A Systematic Review and Meta-Analysis of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Injections in Type-2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Data Analysis
2.6. Risk of Bias
3. Results
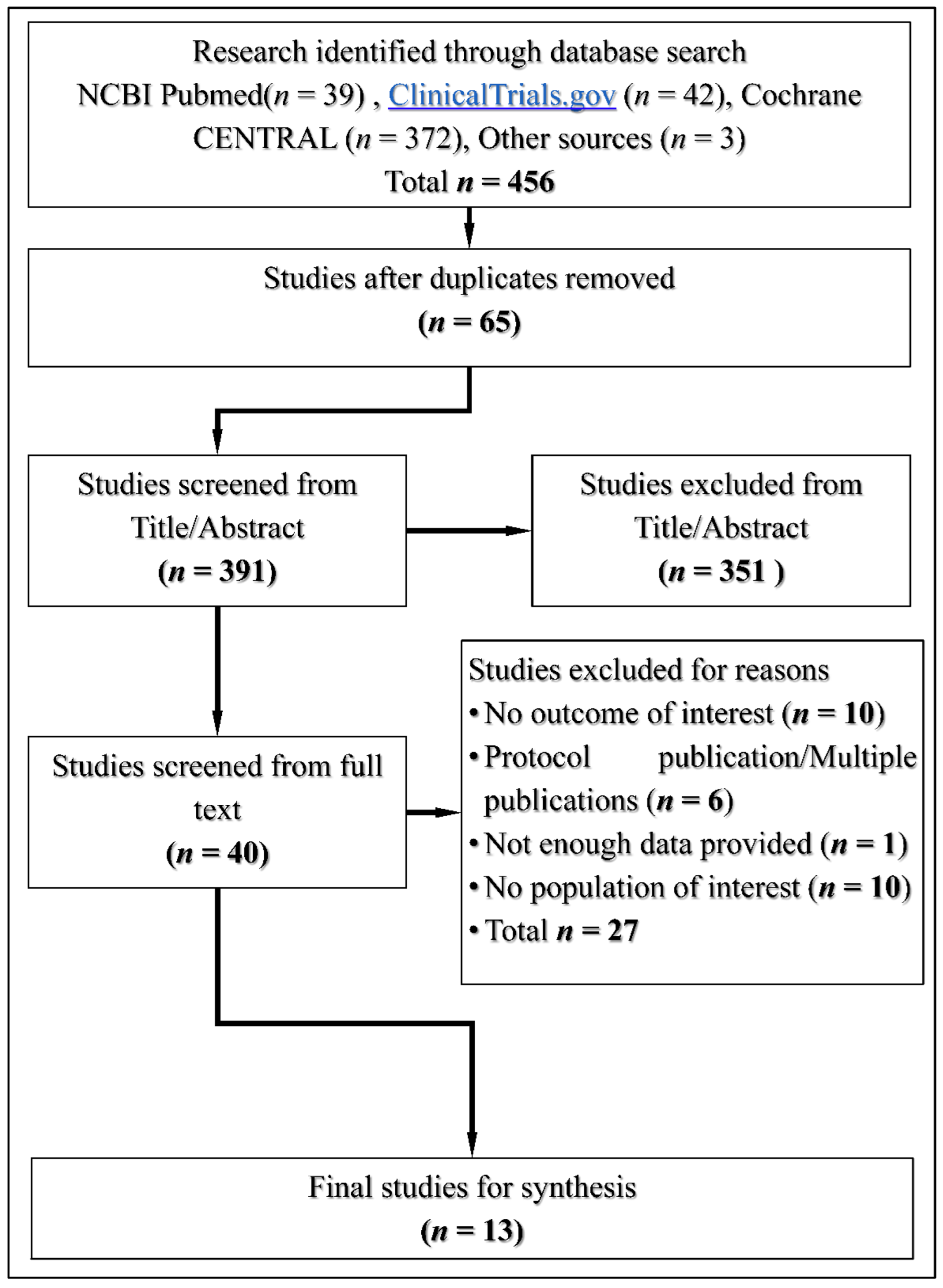
3.1. Search Results
3.2. Study Characteristics
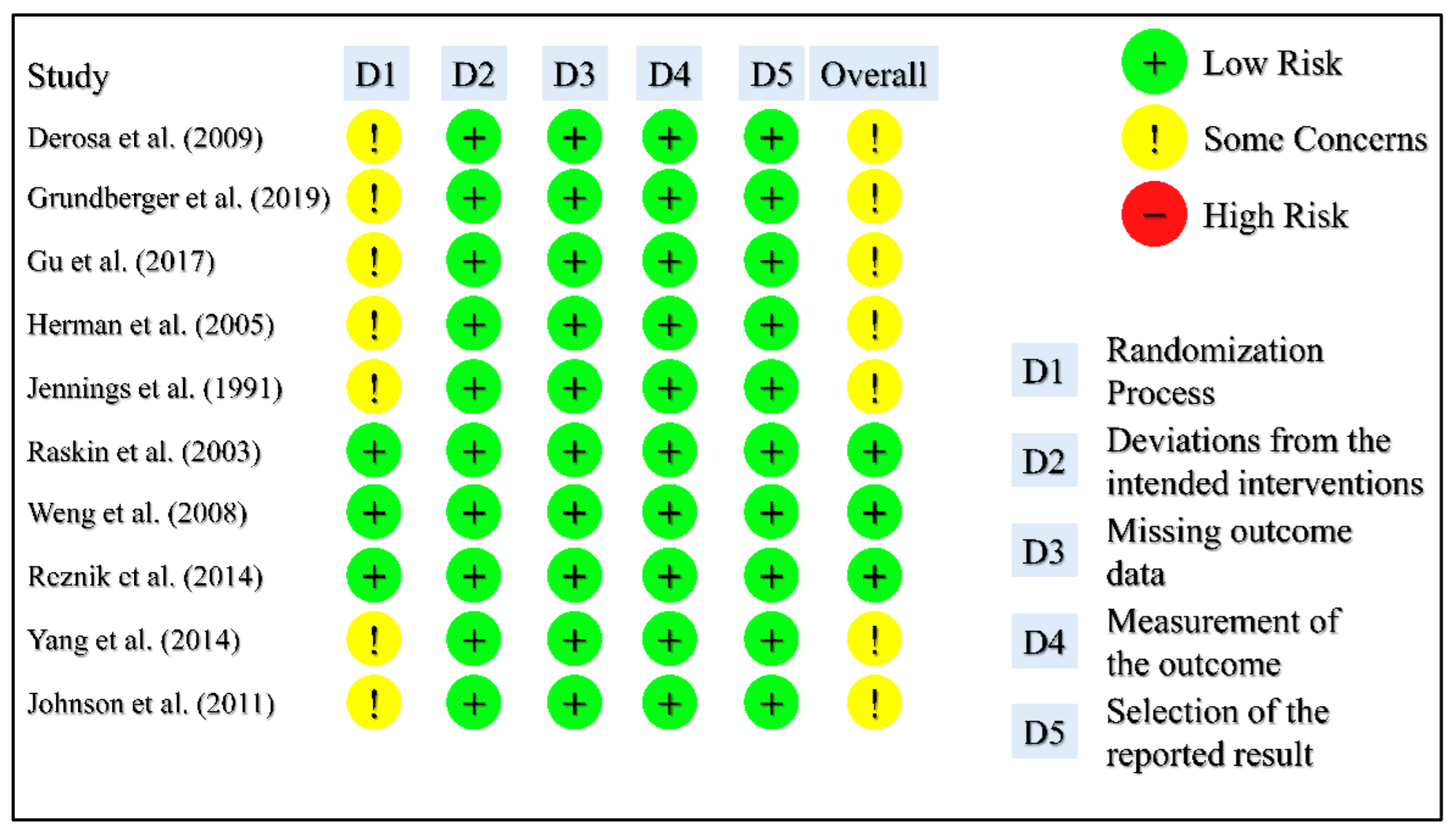
3.3. Risk of Bias Assessment
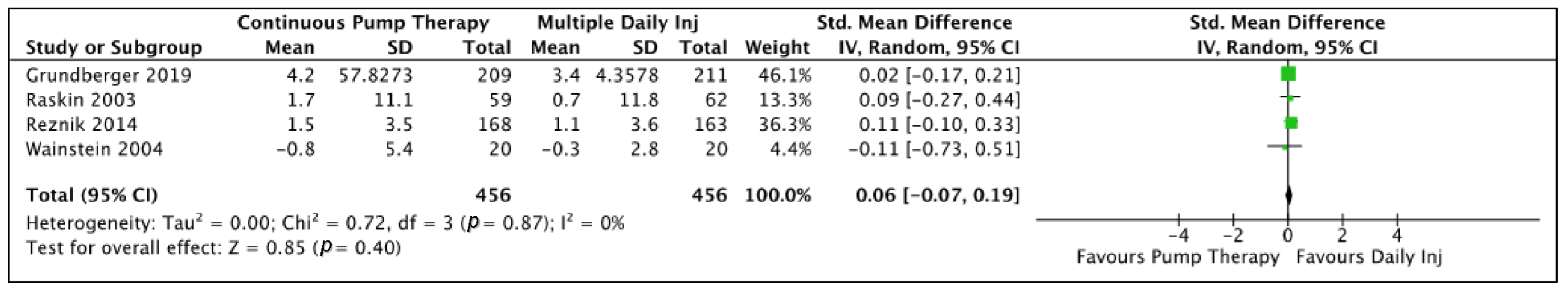
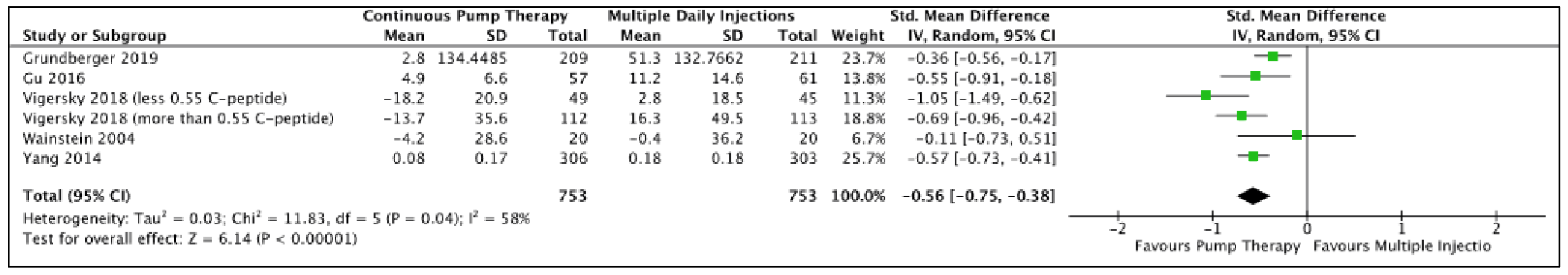
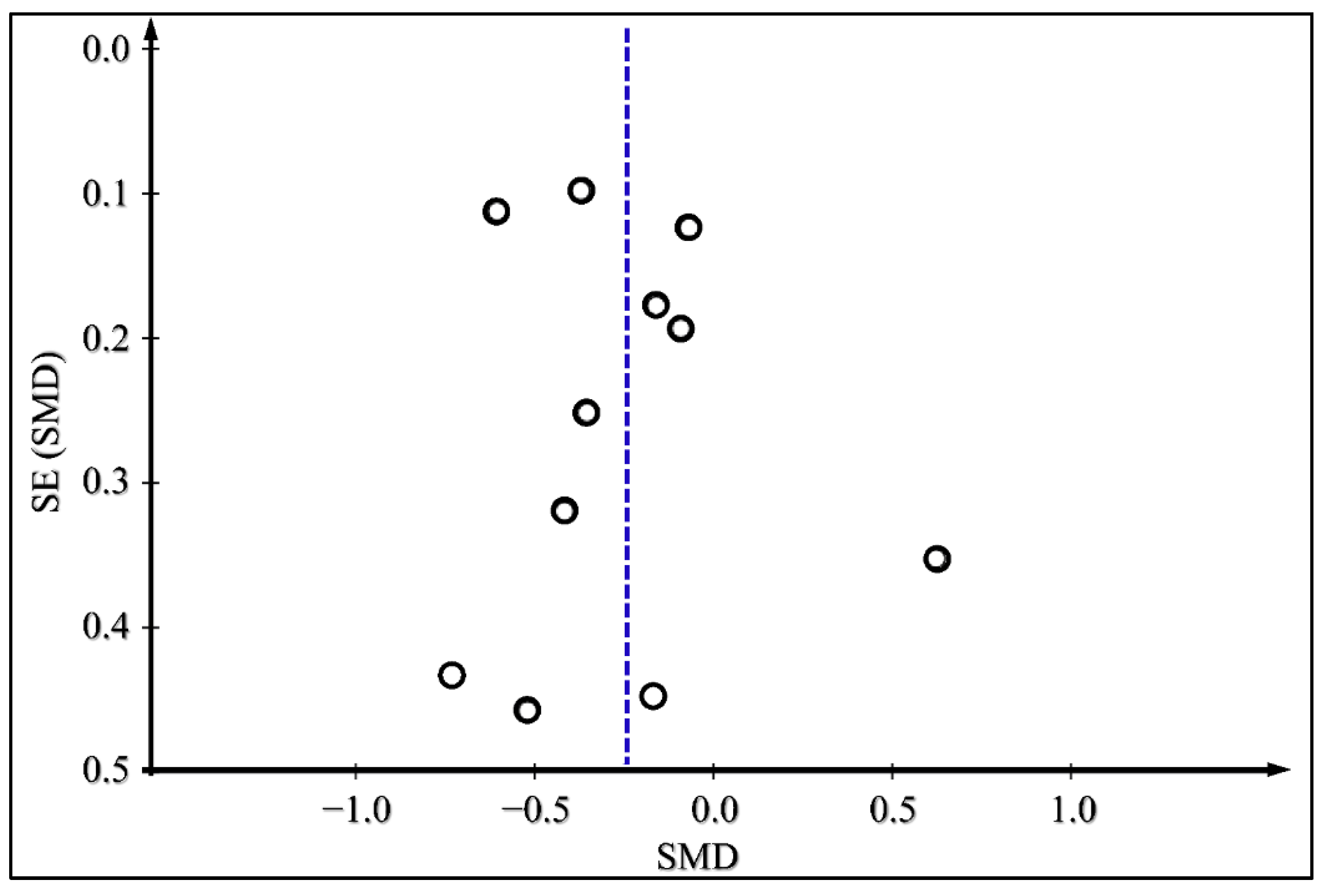
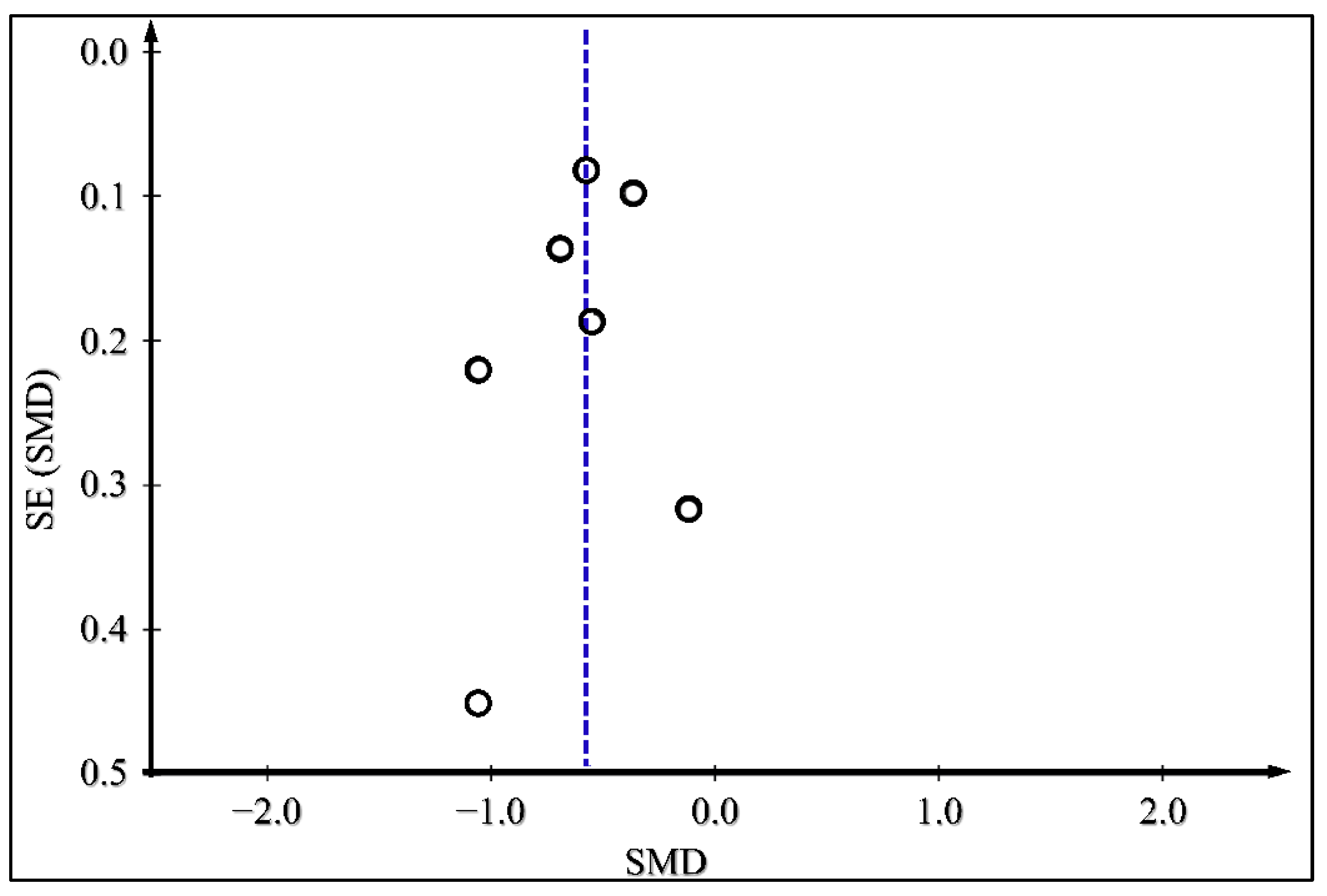
3.4. Meta-Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefebvre, P.; Pierson, A. The global challenge of diabetes. World Hosp. Health Serv. Off. J. Int. Hosp. Fed. 2004, 40, 37–40. [Google Scholar]
- Caliskan, D.; Ozdemir, O.; Ocaktan, E.; Idil, A. Evaluation of awareness of diabetes mellitus and associated factors in four health center areas. Patient Educ. Couns. 2006, 62, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Unwin, N.; Gan, D.; Whiting, D. The idf diabetes atlas: Providing evidence, raising awareness and promoting action. Diabetes Res. Clin. Pract. 2010, 87, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, E.S.; Korytkowski, M.T.; DiNardo, M.; Einhorn, D.; Hellman, R.; Hirsch, I.B.; Inzucchi, S.E.; Ismail-Beigi, F.; Kirkman, M.S.; Umpierrez, G.E.; et al. American association of clinical endocrinologists and american diabetes association consensus statement on inpatient glycemic control. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2009, 15, 353–369. [Google Scholar]
- American Diabetes, A. 3. Prevention or delay of type 2 diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S34–S39. [Google Scholar] [CrossRef]
- Shubrook, J.H.; Bokaie, B.B.; Adkins, S.E. Empagliflozin in the treatment of type 2 diabetes: Evidence to date. Drug Des. Dev. Ther. 2015, 9, 5793–5803. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Hua, J.; Wilson, C.H.; Tallis, G.A.; Zhou, F.H.; Rychkov, G.Y.; Barritt, G.J. The glucagon-like peptide-1 analogue exendin-4 reverses impaired intracellular ca(2+) signalling in steatotic hepatocytes. Biochim. Biophys. Acta 2016, 1863, 2135–2146. [Google Scholar] [CrossRef]
