholar] [CrossRef]
- Dooley, M.A.; Hogan, S.; Jennette, C.; Falk, R. Cyclophosphamide Therapy for Lupus Nephritis: Poor Renal Survival in Black Americans. Kidney. Int. 1997, 51, 1188–1195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ognenovski, V.M.; Marder, W.; Somers, E.C.; Johnston, C.M.; Farrehi, J.G.; Selvaggi, S.M.; McCune, W.J. Increased Incidence of Cervical Intraepithelial Neoplasia in Women with Systemic Lupus Erythematosus Treated with Intravenous Cyclophosphamide. J. Rheumatol. 2004, 31, 1763–1767. [Google Scholar] [PubMed]
- Wang, C.L.; Wang, F.; Bosco, J.J. Ovarian Failure in Oral Cyclophosphamide Treatment for Systemic Lupus Erythematosus. Lupus 1995, 4, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Marder, W.; Christman, G.M.; Ognenovski, V.; McCune, W.J. Use of a Gonadotropin-Releasing Hormone Analog for Protection against Premature Ovarian Failure during Cyclophosphamide Therapy in Women with Severe Lupus. Arthritis Rheum. 2005, 52, 2761–2767. [Google Scholar] [CrossRef]
- Masala, A.; Faedda, R.; Alagna, S.; Satta, A.; Chiarelli, G.; Rovasio, P.P.; Ivaldi, R.; Taras, M.S.; Lai, E.; Bartoli, E. Use of Testosterone to Prevent Cyclophosphamide-Induced Azoospermia. Ann. Intern. Med. 1997, 126, 292–295. [Google Scholar] [CrossRef]
- Wetzels, J.F.M. Cyclophosphamide-Induced Gonadal Toxicity: A Treatment Dilemma in Patients with Lupus Nephritis? Neth. J. Med. 2004, 62, 347–352. [Google Scholar]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular Mechanisms of Acrolein Toxicity: Relevance to Human Disease. Toxicological. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef]
- Korkmaz, A.; Topal, T.; Oter, S. Pathophysiological Aspects of Cyclophosphamide and Ifosfamide Induced Hemorrhagic Cystitis; Implication of Reactive Oxygen and Nitrogen Species as Well as PARP Activation. Cell Biol. Toxicol. 2007, 23, 303–312. [Google Scholar] [CrossRef]
- Yilmaz, N.; Emmungil, H.; Gucenmez, S.; Ozen, G.; Yildiz, F.; Balkarli, A.; Kimyon, G.; Coskun, B.N.; Dogan, I.; Pamuk, O.N.; et al. Incidence of Cyclophosphamide-Induced Urotoxicity and Protective Effect of Mesna in Rheumatic Diseases. J. Rheumatol. 2015, 42, 1661–1666. [Google Scholar] [CrossRef]
- Fu, D.; Ye, S.; Xiao, C.; Xie, Y.; Gao, J.; Liang, L.; Yang, X. Original Article Incidence of Cyclophosphamide-Induced Hemorrhagic Cystitis in Chinese Han Population with Autoimmune Disease. Int. J. Clin. Exp. Med. 2016, 9, 13160–13165. [Google Scholar]
- Yates, M.; Watts, R.A.; Bajema, I.M.; Cid, M.C.; Crestani, B.; Hauser, T.; Hellmich, B.; Holle, J.U.; Laudien, M.; Little, M.A.; et al. EULAR/ERA-EDTA Recommendations for the Management of ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2016, 75, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.G.; Ferreira, J.F.; Loio, M.; Santiago, T.; Serra, S.; Salvador, M.J.; Malcata, A.; da Silva, J.A.P. Bacterial Peritonitis: The Presentation of a Cyclophosphamide-Associated Bladder Carcinoma in a Long-Standing Systemic Lupus Erythematosus Patient. Eur. J. Intern. Med. 2013, 24, e136. [Google Scholar] [CrossRef]
- Subramanian, R.; Pathak, H.; Ravindran, V. Safety of Cyclophosphamide Therapy in Autoimmune Rheumatic Diseases. Indian J. Rheumatol. 2019, 14, 127. [Google Scholar] [CrossRef]
- Tran, A.; Bournerias, F.; le Beller, C.; Mir, O.; Rey, E.; Pons, G.; Delahousse, M.; Tréluyer, J.M. Serious Haematological Toxicity of Cyclophosphamide in Relation to CYP2B6, GSTA1 and GSTP1 Polymorphisms. Br. J. Clin. Pharmacol. 2008, 65, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Woytala, P.J.; Morgiel, E.; Luczak, A.; Czesak-Woytala, K.; Wiland, P. The Safety of Intravenous Cyclophosphamide in the Treatment of Rheumatic Diseases. Adv. Clin. Exp. Med. 2016, 25, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Pryor, B.D.; Bologna, S.G.; Kahl, L.E. Risk Factors for Serious Infection during Treatment with Cyclophosphamide and High-Dose Corticosteroids for Systemic Lupus Erythematosus. Arthritis Rheum. 1996, 39, 1475–1482. [Google Scholar] [CrossRef]
- Pugh, D.; Farrah, T.E.; Gallacher, P.J.; Kluth, D.C.; Dhaun, N. Cyclophosphamide-Induced Lung Injury. Kidney Int. Rep. 2019, 4, 484–486. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Yuan, M.; Tian, C.; Yang, Y.; Wang, X.; Zhang, X.; Sun, Y.; He, T.; Han, S.; et al. Anticancer Therapy-Induced Atrial Fibrillation: Electrophysiology and Related Mechanisms. Front. Pharmacol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Magder, L.; Petri, M. Cyclophosphamide for Lupus during Pregnancy. Lupus 2005, 14, 593–597. [Google Scholar] [CrossRef]
- Food and Drug Administration Cyclophosphamide for Injection; USP Cyclophosphamide Tablets, USP. Package Insert and Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/012142s109lbl.pdf (accessed on 20 December 2022).
- Ogino, M.; Tadi, P. NCBI Bookshelf: Cyclophosphamide. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553087/ (accessed on 20 December 2022).