- Janez, A.; Muzurovic, E.; Stoian, A.P.; Haluzik, M.; Guja, C.; Czupryniak, L.; Duvnjak, L.; Lalic, N.; Tankova, T.; Bogdanski, P.; et al. Translating results from the cardiovascular outcomes trials with glucagon-like peptide-1 receptor agonists into clinical practice: Recommendations from a eastern and southern europe diabetes expert group. Int. J. Cardiol. 2022, 365, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Chiodini, P.; Bellastella, G.; Capuano, A.; Esposito, K.; Giugliano, D. Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2017, 40, 614–624. [Google Scholar] [CrossRef]
- Pickup, J.C. Insulin-pump therapy for type 1 diabetes mellitus. N. Engl. J. Med. 2012, 366, 1616–1624. [Google Scholar] [CrossRef]
- Pickup, J.C.; Yemane, N.; Brackenridge, A.; Pender, S. Nonmetabolic complications of continuous subcutaneous insulin infusion: A patient survey. Diabetes Technol. Ther. 2014, 16, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Freckmann, G.; Buck, S.; Waldenmaier, D.; Kulzer, B.; Schnell, O.; Gelchsheimer, U.; Ziegler, R.; Heinemann, L. Insulin pump therapy for patients with type 2 diabetes mellitus: Evidence, current barriers, and new technologies. J. Diabetes Sci. Technol. 2021, 15, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Hovorka, R. Technology in the management of type 2 diabetes: Present status and future prospects. Diabetes Obes. Metab. 2021, 23, 1722–1732. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Klonoff, D.C. Diabetes technology update: Use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care 2018, 41, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cochrane. Reviewmanager (Revman). 5.0 ed.; Cochrane: West Sussex, UK, 2020. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane handbook for systematic reviews of interventions., 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Salvadeo, S.A.; Ferrari, I.; Fogari, E.; Mereu, R.; Gravina, A.; Palumbo, I.; Randazzo, S.; et al. Effects of insulin therapy with continuous subcutaneous insulin infusion (csii) in diabetic patients: Comparison with multi-daily insulin injections therapy (mdi). Endocr. J. 2009, 56, 571–578. [Google Scholar] [CrossRef]
- Grunberger, G.; Bhargava, A.; Ly, T.; Zisser, H.; Ilag, L.L.; Malone, J.; Fan, L.; Zhang, S.; Johnson, J. Human regular u-500 insulin via continuous subcutaneous insulin infusion versus multiple daily injections in adults with type 2 diabetes: The vivid study. Diabetes Obes. Metab. 2020, 22, 434–441. [Google Scholar] [CrossRef]