- Fraiser, L.H.; Kanekal, S.; Kehrer, J.P. Cyclophosphamide Toxicity: Characterising and Avoiding the Problem. Drugs 1991, 42, 781–795. [Google Scholar] [CrossRef]
- Maltzman, J.S.; Koretzky, G.A. Azathioprine: Old Drug, New Actions. J. Clin. Investig. 2003, 111, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B. The Purine Path to Chemotherapy. Science 1989, 244, 41–47. [Google Scholar] [CrossRef]
- Croyle, L.; Hoi, A.; Morand, E.F. Characteristics of Azathioprine Use and Cessation in a Longitudinal Lupus Cohort. Lupus Sci. Med. 2015, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shakra, M.; Shoenfeld, Y. Azathioprine Therapy for Patients with Systemic Lupus Erythematosus. Lupus 2001, 10, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Aarbakke, J.; Janka-Schaub, G.; Elion, G.B. Thiopurine Biology and Pharmacology. In Proceedings of the Trends in Pharmacological Sciences. Trends Pharmacol. Sci. 1997, 18, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Broen, J.C.A.; Laar, J.M. Mycophenolate Mofetil, Azathioprine and Tacrolimus: Mechanisms in Rheumatology. Nat. Rev. Rheumatol. 2020, 16, 167–178. [Google Scholar] [CrossRef]
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-Dependent Rac1 Activation Is the Molecular Target of Azathioprine in Primary Human CD4+ T Lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145. [Google Scholar] [CrossRef]
- Moeslinger, T.; Friedl, R.; Spieckermann, P.G. Inhibition of Inducible Nitric Oxide Synthesis by Azathioprine in a Macrophage Cell Line. Life Sci. 2006, 79, 374–381. [Google Scholar] [CrossRef]
- Houssiau, F.A.; D’Cruz, D.; Sangle, S.; Remy, P.; Vasconcelos, C.; Petrovic, R.; Fiehn, C.; Garrido, E.D.R.; Gilboe, I.M.; Tektonidou, M.; et al. Azathioprine versus Mycophenolate Mofetil for Long-Term Immunosuppression in Lupus Nephritis: Results from the MAINTAIN Nephritis Trial. Ann. Rheum. Dis. 2010, 69, 2083–2089. [Google Scholar] [CrossRef]
- Man, B.L.; Mok, C.C.; Fu, Y.P. Neuro-Ophthalmologic Manifestations of Systemic Lupus Erythematosus: A Systematic Review. Int. J. Rheum. Dis. 2014, 17, 494–501. [Google Scholar] [CrossRef]
- O’connor, A.; Qasim, A.; O’morain, C.A. The Long-Term Risk of Continuous Immunosuppression Using Thioguanides in Inflammatory Bowel Disease. Ther. Adv. Chronic. Dis. 2010, 1, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Magro-Checa, C.; Zirkzee, E.J.; Huizinga, T.W.; Steup-Beekman, G.M. Management of Neuropsychiatric Systemic Lupus Erythematosus: Current Approaches and Future Perspectives. Drugs 2016, 76, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Simms, R.W.; Kwoh, C.K.; Anderson, L.G.; Erlandson, D.M.; Greene, J.M.; Kelleher, M.; O’Dell, J.R.; Partridge, A.J.; Roberts, W.N.; Robbins, M.L.; et al. Guidelines for Monitoring Drug Therapy in Rheumatoid Arthritis. Arthritis Rheum. 1996, 39, 723–731. [Google Scholar] [CrossRef]
- Jordan, A.; Gresser, U. Side Effects and Interactions of the Xanthine Oxidase Inhibitor Febuxostat. Pharmaceuticals 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.H.; Marty, F.M.; Winkelmayer, W.C.; Guan, H.; Franklin, J.M.; Solomon, D.H.; Costenbader, K.H.; Kim, S.C. Comparative Rates of Serious Infections Among Patients with Systemic Lupus Erythematosus Receiving Immunosuppressive Medications. Arthritis Rheumatol. 2017, 69, 387–397. [Google Scholar] [CrossRef]
- Singh, J.A.; Hossain, A.; Kotb, A.; Wells, G. Risk of Serious Infections with Immunosuppressive Drugs and Glucocorticoids for Lupus Nephritis: A Systematic Review and Network Meta-Analysis. BMC Med. 2016, 14, 137. [Google Scholar] [CrossRef]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European Evidence-Based Consensus on the Prevention, Diagnosis and Management of Opportunistic Infections in Inflammatory Bowel Disease. J. Crohns. Colitis. 2014, 8, 443–468. [Google Scholar] [CrossRef]
- Wilson, A.; Jansen, L.E.; Rose, R.V.; Gregor, J.C.; Ponich, T.; Chande, N.; Khanna, R.; Yan, B.; Jairath, V.; Khanna, N.; et al. HLA-DQA1-HLA-DRB1 Polymorphism Is a Major Predictor of Azathioprine-Induced Pancreatitis in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 47, 615–620. [Google Scholar] [CrossRef]
- Teich, N.; Mohl, W.; Bokemeyer, B.; Bündgens, B.; Büning, J.; Miehlke, S.; Hüppe, D.; Maaser, C.; Klugmann, T.; Kruis, W.; et al. Azathioprine-Induced Acute Pancreatitis in Patients with Inflammatory Bowel Diseases-a Prospective Study on Incidence and Severity. J. Crohns. Colitis 2016, 10, 61–68. [Google Scholar] [CrossRef]
- Horning, K.; Schmidt, C. Azathioprine-Induced Rapid Hepatotoxicity. J. Pharm. Technol. 2014, 30, 18–20. [Google Scholar] [CrossRef]
- Kraaij, T.; Bredewold, O.W.; Trompet, S.; Huizinga, T.W.J.; Rabelink, T.J.; de Craen, A.J.M.; Teng, Y.K.O. TAC-TIC Use of Tacrolimus-Based Regimens in Lupus Nephritis. Lupus Sci. Med. 2016, 3, e000169. [Google Scholar] [CrossRef] [PubMed]
- Alstead, E.M.; Ritchie, J.K.; Lennard-Jones, J.E.; Farthing, M.J.G.; Clark, M.L. Safety of Azathioprine in Pregnancy in Inflammatory Bowel Disease. Gastroenterology 1990, 99, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Cleary, B.J.; Källén, B. Early Pregnancy Azathioprine Use and Pregnancy Outcomes. Birth. Defects Res. A Clin. Mol. Teratol. 2009, 85, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, N.; Bermas, B.L. Pharmacological Approach to Managing Childhood-Onset Systemic Lupus Erythematosus During Conception, Pregnancy and Breastfeeding. Pediatric. Drugs. 2018, 20, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.C.; Cheetham, T.C.; Stein, C.M.; Callahan, S.T.; Morgan, T.M.; Shintani, A.K.; Chen, N.; Griffin, M.R.; Ray, W.A. Adverse Fetal Outcomes Associated with Immunosuppressive Medications for Chronic Immune Mediated Diseases in Pregnancy. Arthritis Rheumatol. 2014, 66, 444–450. [Google Scholar] [CrossRef]
- van Scoik, K.G.; Johnson, C.A.; Porter, W.R. The Pharmacology and Metabolism of the Thiopurine Drugs 6-Mercaptopurine and Azathioprine. Drug. Metab. Rev. 1985, 16, 157–174. [Google Scholar] [CrossRef]
- Weiner, S.M.; Bergner, R. Dosierung Und Toxizität von Antirheumatika Bei Niereninsuffizienz. Z. Rheumatol. 2015, 74, 300–309. [Google Scholar] [CrossRef]
- Weiner, S.M. Treatment of Rheumatic Disease with Renal Insufficiency. Orthopade 2019, 48, 927–935. [Google Scholar] [CrossRef]
- Gaffney, K.; Scott, D.G. Azathioprine and Cyclophosphamide in the Treatment of Rheumatoid Arthritis. Rheumatology 1998, 37, 824–836. [Google Scholar] [CrossRef]
- Felten, R.; Scher, F.; Sibilia, J.; Ois Chasset, F.; Arnaud, L. Advances in the Treatment of Systemic Lupus Erythematosus: From Back to the Future, to the Future and Beyond. Joint Bone Spine 2019, 86, 429–436. [Google Scholar] [CrossRef]
- Dall’Era, M. Mycophenolate Mofetil in the Treatment of Systemic Lupus Erythematosus. Curr. Opin. Rheumatol. 2011, 23, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Cortinovis, M.; Baldelli, S.; Bitto, A.; Gotti, E.; Remuzzi, G.; Perico, N. Pharmacokinetics of Mycophenolate Sodium and Comparison with the Mofetil Formulation in Stable Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2007, 2, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Olech, E.; Merrill, J.T. Mycophenolate Mofetil for Lupus Nephritis. Expert. Rev. Clin. Immunol. 2008, 4, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Appel, G.B.; Contreras, G.; Dooley, M.A.; Ginzler, E.M.; Isenberg, D.; Jayne, D.; Li, L.S.; Mysler, E.; Sánchez-Guerrero, J.; Solomons, N.; et al. Mycophenolate Mofetil versus Cyclophosphamide for Induction Treatment of Lupus Nephritis. J. Am. Soc. Nephrol. 2009, 20, 1103–1112. [Google Scholar] [CrossRef]
- Rathi, M.; Goyal, A.; Jaryal, A.; Sharma, A.; Gupta, P.K.; Ramachandran, R.; Kumar, V.; Kohli, H.S.; Sakhuja, V.; Jha, V.; et al. Comparison of Low-Dose Intravenous Cyclophosphamide with Oral Mycophenolate Mofetil in the Treatment of Lupus Nephritis. Kidney Int. 2016, 89, 235–242. [Google Scholar] [CrossRef]
- Deng, J.; Xie, H.; Zhu, L.; Luo, L.; Xie, H. Maintenance Therapy for Lupus Nephritis with Mycophenolate Mofetil or Azathioprine. A Meta-Analysis. Clin. Nephrol. 2019, 91, 172–179. [Google Scholar] [CrossRef]
- Soares, P.M.F.; Borba, E.F.; Bonfa, E.; Hallak, J.; Corrêa, A.L.; Silva, C.A.A. Gonad Evaluation in Male Systemic Lupus Erythematosus. Arthritis Rheum. 2007, 56, 2352–2361. [Google Scholar] [CrossRef]
- Dall’Era, M.; Solomons, N.; Federico, R.; Truman, M. Comparison of Standard of Care Treatment with a Low Steroid and Mycophenolate Mofetil Regimen for Lupus Nephritis in the ALMS and AURA Studies. Lupus 2019, 28, 591–596. [Google Scholar] [CrossRef]
- Dooley, M.A.; Jayne, D.; Ginzler, E.M.; Isenberg, D.; Olsen, N.J.; Wofsy, D.; Eitner, F.; Appel, G.B.; Contreras, G.; Lisk, L.; et al. Mycophenolate versus Azathioprine as Maintenance Therapy for Lupus Nephritis. N. Engl. J. Med. 2011, 365, 1886–1895. [Google Scholar] [CrossRef]
- Tamirou, F.; D’Cruz, D.; Sangle, S.; Remy, P.; Vasconcelos, C.; Fiehn, C.; del Mar Ayala Guttierez, M.; Gilboe, I.M.; Tektonidou, M.; Blockmans, D.; et al. Long-Term Follow-up of the MAINTAIN Nephritis Trial, Comparing Azathioprine and Mycophenolate Mofetil as Maintenance Therapy of Lupus Nephritis. Ann. Rheum. Dis. 2016, 75, 526–531. [Google Scholar] [CrossRef]
- Lourdudoss, C.; van Vollenhoven, R. Mycophenolate Mofetil in the Treatment of SLE and Systemic Vasculitis: Experience at a Single University Center. Lupus 2014, 23, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C. Mycophenolate Mofetil for Non-renal Manifestations of Systemic Lupus Erythematosus: A Systematic Review. Scand. J. Rheumatol. 2007, 36, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.Y. Mycophenolate Mofetil in the Treatment of Non-Renal Manifestations of Systemic Lupus Erythematosus: A Review. APLAR J. Rheumatol. 2006, 9, 408–412. [Google Scholar] [CrossRef]
- Henderson, L.K.; Masson, P.; Craig, J.C.; Roberts, M.A.; Flanc, R.S.; Strippoli, G.F.M.; Webster, A.C. Induction and Maintenance Treatment of Proliferative Lupus Nephritis: A Meta-Analysis of Randomized Controlled Trials. Am. J. Kidney Dis. 2013, 61, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C. Mycophenolate Mofetil for Lupus Nephritis: An Update. Expert. Rev. Clin. Immunol. 2015, 11, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, A.M.; Bonaci-Nikolic, B.; Kozic, D.; Ostojic, J.; Abinun, M.; Svabic-Medjedovic, T.; Nikolic, M.; Sternic, N. Progressive Multifocal Leukoencephalopathy Associated with Mycophenolate Mofetil Treatment in a Woman with Lupus and CD4+ T-Lymphocyte Deficiency. Lupus 2012, 21, 100–102. [Google Scholar] [CrossRef]
- Le, H.L.; Francke, M.I.; Andrews, L.M.; de Winter, B.C.M.; van Gelder, T.; Hesselink, D.A. Usage of Tacrolimus and Mycophenolic Acid During Conception, Pregnancy, and Lactation, and Its Implications for Therapeutic Drug Monitoring: A Systematic Critical Review. Ther. Drug. Monit. 2020, 42, 518–531. [Google Scholar] [CrossRef]
- Skorpen, C.G.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefant, E.; Chambers, C.; da Silva, J.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR Points to Consider for Use of Antirheumatic Drugs before Pregnancy, and during Pregnancy and Lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef]
- Food and Drug Administration. CellCept ® (Mycophenolate Mofetil Capsules) (Mycophenolate Mofetil Tablets), CellCept ® Oral Suspension (Mycophenolate Mofetil for Oral Suspension), CellCept ® Intravenous (Mycophenolate Mofetil Hydrochloride for Injection). Package Insert and Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050722s030s031,050723s029s030,050758s028s029,.pdf (accessed on 20 December 2022).