- Gu, W.; Liu, Y.; Chen, Y.; Deng, W.; Ran, X.; Chen, L.; Zhu, D.; Yang, J.; Shin, J.; Lee, S.W.; et al. Multicentre randomized controlled trial with sensor-augmented pump vs. multiple daily injections in hospitalized patients with type 2 diabetes in china: Time to reach target glucose. Diabetes Metab. 2017, 43, 359–363. [Google Scholar] [CrossRef]
- Herman, W.H.; Ilag, L.L.; Johnson, S.L.; Martin, C.L.; Sinding, J.; Al Harthi, A.; Plunkett, C.D.; LaPorte, F.B.; Burke, R.; Brown, M.B.; et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005, 28, 1568–1573. [Google Scholar] [CrossRef]
- Jennings, A.M.; Lewis, K.S.; Murdoch, S.; Talbot, J.F.; Bradley, C.; Ward, J.D. Randomized trial comparing continuous subcutaneous insulin infusion and conventional insulin therapy in type 2 diabetic patients poorly controlled with sulfonylureas. Diabetes Care 1991, 14, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; McEwen, L.N.; Newton, C.A.; Martin, C.L.; Raskin, P.; Halter, J.B.; Herman, W.H. The impact of continuous subcutaneous insulin infusion and multiple daily injections of insulin on glucose variability in older adults with type 2 diabetes. J. Diabetes Its Complicat. 2011, 25, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Raskin, P.; Bode, B.W.; Marks, J.B.; Hirsch, I.B.; Weinstein, R.L.; McGill, J.B.; Peterson, G.E.; Mudaliar, S.R.; Reinhardt, R.R. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: A randomized, parallel-group, 24-week study. Diabetes Care 2003, 26, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Reznik, Y.; Cohen, O.; Aronson, R.; Conget, I.; Runzis, S.; Castaneda, J.; Lee, S.W.; Op, T.m.S.G. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (opt2mise): A randomised open-label controlled trial. Lancet 2014, 384, 1265–1272. [Google Scholar] [CrossRef]
- Weng, J.; Li, Y.; Xu, W.; Shi, L.; Zhang, Q.; Zhu, D.; Hu, Y.; Zhou, Z.; Yan, X.; Tian, H.; et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet 2008, 371, 1753–1760. [Google Scholar] [CrossRef]
- Yang, H.; Heng, X.; Liang, C.; Liu, X.; Du, W.; Li, S.; Wang, Y.; Dong, Q.; Li, W.; Pan, Z.; et al. Comparison of continuous subcutaneous insulin infusion and multiple daily insulin injections in chinese patients with type 2 diabetes mellitus. J. Int. Med. Res. 2014, 42, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Berthe, E.; Lireux, B.; Coffin, C.; Goulet-Salmon, B.; Houlbert, D.; Boutreux, S.; Fradin, S.; Reznik, Y. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: A comparison study in type 2 diabetes with conventional insulin regimen failure. Horm. Metab. Res. Horm.-Und Stoffwechs. Horm. Metab. 2007, 39, 224–229. [Google Scholar] [CrossRef]