- European Medicines Agency. EMA Recommends Additional Measures to Prevent Use of Mycophenolate in Pregnancy. London, UK. Available online: https://www.ema.europa.eu/en/news/ema-recommends-additional-measures-prevent-use-mycophenolate-pregnancy (accessed on 20 December 2022).
- Mok, C.C. Pro: The Use of Calcineurin Inhibitors in the Treatment of Lupus Nephritis. Nephrol. Dial. Transplant. 2016, 31, 1561–1566. [Google Scholar] [CrossRef]
- Gold, B.G. FK506 and the Role of Immunophilins in Nerve Regeneration. Mol. Neurobiol. 1997, 15, 285–306. [Google Scholar] [CrossRef]
- Rothenberg, R.J.; Graziano, F.M.; Grandone, J.T.; Goldberg, J.W.; Bjarnason, D.F.; Finesilver, A.G. The Use of Methotrexate in Steroid-resistant Systemic Lupus Erythematosus. Arthritis Rheum. 1988, 31, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Nagai, K.; Takahashi, N.; Watanabe, T.; Matsumoto, T.; Asai, N.; Sobue, Y.; Ishiguro, N.; Kojima, T. Influence of Methotrexate on Gastrointestinal Symptoms in Patients with Rheumatoid Arthritis. Int. J. Rheum. Dis. 2019, 22, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Jegasothy, B.V.; Ackerman, C.D.; Todo, S.; Fung, J.J.; Abu Elmagd, K.; Starzl, T.E. Tacrolimus (FK 506)—A New Therapeutic Agent for Severe Recalcitrant Psoriasis. Arch. Dermatol. 1992, 128, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, K.; Koike, T.; Kurasawa, K.; Matsumura, R.; Sato, T.; Tomioka, H.; Ito, I.; Yoshiki, T.; Yoshida, S. Effect of FK-506, a Novel Immunosuppressive Drug on Murine Systemic Lupus Erythematosus. Clin. Immunol. Immunopathol. 1989, 51, 110–117. [Google Scholar] [CrossRef]
- Kondo, H.; Abe, T.; Hashimoto, H.; Uchida, S.; Irimajiri, S.; Hara, M.; Sugawara, S. Efficacy and Safety of Tacrolimus (FK506) in Treatment of Rheumatoid Arthritis: A Randomized, Double Blind, Placebo Controlled Dose-Finding Study. J. Rheumatol. 2004, 31, 243–251. [Google Scholar]
- Tedesco, D.; Haragsim, L. Cyclosporine: A Review. J. Transplant. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Shaw, K.T.Y.; Ho, A.M.; Raghavan, A.; Kim, J.; Jain, J.; Park, J.; Sharma, S.; Rao, A.; Hogan, P.G. Immunosuppressive Drugs Prevent a Rapid Dephosphorylation of Transcription Factor NFAT1 in Stimulated Immune Cells. Proc. Natl. Acad. Sci. USA 1995, 92, 11205–11209. [Google Scholar] [CrossRef]
- Lan, C.C.E.; Yu, H.S.; Wu, C.S.; Kuo, H.Y.; Chai, C.Y.; Chen, G.S. FK506 Inhibits Tumour Necrosis Factor-α Secretion in Human Keratinocytes via Regulation of Nuclear Factor-ΚB. Br. J. Dermatol. 2005, 153, 725–732. [Google Scholar] [CrossRef]
- Hoyos, B.; Ballard, D.W.; Böhnlein, E.; Siekevitz, M.; Greene, W.C. Kappa B-Specific DNA Binding Proteins: Role in the Regulation of Human Interleukin-2 Gene Expression. Science 1989, 244, 457–460. [Google Scholar] [CrossRef]
- Maluccio, M.; Sharma, V.; Lagman, M.; Vyas, S.; Yang, H.; Li, B.; Suthanthiran, M. Tacrolimus Enhances Transforming Growth Factor-Β1 Expression and Promotes Tumor Progression. Transplantation 2003, 76, 597–602. [Google Scholar] [CrossRef]
- Iwasaki, K. Metabolism of Tacrolimus (FK506) and Recent Topics in Clinical Pharmacokinetics. Drug. Metab. Pharmacokinet. 2007, 22, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulos, C.E.; Sangle, S.; Harrison, P.; Hughes, G.R.V.; D’Cruz, D.P. Topical Tacrolimus Therapy of Resistant Cutaneous Lesions in Lupus Erythematosus: A Possible Alternative. Rheumatology 2004, 43, 1383–1385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vesely, M.D. Getting under the Skin: Targeting Cutaneous Autoimmune Disease. Yale J. Biol. Med. 2020, 93, 197–206. [Google Scholar]
- Liu, Z.; Zhang, H.; Liu, Z.; Xing, C.; Fu, P.; Ni, Z.; Chen, J.; Lin, H.; Liu, F.; He, Y.; et al. Multitarget Therapy for Induction Treatment of Lupus Nephritis: A Randomized Trial. Ann. Intern. Med. 2015, 162, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Kukla, A.; Ibrahim, H.N. Calcineurin Inhibitor Nephrotoxicity: A Review and Perspective of the Evidence. Am. J. Nephrol. 2013, 37, 602–612. [Google Scholar] [CrossRef]
- Macphee, I.; Fredericks, S.; Tai, T.; Syrris, P.; Carter, N.; Johnston, A.; Goldberg, L.; Holt, D. Tacrolimus Pharmacogenetics: Polymorphisms Associated with Expression of Cytochrome P4503A5 and P-Glycoprotein Correlate with Dose Requirement. Transplantation 2002, 74, 1486. [Google Scholar] [CrossRef] [PubMed]
- Christians, U.; Jacobsen, W.; Benet, L.Z.; Lampen, A. Mechanisms of Clinically Relevant Drug Interactions Associated with Tacrolimus. Clin. Pharmacokinet. 2002, 41, 813–851. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Kuypers, D.; Sarwal, M. Calcineurin Inhibitor Nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481. [Google Scholar] [CrossRef]
- Myers, B.D.; Ross, J.; Newton, L.; Luetscher, J.; Perlroth, M. Cyclosporine-Associated Chronic Nephropathy. N. Engl. J. Med. 1984, 311, 699–705. [Google Scholar] [CrossRef]
- Starzl, T.E.; Fung, J.; Jordan, M.; Shapiro, R.; Tzakis, A.; McCauley, J.; Johnston, J.; Iwaki, Y.; Jain, A.; Alessiani, M.; et al. Kidney Transplantation Under FK 506. JAMA: J. Am. Med. Assoc. 1990, 264, 63–67. [Google Scholar] [CrossRef]
- Wu, G.; Weng, F.L.; Balaraman, V. Tacrolimus-Induced Encephalopathy and Polyneuropathy in a Renal Transplant Recipient. BMJ Case Rep. 2013, 2013, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, B.; Abu-Elmagd, K.; Wilson, J.; Fung, J.; Alessiani, M.; Jain, A.; Takaya, S.; Todo, S.; Tzakis, A.; van Thiel, D. Neurologic Complications of FK 506. Transplant Proc. 1991, 23, 3175. [Google Scholar] [PubMed]
- Hinchey, J.; Chaves, C.; Appignani, B.; Breen, J.; Pao, L.; Wang, A.; Pessin, M.; Lamy, C.; Mas, J.; Caplan, L. A Reversible Posterior Leukoencephalopathy Syndrome. N. Engl. J. Med. 1996, 334, 494. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, P.J.; Restrepo, L.; Mitchell, S.A.; Tornatore, C.S.; Frankel, S.R. Reversible Tacrolimus-Induced Neurotoxicity Isolated to the Brain Stem from the Departments of Radiology. Am. J. Neuroradiol. 2000, 21, 1251–1254. [Google Scholar] [PubMed]
- Marchetti, P.; Navalesi, R. The Metabolic Effects of Cyclosporin and Tacrolimus. J. Endocrinol. Investig. 2000, 23, 482–490. [Google Scholar] [CrossRef]
- Chang, H.L.; Kim, G.H. Electrolyte and Acid-Base Disturbances Induced by Clacineurin Inhibitors. Electrolyte Blood Press. 2007, 5, 126–130. [Google Scholar]
- Redmon, J.B.; Olson, L.K.; Armstrong, M.B.; Greene, M.J.; Robertson, R.P. Effects of Tacrolimus (FK506) on Human Insulin Gene Expression, Insulin MRNA Levels, and Insulin Secretion in HIT-T15 Cells. J. Clin. Investig. 1996, 98, 2786–2793. [Google Scholar] [CrossRef]
- Hirano, Y.; Fujihira, S.; Ohara, K.; Katsuki, S.; Noguchi, H. Morphological and Functional Changes of Islets of Langerhans in FK506-Treated Rats. Transplantation 1992, 53, 889–894. [Google Scholar] [CrossRef]
- Malpica, L.; Moll, S. Practical Approach to Monitroing and Prevention of Infectious Complications Associated with Ssytemic Corticosteroids, Antimetabolites, Cyclosporina and Cyclophosphamide in Nonmalignant Hematologic Diseases. Hematol. Am. Soc. Hematol. Educ. Program. 2020, 1, 319–327. [Google Scholar] [CrossRef]
- Barber, M.R.W.; Clarke, A.E. Systemic Lupus Erythematosus and Risk of Infection. Expert. Rev. Clin. Immunol. 2020, 16, 527–538. [Google Scholar] [CrossRef]
- Mok, C.C. Calcineurin Inhibitors in Systemic Lupus Erythematosus. Best. Pract. Res. Clin. Rheumatol. 2017, 31, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Noviani, M.; Wasserman, S.; Clowse, M.E.B. Breastfeeding in Mothers with Systemic Lupus Erythematosus. Lupus 2016, 25, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Wentzell, J.; Trinacty, M.; Giguère, P.; Patel, P.; Kekre, N.; Nguyen, T. Efficacy, Safety, and Practicality of Tacrolimus Monitoring after Bone Marrow Transplant: Assessment of a Change in Practice. Can J. Hosp. Pharm. 2020, 73, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cosansu, K.; Cakmak, H.A.; Karadag, B.; Aivazov, M.; Seyahi, N.; Vural, V.A. Impact of Different Immunosuppressive Drugs on QT Interval in Renal Transplant Patients. Heart 2011, 97, A186. [Google Scholar] [CrossRef][Green Version]
- Filler, G. Calcineurin Inhibitors in Pediatric Renal Transplant Recipients. Pediatric. Drugs 2007, 9, 165–174. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Ponticelli, C.; Reggiani, F.; Moroni, G. Old and New Calcineurin Inhibitors in Lupus Nephritis. J. Clin. Med. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Betancourt, B.Y.; Biehl, A.; Katz, J.D.; Subedi, A. Pharmacotherapy Pearls in Rheumatology for the Care of Older Adult Patients: Focus on Oral Disease-Modifying Antirheumatic Drugs and the Newest Small Molecule Inhibitors. Rheum. Dis. Clin. North Am. 2018, 44, 371–391. [Google Scholar] [CrossRef]
- Fortin, P.R.; Abrahamowicz, M.; Ferland, D.; Lacaille, D.; Smith, C.D.; Zummer, M. Steroid-Sparing Effects of Methotrexate in Systemic Lupus Erythematosus: A Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis Rheum. 2008, 59, 1796–1804. [Google Scholar] [CrossRef]
- Moutsopoulos, H.M.; Zampeli, E. Medications, Therapeutic Modalities, and Regimens Used in the Management of Rheumatic Diseases. In Immunology and Rheumatology in Questions; Springer International Publishing: Cham, Switzerland, 2021; pp. 205–243. ISBN 978-3-030-56669-2. [Google Scholar]
- Haustein, U.F.; Rytter, M. Methotrexate in Psoriasis: 26 Years’ Experience with Low-Dose Long-Term Treatment. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 382–388. [Google Scholar] [CrossRef]
- Ceponis, A.; Kavanaugh, A. Use of Methotrexate in Patients with Psoriatic Arthritis. Clin. Exp. Rheumatol. 2010, 28, 132–137. [Google Scholar]
- Cipriani, P.; Ruscitti, P.; Carubbi, F.; Liakouli, V.; Giacomelli, R. Methotrexate: An Old New Drug in Autoimmune Disease. Expert. Rev. Clin. Immunol. 2014, 10, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; Hoogendijk, J.E.; Lecky, B.; Winer, J.B.; Gordon, P. Immunosuppressant and Immunomodulatory Treatment for Dermatomyositis and Polymyositis. Cochrane Database Syst. Rev. 2009, 4, 1465–1858. [Google Scholar] [CrossRef]
- Lee, S.D.; Shivashankar, R.; Quirk, D.; Zhang, H.; Telliez, J.B.; Andrews, J.; Marren, A.; Mukherjee, A.; Loftus, E.V. Therapeutic Drug Monitoring for Current and Investigational Inflammatory Bowel Disease Treatments. J. Clin. Gastroenterol. 2021, 55, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Y.; Zhang, Z.; Huang, C.; Liu, Y.; Gu, J.; Zhang, X.; Xu, H.; Li, X.; Wu, L.; et al. 2020 Chinese Guidelines for the Diagnosis and Treatment of Systemic Lupus Erythematosus. Rheumatol. Immunol. Res. 2020, 1, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Bertsias, G. Treating Systemic Lupus Erythematosus in the 21st Century: New Drugs and New Perspectives on Old Drugs. Rheumatology 2020, 59, v69–v81. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Aune, T.M. Methotrexate and Its Mechanisms of Action in Inflammatory Arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef]
- Wallace, D.J. Systemic and Biologic Agents for Lupus Erythematosus. In Biologic and Systemic Agents in Dermatology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 377–390. ISBN 9783319668840. [Google Scholar]
- Sakthiswary, R.; Suresh, E. Methotrexate in Systemic Lupus Erythematosus: A Systematic Review of Its Efficacy. Lupus 2014, 23, 225–235. [Google Scholar] [CrossRef]