- Wainstein, J.; Metzger, M.; Boaz, M.; Minuchin, O.; Cohen, Y.; Yaffe, A.; Yerushalmy, Y.; Raz, I.; Harman-Boehm, I. Insulin pump therapy vs. Multiple daily injections in obese type 2 diabetic patients. Diabet. Med. A J. Br. Diabet. Assoc. 2005, 22, 1037–1046. [Google Scholar] [CrossRef]
- Vigersky, R.A.; Huang, S.; Cordero, T.L.; Shin, J.; Lee, S.W.; Chhabra, H.; Kaufman, F.R.; Cohen, O.; Op, T.m.S.G. Improved hba1c, total daily insulin dose, and treatment satisfaction with insulin pump therapy compared to multiple daily insulin injections in patients with type 2 diabetes irrespective of baseline c-peptide levels. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2018, 24, 446–452. [Google Scholar] [CrossRef]
- Chlup, R.; Runzis, S.; Castaneda, J.; Lee, S.W.; Nguyen, X.; Cohen, O. Complex assessment of metabolic effectiveness of insulin pump therapy in patients with type 2 diabetes beyond hba1c reduction. Diabetes Technol. Ther. 2018, 20, 153–159. [Google Scholar] [CrossRef]
- Wilson, D.M.; Calhoun, P.M.; Maahs, D.M.; Chase, H.P.; Messer, L.; Buckingham, B.A.; Aye, T.; Clinton, P.K.; Hramiak, I.; Kollman, C.; et al. Factors associated with nocturnal hypoglycemia in at-risk adolescents and young adults with type 1 diabetes. Diabetes Technol. Ther. 2015, 17, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Weykamp, C. Hba1c: A review of analytical and clinical aspects. Ann. Lab. Med. 2013, 33, 393–400. [Google Scholar] [CrossRef]
- Saudek, C.D.; Duckworth, W.C.; Giobbie-Hurder, A.; Henderson, W.G.; Henry, R.R.; Kelley, D.E.; Edelman, S.V.; Zieve, F.J.; Adler, R.A.; Anderson, J.W.; et al. Implantable insulin pump vs. multiple-dose insulin for non-insulin-dependent diabetes mellitus: A randomized clinical trial. Department of veterans affairs implantable insulin pump study group. JAMA 1996, 276, 1322–1327. [Google Scholar] [CrossRef]
- Nauck, M.A.; Kahle-Stephan, M.; Lindmeyer, A.M.; Wenzel, S.; Meier, J.J. Prediction of individual basal rate profiles from patient characteristics in type 1 diabetes on insulin pump therapy. J. Diabetes Sci. Technol. 2021, 15, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Wahlqvist, P.; Warner, J.; Morlock, R. Cost-effectiveness of simple insulin infusion devices compared to multiple daily injections in uncontrolled type 2 diabetics in the united states based on a simulation model. J. Health Econ. Outcomes Res. 2018, 6, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Roze, S.; Buompensiere, M.I.; Ozdemir, Z.; de Portu, S.; Cohen, O. Cost-effectiveness of a novel hybrid closed-loop system compared with continuous subcutaneous insulin infusion in people with type 1 diabetes in the uk. J. Med. Econ. 2021, 24, 883–890. [Google Scholar] [CrossRef]
- Reutrakul, S.; Wroblewski, K.; Brown, R.L. Clinical use of u-500 regular insulin: Review and meta-analysis. J. Diabetes Sci. Technol. 2012, 6, 412–420. [Google Scholar] [CrossRef]
- Li, H.; Yang, A.; Zhao, S.; Chow, E.Y.; Javanbakht, M.; Li, Y.; Lin, D.; Xu, L.; Zang, D.; Wang, K.; et al. Continuous subcutaneous insulin infusion (csii) combined with oral glucose-lowering drugs in type 2 diabetes: A systematic review and network meta-analysis of randomized, controlled trials. Pharmaceuticals 2022, 15, 953. [Google Scholar] [CrossRef]
- Medical Advisory, S. Continuous subcutaneous insulin infusion (csii) pumps for type 1 and type 2 adult diabetic populations: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2009, 9, 1–58. [Google Scholar]
- Jeitler, K.; Horvath, K.; Berghold, A.; Gratzer, T.W.; Neeser, K.; Pieber, T.R.; Siebenhofer, A. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: Systematic review and meta-analysis. Diabetologia 2008, 51, 941–951. [Google Scholar] [CrossRef]
- Dicembrini, I.; Mannucci, E.; Monami, M.; Pala, L. Impact of technology on glycaemic control in type 2 diabetes: A meta-analysis of randomized trials on continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Obes. Metab. 2019, 21, 2619–2625. [Google Scholar] [CrossRef] [PubMed]





| Database | Search Strategy |
|---|---|
| NCBI Pubmed (n = 39) | ((((type 2 diabetes[Title/Abstract]) AND (insulin pump[Title/Abstract])) OR (continuous subcutaneous infusion[Title/Abstract])) AND (daily injections[Title/Abstract])) OR (multiple insulin injection[Title/Abstract]) Filters Clinical trial and Randomized controlled trial |