- Otón, T.; Carmona, L.; Loza, E.; Rosario, M.P.; Andreu, J.L. Use of Parenteral Methotrexate in Rheumatic Diseases: A Systematic Review. Reumatol. Clin. 2022, 18, 207–226. [Google Scholar] [CrossRef]
- Carneiro, J.R.M.; Sato, E.I. Double Blind, Randomized, Placebo Controlled Clinical Trial of Methotrexate in Systemic Lupus Erythematosus. J. Rheumatol. 1999, 26, 1275–1279. [Google Scholar]
- Islam, M.N.; Hossain, M.; Haq, S.A.; Alam, M.N.; ten Klooster, P.M.; Rasker, J.J. Efficacy and Safety of Methotrexate in Articular and Cutaneous Manifestations of Systemic Lupus Erythematosus. Int. J. Rheum. Dis. 2012, 15, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Abeles, M. A 2 Year, Open Ended Trial of Methotrexate in Systemic Lupus Erythematosus. J. Rheumatol. 1994, 21, 1674–1677. [Google Scholar] [PubMed]
- Gansauge, S.; Breitbart, A.; Rinaldi, N.; Schwarz-Eywill, M. Methotrexate in Patients with Moderate Systemic Lupus Erythematosus (Exclusion of Renal and Central Nervous System Disease). Ann. Rheum. Dis. 1997, 56, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Kipen, Y.; Littlejohn, G.O.; Morand, E.F. Methotrexate Use in Systemic Lupus Erythematosus. Lupus 1997, 6, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.; Humphrey-Murto, S.; Gladman, D.D.; Urowitz, M.B. Efficacy and Tolerability of Methotrexate in Antimalarial Resistant Lupus Arthritis. J. Rheumatol. 1998, 25, 243–246. [Google Scholar]
- Miyawaki, S.; Nishiyama, S.; Aita, T.; Yoshinaga, Y. The Effect of Methotrexate on Improving Serological Abnormalities of Patients with Systemic Lupus Erythematosus. Mod. Rheumatol. 2013, 23, 659–666. [Google Scholar] [CrossRef]
- Wise, C.M.; Vuyyuru, S.; Roberts, W.N. Methotrexate in Nonrenal Lupus and Undifferentiated Connective Tissue Disease—A Review of 36 Patients. J. Rheumatol. 1996, 23, 1005–1010. [Google Scholar]
- Wollina, U.; Ständer, K.; Barta, U. Toxicity of Methotrexate Treatment in Psoriasis Arthritis—Short- and Long-Term Toxicity in 104 Patients. Clin. Rheumatol. 2001, 20, 406–410. [Google Scholar] [CrossRef]
- Wong, J.M.; Esdaile, J.M. Methotrexate in Systemic Lupus Erythematosus. Lupus 2005, 14, 101–105. [Google Scholar] [CrossRef]
- Solomon, D.H.; Glynn, R.J.; Karlson, E.W.; Lu, F.; Corrigan, C.; Colls, J.; Xu, C.; MacFadyen, J.; Barbhaiya, M.; Berliner, N.; et al. Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann. Intern. Med. 2020, 172, 369–380. [Google Scholar] [CrossRef]
- Shea, B.; Swinden, M.V.; Tanjong Ghogomu, E.; Ortiz, Z.; Katchamart, W.; Rader, T.; Bombardier, C.; Wells, G.A.; Tugwell, P. Folic Acid and Folinic Acid for Reducing Side Effects in Patients Receiving Methotrexate for Rheumatoid Arthritis. Cochrane Database Syst. Rev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Visser, K.; Katchamart, W.; Loza, E.; Martinez-Lopez, J.A.; Salliot, C.; Trudeau, J.; Bombardier, C.; Carmona, L.; van der Heijde, D.; Bijlsma, J.W.J.; et al. Multinational Evidence-Based Recommendations for the Use of Methotrexate in Rheumatic Disorders with a Focus on Rheumatoid Arthritis: Integrating Systematic Literature Research and Expert Opinion of a Broad International Panel of Rheumatologists in the 3E. Ann. Rheum. Dis. 2009, 68, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Katchamart, W.; Bourré-Tessier, J.; Donka, T.; Drouin, J.; Rohekar, G.; Bykerk, V.P.; Haraoui, B.; LeClerq, S.; Mosher, D.P.; Pope, J.E.; et al. Canadian Recommendations for Use of Methotrexate in Patients with Rheumatoid Arthritis. J. Rheumatol. 2010, 37, 1422–1430. [Google Scholar] [CrossRef]
- Albrecht, K.; Müller-Ladner, U. Side Effects and Management of Side Effects of Methotrexate in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2010, 28, 502–516. [Google Scholar]
- Conway, R.; Carey, J.J. Risk of Liver Disease in Methotrexate Treated Patients. World J. Hepatol. 2017, 9, 1092–1100. [Google Scholar] [CrossRef]
- Kevat, S.; Ahern, M.; Hall, P. Hepatotoxicity of Methotrexate in Rheumatic Diseases. Med. Toxicol. Adverse Drug. Exp. 1988, 3, 197–208. [Google Scholar] [CrossRef]
- Cansu, D.Ü.; Teke, H.Ü.; Bodakçi, E.; Korkmaz, C. How Should We Manage Low-Dose Methotrexate-Induced Pancytopenia in Patients with Rheumatoid Arthritis? Clin. Rheumatol. 2018, 37, 3419–3425. [Google Scholar] [CrossRef]
- Berkun, Y.; Levartovsky, D.; Rubinow, A.; Orbach, H.; Aamar, S.; Grenader, T.; Abou Atta, I.; Mevorach, D.; Friedman, G.; Ben-Yehuda, A. Methotrexate Related Adverse Effects in Patients with Rheumatoid Arthritis Are Associated with the A1298C Polymorphism of the MTHFR Gene. Ann. Rheum. Dis. 2004, 63, 1227–1231. [Google Scholar] [CrossRef]
- Al-Anazi, K.A.; Eltayeb, K.I.; Bakr, M.; Al-Mohareb, F.I. Methotrexate-Induced Acute Leukemia: Report of Three Cases and Review of the Literature. Clin. Med. Case Rep. 2009, 2009, 43–49. [Google Scholar] [CrossRef]
- Jakubovic, B.D.; Donovan, A.; Webster, P.M.; Shear, N.H. Methotrexate-Induced Pulmonary Toxicity. Can Respir. J. 2013, 20, 153–155. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Nikiphorou, E.; Larsen, J.; Korsten, P.; Conway, R. Methotrexate-Associated Pneumonitis and Rheumatoid Arthritis-Interstitial Lung Disease: Current Concepts for the Diagnosis and Treatment. Front. Med. 2019, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Widemann, B.C.; Adamson, P.C. Understanding and Managing Methotrexate Nephrotoxicity. Oncologist 2006, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; de Groot, K. Nierenschädigung Durch Methotrexat? Dosisabhängigkeit, Komorbidität Und Komedikation. Z. Rheumatol. 2011, 70, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Vezmar, S.; Becker, A.; Bode, U.; Jaehde, U. Biochemical and Clinical Aspects of Methotrexate Neurotoxicity. Chemotherapy 2003, 49, 92–104. [Google Scholar] [CrossRef]