| ClinicalTrials.gov (n = 42) | Type 2 Diabetes AND insulin Pump Filters Completed studies |
| Cochrane CENTRAL (n = 372) | type 2 diabetes in Title Abstract Keyword AND continuous insulin in Title Abstract Keyword AND multiple injection in Title Abstract Keyword—(Word variations have been searched) |
| Population | Intervention | Comparator | Outcome |
|---|---|---|---|
| T2DM | Continuous, Subcutaneous Insulin Infusion | Multiple Daily Insulin Injection | Difference in HbA1c Difference in Fasting plasma glucose Difference in Body Weight Difference in Total daily insulin dose |
| Author | Study Design | Population | Intervention/ Comparator | Pump Type | Insulin type | No Intervention | No Comparator | Route, Dose, Frequency I/C | Treatment Duration I/C MD (IQR) | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berthe et al. (2007) [29] | Open label RCT crossover | 33.7 (4.6) | 9 (1.6) | 55 (6) | CSII/MDI | Medronic 508 | Lispro plus NPH | 17 | 17 | 70% daily + 30% prandial bolus/ 3 daily inj 50/50 | 12 w | HbA1c, capillary blood glu, hyperglycemic AUC, pt satisfaction, chol, Tg |
| Derosa et al. (2009) [19] | Randomized case-control trial (Type 1 & 2) | 29.5(5.1)/29.8(5.4) | 9.2(2)/9.3(2.1) | 49.8(14.6)/50.4(14.2) | CSII/ MDI | Lispro /glargine | 32 | 32 | 47 UI 50–50/33 UI 3 shots daily lispro + 22 UI 1 shot glargine ins | 12 m | HbA1c, fasting plasma glu, post-prandial glu, total chol, HDL, Tg | |
| Grunberger et al. (2019) [20] | Open label RCT-parallel (VIVID study) | 39.3(5.6)/40.1(5.8) | 8.75(1.03)/ 8.77(1.08) | 57.6(10.3)/56.7(10.1) | CSII/MDI | Omnipod DASH U-500 | U-100 rapid/U-500 R + other glu lowering agents | 209 | 211 | 50–50/3 daily inj | 26 w | HbA1c, fasting plasma glu, proportion achieving target HbA1c |
| Gu et al. (2016) [21] | Open label RCT parallel NCT01921322 | 25(3.1)/25(3.3) | 10(1.6)/10(1.2) | 51(10.2)/49(9.6) | CSII/MDI | Medronic MiniMed Paradigm sensor augmented pump | Novo Nordisk A/S fast and long acting | 57 | 61 | 50–50 fast acting/3 daily inj + 1 bed-time inj | Time to achieve blood glu levels, pts achieving target glu, AUC, | |
| Herman et al. (2005) [22] | RCT parallel | 32.5(5.8)/31.8(5.8) | 8.4(1.1)/ 8.1(1.2) | 66.6(5.9)/66.2(4.5) | CSII/MDI | Medronic MiniMed 508 | Lispro and glargine | 53 | 54 | 50–50/3 daily +1 before bed-time | 12 m | HbA1c, QoL |
| Jennings et al. (1991) [23] | RCT parallel | 64.5/62.5 | 58/61 | CSII/MDI | Regular and NPH | 10 | 10 | 2 daily | 4 m | HbA1c, Fasting glu, capillary blood glu, chol, Tg, HDL, satisfaction | ||
| Johnson et al. (2011) [24] | RCT Parallel | 33.5(5.7)/31.8(5.9) | 8.3(1.1)/8.1(1.3) | 66(6)/66(4.6) | CSII/MDI | Medronic MiniMed 508 | Lispro /glargine | 53 | 54 | 12 m | Mean day glu, mean pre-prandial glu, AUC high, AUC-low | |
| Reznik et al. (2014) [26] | Open label-RCT parallel with single arm crossover OpT2mise NCT01182493 | 33.5(7.5)/33.2(7) | 9 | 55.5(9.7)/56.4(9.5) | CSII/MDI | Medronic MiniMed Paradigm Veo | Lispro or aspart or glulisine & glargine or detemir | 168 | 163 | 50–50/Inj at investigator’s clinical practice | 6 m/6 m crossover of MDI to CSII | HbA1c, AUC hypoglycemia/hyperglycemia |
| Vigersky et al. (2018) [31] | OpT2mise- subgroup analysis | CSII/MDI according to C-peptide level & Age | HbA1c, TDD, satisfaction | |||||||||
| Raskin et al. (2003) [25] | Open label RCT parallel | 32.2(4.2)/32.2(5.1) | 8.2(1.4)/ 8(1.1) | 55.1(10.2)/56(8.18) | CSII/MDI | Medronic MiniMed 507C | Insulin aspart & NPH | 66 | 61 | Ins aspart continuous/ Ins aspart after meals + once or twice long acting ins | 24 w | HbA1c, BG, TDD, satisfaction |
| Wainstein et al. (2004) [30] | RCT crossover | 30–45 | >8.5% | 30–70 | CSII/MDI | Medronic MiniMed | Lispro /Regular ins or Humulin R & NPH | 20 | 20 | 4 daily inj | 18 w/18 w | HbA1c, AUC, chol, HDL, LDL, Tg, C-pept, weight |
| Weng et al. (2008) [27] | RCT parallel NTC00147836 | 25.1(3)/ 24.4(2.7)/25.1(3.3) | 9.8(2.3)/9.7(2.3)/9.5(2.5) | 50(11)/51(10)/52(9) | CSII/ MDI/ oral agents | Human ins (Novo Nordisk)/ Novolin-R & NPH/ gliclazide or metformin + gliclazide | 137 | 124/121 | 50–50/ 30–20-20–30/ 80 mg twice daily gliclazide ± 0.5–2 g metformin | 12 m | Fasting plasma glu, β-cell function, HbA1c, Tg, chol, LDL, HDL, | |
| Yang et al. (2014) [28] | RCT parallel | 24.41(3.63)/24.89(3.48) | 10.46(2.12)/10.34(2.15) | 51.38(11.74)/50.58(12.68) | CSII/MDI | Medronic | Ins aspart/ Human ins short-acting (Novo Nordisk) & NPH | 306 | 303 | 40–60/ 3 times/d fast + 2 times/d long (40–60) | 12 w | Days to achieve target glu, BG levels, TDD, hypoglycemia |
| Test of Association | Test of Heterogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interventions | Outcomes | Subgroups | Effect Sizes | Pooled SMD(CI) | p-Value | Model | Z-Test | X2 | p-Value | I2(%) |