- Watanabe, K.; Arakawa, Y.; Oguma, E.; Uehara, T.; Yanagi, M.; Oyama, C.; Ikeda, Y.; Sasaki, K.; Isobe, K.; Mori, M.; et al. Characteristics of Methotrexate-Induced Stroke-like Neurotoxicity. Int. J. Hematol. 2018, 108, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Wernick, R.; Smith, D.L. Central Nervous System Toxicity Associated with Weekly Low-dose Methotrexate Treatment. Arthritis Rheum. 1989, 32, 770–775. [Google Scholar] [CrossRef]
- Lewden, B.; Vial, T.; Elefant, E.; Nelva, A.; Carlier, P.; Descotes, J. Low Dose Methotrexate in the First Trimester of Pregnancy: Results of a French Collaborative Study. J. Rheumatol. 2004, 31, 2360–2365. [Google Scholar]
- Valerio, V.; Kwok, M.; Loewen, H.; Winkler, J.; Mody, G.M.; Scuccimarri, R.; Meltzer, M.; Mengistu, Y.; Feldman, C.H.; Weinblatt, M.E.; et al. Systematic Review of Recommendations on the Use of Methotrexate in Rheumatoid Arthritis. Clin. Rheumatol. 2021, 40, 1259–1271. [Google Scholar] [CrossRef]
- Contestable, J.J.; Edhegard, K.D.; Meyerle, J.H. Bullous Systemic Lupus Erythematosus: A Review and Update to Diagnosis and Treatment. Am. J. Clin. Dermatol. 2014, 15, 517–524. [Google Scholar] [CrossRef]
- Fairley, J.L.; Oon, S.; Saracino, A.M.; Nikpour, M. Management of Cutaneous Manifestations of Lupus Erythematosus: A Systematic Review. Semin. Arthritis. Rheum. 2020, 50, 95–127. [Google Scholar] [CrossRef]
- Wozel, V.E.G. Innovative Use of Dapsone. Dermatol. Clin. 2010, 28, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Long, H.; Chow, S.; Hidayat, S.; Danarti, R.; Listiawan, Y.; Deng, D.; Guo, Q.; Fang, H.; Tao, J.; et al. Guideline for the Diagnosis, Treatment and Long-Term Management of Cutaneous Lupus Erythematosus. J. Autoimmun. 2021, 123, 102707. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.; Hilbers-Modderman, E.S.M.; Merkus, F.W.H.M. Clinical Pharmacokinetics of Dapsone. Clinical. Pharmacokinet. 2012, 11, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Paolinelli, M.; Campanati, A.; Brisigotti, V.; Offidani, A. Metabolic, Pharmacokinetic, and Toxicological Issues Surrounding Dapsone. 2019, 15, 367–379. [CrossRef]
- Schmidt, E.; Reimer, S.; Kruse, N.; Bröcker, E.B.; Zillikens, D. The IL-8 Release from Cultured Human Keratinocytes, Mediated by Antibodies to Bullous Pemphigoid Autoantigen 180, Is Inhibited by Dapsone. Clin. Exp. Immunol. 2001, 124, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Debol, S.M.; Herron, M.J.; Nelson, R.D. Anti-Inflammatory Action of Dapsone: Inhibition of Neutrophil Adherence Is Associated with Inhibition of Chemoattractant-Induced Signal Transduction. J. Leukoc. Biol. 1997, 62, 827–836. [Google Scholar] [CrossRef]
- Bozeman, P.M.; Learn, D.B.; Thomas, E.L. Inhibition of the Human Leukocyte Enzymes Myeloperoxidase and Eosinophil Peroxidase by Dapsone. Biochem. Pharmacol. 1992, 44, 553–563. [Google Scholar] [CrossRef]
- Verdelli, A.; Corrà, A.; Mariotti, E.B.; Aimo, C.; Ruffo di Calabria, V.; Volpi, W.; Quintarelli, L.; Caproni, M. An Update on the Management of Refractory Cutaneous Lupus Erythematosus. Front. Med. 2022, 9, 941003. [Google Scholar] [CrossRef]
- Hall, R.P.; Lawley, T.J.; Smith, H.R.; Katz, S.I. Bullous Eruption of Systemic Lupus Erythematosus. Dramatic Response to Dapsone Therapy. Ann. Intern. Med. 1982, 97, 165–170. [Google Scholar] [CrossRef]
- Lalova, A.; Pramatarov, K.; Vassileva, S. Facial Bullous Systemic Lupus Erythematosus. Int. J. Dermatol. 1997, 36, 369–371. [Google Scholar] [CrossRef]
- Ludgate, M.W.; Greig, D.E. Bullous Systemic Lupus Erythematosus Responding to Dapsone. Australas. J. Dermatol. 2008, 49, 91–93. [Google Scholar] [CrossRef]
- Lourenço, D.M.R.; Cunha Gomes, R.; Aikawa, N.E.; Campos, L.M.A.; Romiti, R.; Silva, C.A. Childhood-Onset Bullous Systemic Lupus Erythematosus. Lupus 2014, 23, 1422–1425. [Google Scholar] [CrossRef]
- de Risi-Pugliese, T.; Cohen Aubart, F.; Haroche, J.; Moguelet, P.; Grootenboer-Mignot, S.; Mathian, A.; Ingen-Housz-Oro, S.; Hie, M.; Wendremaire, N.; Aucouturier, F.; et al. Clinical, Histological, Immunological Presentations and Outcomes of Bullous Systemic Lupus Erythematosus: 10 New Cases and a Literature Review of 118 Cases. Semin. Arthritis. Rheum. 2018, 48, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hanuschk, D.; Kozyreff, A.; Tafzi, N.; Tennstedt, D.; Hantson, P.; Saint-Marcoux, F. Acute Visual Loss Following Dapsone-Induced Methemoglobinemia and Hemolysis. Clin. Toxicol. 2015, 53, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Satapornpong, P.; Pratoomwun, J.; Rerknimitr, P.; Klaewsongkram, J.; Nakkam, N.; Rungrotmongkol, T.; Konyoung, P.; Saksit, N.; Mahakkanukrauh, A.; Amornpinyo, W.; et al. HLA-B*13: 01 Is a Predictive Marker of Dapsone-Induced Severe Cutaneous Adverse Reactions in Thai Patients. Front. Immunol. 2021, 12, 661135. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Agarwalla, A. Dapsone Hypersensitivity Syndrome: A Clinico-Epidemiological Review. J. Dermatol. 2005, 32, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.S.; Chu, C.Y.; Wang, S.H.; Tsai, T.F. Dapsone Hypersensitivity Syndrome in Non-Leprosy Patients: A Retrospective Study of Its Incidence in a Tertiary Referral Center in Taiwan. J. Dermatol. Treat. 2009, 20, 340–343. [Google Scholar] [CrossRef]
- Pahadiya, H.R.; Lakhotia, M. Dapsone Hypersensitivity Syndrome with Leukemoid Reaction and Severe Thrombocytosis. Cureus 2021, 13, 1–4. [Google Scholar] [CrossRef]
- Lorenz, M.; Wozel, G.; Schmit, J. Hypersensitivity Reactions to Dapsone: A Systematic Review. Acta Derm. Venereol. 2012, 92, 194–199. [Google Scholar] [CrossRef]
- Fine, J.D.; Katz, S.I.; Donahue, M.J.; Hendricks, A.A. Psychiatric Reaction to Dapsone and Sulfapyridine. J. Am. Acad. Dermatol. 1983, 9, 274–275. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Dapsone-Induced Hepatic Complications: It’s Time to Think beyond Methemoglobinemia. Drug Chem. Toxicol. 2019, 44, 330–333. [Google Scholar] [CrossRef]