| Continuous Subcutaneous Insulin Infusion v.s Multiple Daily Injections | Difference in HbA1c | 12 | −0.26(−0.42, −0.10) | 0.002 | RE | 3.11 | 22.14 | 0.02 | 50 | |
| Sensitivity Analysis | ||||||||||
| After 2 larger studies removed | 10 | −0.14(−0.28,0) | 0.05 | RE | 1.99 | 9.05 | 0.43 | 1 | ||
| Parallel design only | 7 | −0.28(−0.47, −0.10) | 0.002 | RE | 3.08 | 14.76 | 0.02 | 59 | ||
| Crossover design only | 5 | −0.17(−0.57, 0.23) | 0.41 | RE | 0.82 | 6.73 | 0.15 | 41 | ||
| Difference in Body Weight | 6 | 0.20(−0.16, 0.55) | 0.28 | RE | 1.09 | 23.72 | 0.0002 | 79 | ||
| Parallel design only | 4 | 0.06(−0.07, 0.19) | 0.34 | RE | 0.95 | 0.42 | 0.94 | 0 | ||
| Fasting Plasma Glucose Difference | 3 | −0.01(−0.14, 0.13) | 0.94 | RE | 0.08 | 0.36 | 0.83 | 0 | ||
| Daily Insulin Dose Difference | 7 | −0.58(−0.76, −0.40) | <0.00001 | RE | 6.41 | 13.12 | 0.04 | 54 | ||
| Parallel design only | 5 | −0.54(−0.69, -0.40) | <0.00001 | RE | 7.38 | 5.93 | 0.2 | 32 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatziravdeli, V.; Lambrou, G.I.; Samartzi, A.; Kotsalas, N.; Vlachou, E.; Komninos, J.; Tsartsalis, A.N. A Systematic Review and Meta-Analysis of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Injections in Type-2 Diabetes. Medicina 2023, 59, 141. https://doi.org/10.3390/medicina59010141
Chatziravdeli V, Lambrou GI, Samartzi A, Kotsalas N, Vlachou E, Komninos J, Tsartsalis AN. A Systematic Review and Meta-Analysis of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Injections in Type-2 Diabetes. Medicina. 2023; 59(1):141. https://doi.org/10.3390/medicina59010141
Chicago/Turabian StyleChatziravdeli, Vasiliki, George I. Lambrou, Athanasia Samartzi, Nikolaos Kotsalas, Eugenia Vlachou, John Komninos, and Athanasios N. Tsartsalis. 2023. "A Systematic Review and Meta-Analysis of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Injections in Type-2 Diabetes" Medicina 59, no. 1: 141. https://doi.org/10.3390/medicina59010141
APA StyleChatziravdeli, V., Lambrou, G. I., Samartzi, A., Kotsalas, N., Vlachou, E., Komninos, J., & Tsartsalis, A. N. (2023). A Systematic Review and Meta-Analysis of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Injections in Type-2 Diabetes. Medicina, 59(1), 141. https://doi.org/10.3390/medicina59010141