- Soliman, Y.Y.; Soliman, M.S.; Abbas, F. Why Now? Delayed Drug-Induced Pancreatitis Due to Dapsone for Dermatitis Herpetiformis. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Imadojemu, S.; Werth, V.P. Management of Rheumatic and Autoimmune Blistering Disease in Pregnancy and Postpartum. Clin. Dermatol. 2016, 34, 344–352. [Google Scholar] [CrossRef] [PubMed]
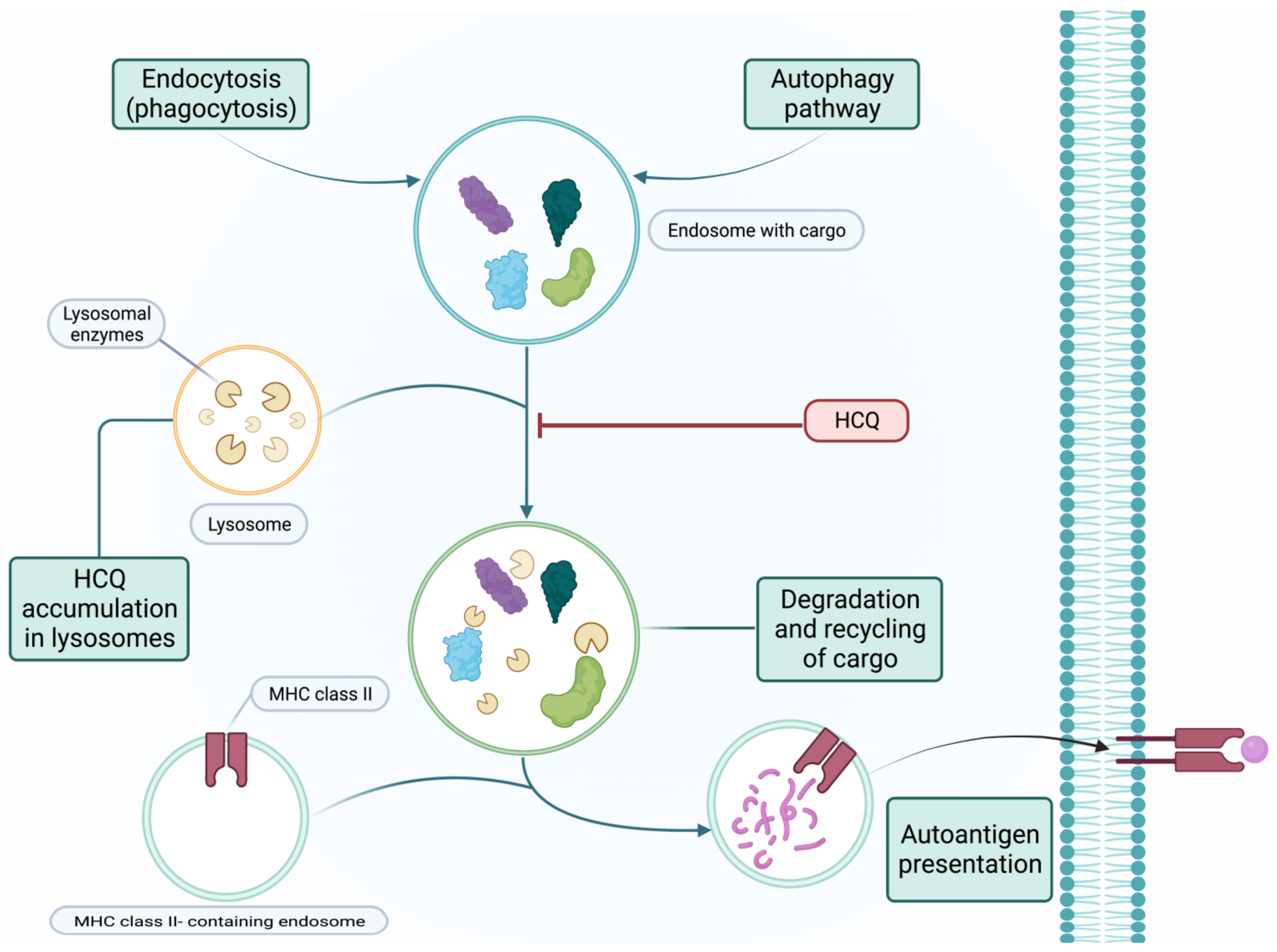
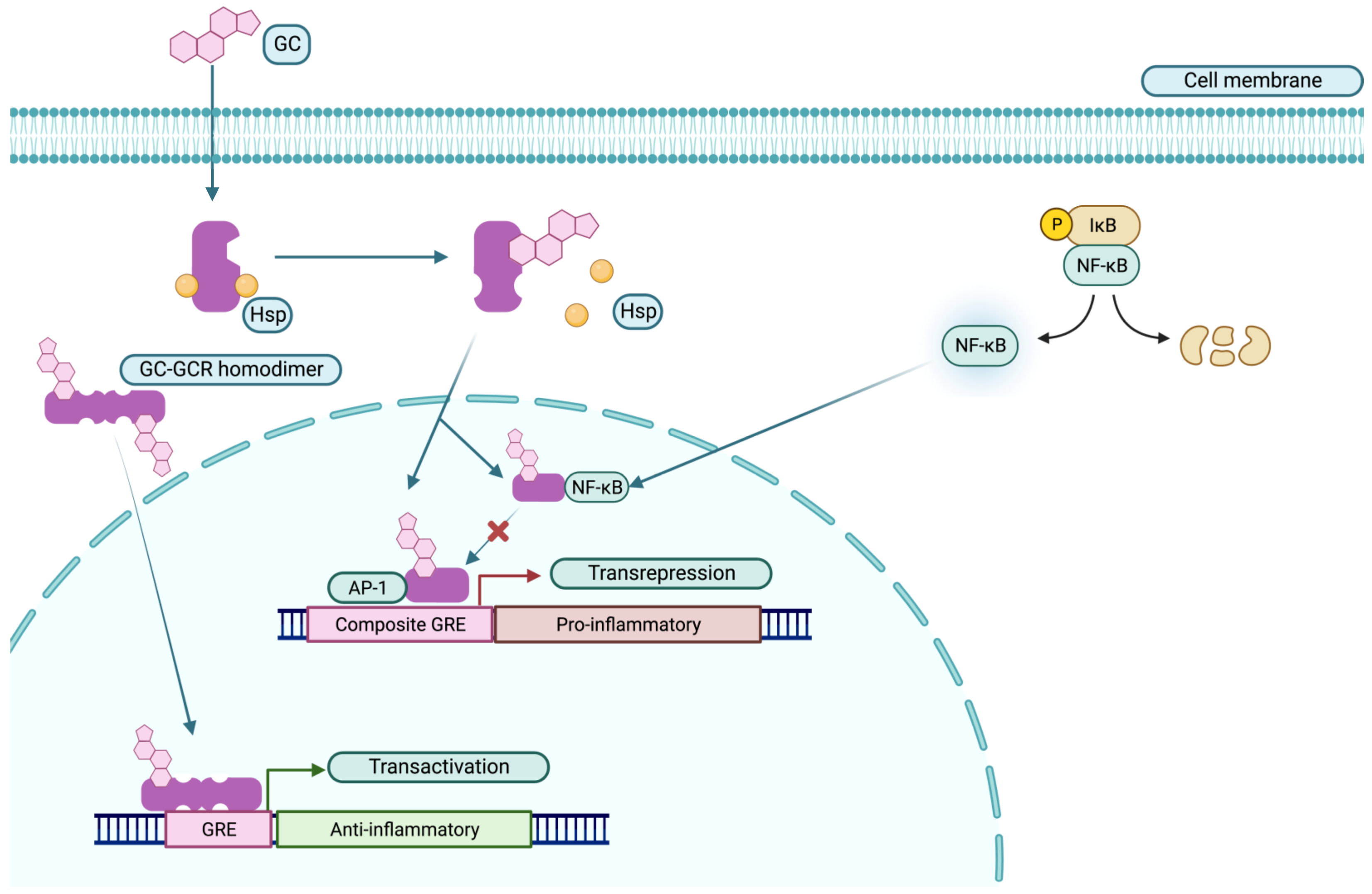
|
| Organ | Side Effects |
|---|---|
| Gastrointestinal tract | Nausea, vomiting, abdominal discomfort, diarrhea, hepatotoxicity |
| Skin | Pruritus (generalized, aquagenic), hyper- or depigmentation, ecchymosis, DRESS, erythema multiforme, erythroderma. |
| Eye | Retinopathy, diminished peripheral and nocturnal vision, bulls-eye maculopathy, difficulty reading, altered color perception. |
| Heart | Cardiomyopathy, bradycardia, tachycardia, T-wave flattening, left anterior fascicular block, complete atrioventricular block. |
| Neuromuscular system | Headaches, dizziness, insomnia, vertigo, tinnitus, hearing loss, psychosis, delirium, depression, reversible proximal myopathy, non-painful neuropathy. |
| Organ | Side Effects |
|---|---|
| Kidney | Increased sodium retention and potassium excretion |
| Musculoskeletal system | Osteoporosis, osteonecrosis, myopathy and atrophy, and growth retardation, |
| Cardiovascular system | Dyslipidemia, hypertension, hyperglycemia, lipodystrophy and weight gain, thrombosis, and vasculitis |
| Adrenal gland | Adrenal atrophy and Cushing’s syndrome |
| Skin | Atrophy, delayed wound healing, erythema, hypertrichosis, perioral dermatitis, petechiae, glucocorticoid-induced acne, striae rubrae distensae, and telangiectasia |
| Eyes | Cataracts, glaucoma, myopia, exophthalmos, papilledema, chorioretinopathy, and subconjunctival hemorrhages. |
| Central nervous system | Depression, psychosis, bipolar disorders, delirium, panic attacks, obsessive–compulsive disorder, anxiety, insomnia, catatonia, and cognitive impairment |
| Gastrointestinal tract | Bleeding, pancreatitis, and peptic ulcer |
| Immune system | Broad immunosuppression; activation of latent viruses; increased risk of bacterial, fungal, and viral infections. |
| Reproductive system | Delayed puberty, fetal growth retardation, hypogonadism, gestational diabetes, hypertension, preeclampsia, premature rupture of membranes, and risk of cleft palate |
| Organ | Side Effect |
|---|---|
| Gastrointestinal tract | Nausea, gastrointestinal dysmotility, emesis, and hepatotoxicity |
| Reproductive system | Ovarian failure (reduced estradiol, progesterone, maturation of oocytes, and number of ovarian follicles), amenorrhea, azoospermia, spontaneous abortions, congenital malformation, growth retardation, anatomical abnormalities, and cervical atypia |
| Urinary tract | Necrosis of bladder mucosa, hematuria, hemorrhagic cystitis, and bladder carcinoma |
| Immune system | Hematologic malignancies, neutropenia, lymphopenia, thrombocytopenia, anemia, and increased risk of infections. |
| Lung | Interstitial pneumonitis and fibrosis |
| Cardiovascular system | Hypertrophy, myocardial fibrosis, tachyarrhythmias, hypotension, heart failure, myocarditis, and perimyocarditis |
| Organ | Side Effect |
|---|---|
| Reproductive system | Developmental delays, pancytopenia, premature birth, mild malformations |
| Gastrointestinal tract | Anorexia, nausea, vomiting, and diarrhea, pancreatitis, hepatotoxicity |
| Immune system | Myelosuppression (leucopenia and thrombocytopenia), anemia, bleeding, increased risk of herpesvirus (CMV, VZV, HSV, EBV) and bacterial infection, non-Hodgkin’s lymphoma |
| Organ | Side Effect |
|---|---|
| Kidney | Acute nephrotoxicity (hypomagnesemia, hyperkalemia, hyperuricemia, hyperchloremic metabolic acidosis), chronic nephrotoxicity (arteriolar hyalinosis, tubular atrophy, interstitial fibrosis, glomerular sclerosis) |
| Central nervous system | Tremor, headache, vertigo, photophobia, dysesthesia, paresthesia, mood disturbances, insomnia, confusion, seizures, cortical blindness, encephalopathy, coma, peripheral neuropathy, posterior cerebral edema syndrome |
| Cardiovascular system | Hyperlipidemia (high LDL cholesterol and triglycerides), hyperglycemia |
| Immune system | Slightly increased risk of bacteria and herpesvirus infection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téllez Arévalo, A.M.; Quaye, A.; Rojas-Rodríguez, L.C.; Poole, B.D.; Baracaldo-Santamaría, D.; Tellez Freitas, C.M. Synthetic Pharmacotherapy for Systemic Lupus Erythematosus: Potential Mechanisms of Action, Efficacy, and Safety. Medicina 2023, 59, 56. https://doi.org/10.3390/medicina59010056
Téllez Arévalo AM, Quaye A, Rojas-Rodríguez LC, Poole BD, Baracaldo-Santamaría D, Tellez Freitas CM. Synthetic Pharmacotherapy for Systemic Lupus Erythematosus: Potential Mechanisms of Action, Efficacy, and Safety. Medicina. 2023; 59(1):56. https://doi.org/10.3390/medicina59010056
Chicago/Turabian StyleTéllez Arévalo, Angélica María, Abraham Quaye, Luis Carlos Rojas-Rodríguez, Brian D. Poole, Daniela Baracaldo-Santamaría, and Claudia M. Tellez Freitas. 2023. "Synthetic Pharmacotherapy for Systemic Lupus Erythematosus: Potential Mechanisms of Action, Efficacy, and Safety" Medicina 59, no. 1: 56. https://doi.org/10.3390/medicina59010056
APA StyleTéllez Arévalo, A. M., Quaye, A., Rojas-Rodríguez, L. C., Poole, B. D., Baracaldo-Santamaría, D., & Tellez Freitas, C. M. (2023). Synthetic Pharmacotherapy for Systemic Lupus Erythematosus: Potential Mechanisms of Action, Efficacy, and Safety. Medicina, 59(1), 56. https://doi.org/10.3390/medicina59010056








