Clinical Presentations, Predictive Factors, and Outcomes of Clostridioides difficile Infection among COVID-19 Hospitalized Patients—A Single Center Experience from the COVID Hospital of the University Clinical Center of Vojvodina, Serbia
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
Risk Factors for the Onset of CDI in COVID-19 Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Redd, W.D.; Zhou, J.C.; Hathorn, K.E.; Mccarty, T.R.; Bazarbashi, A.N.; Thompson, C.C.; Shen, L.; Chan, W.W. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020, 159, 765–767.e2. [Google Scholar] [CrossRef] [PubMed]
- Cojocariu, C.; Girleanu, I.; Trifan, A.; Olteanu, A.; Muzica, C.M.; Huiban, L.; Chiriac, S.; Singeap, A.M.; Cuciureanu, T.; Sfarti, C.; et al. Did the severe acute respiratory syndrome-coronavirus 2 pandemic cause an endemic Clostridium difficile infection? World J. Clin. Cases 2021, 9, 10180–10188. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Nije, C.; McClure, E.; Redd, W.D.; Wong, D.; Zhou, J.C.; Bazarbashi, A.N.; McCarty, T.R.; E Hathorn, K.; Shen, L.; et al. Prevalence and impact of Clostridioides difficile infection among hospitalized patients with coranavirus disease 2019. JGH Open 2021, 5, 622–625. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A.H. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sartelli, M.; McKimm, J.; Bin Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug Resist. 2018, ume 11, 2321–2333. [Google Scholar] [CrossRef]
- Singh, T.; Bedi, P.; Bumrah, K.; Singh, J.; Rai, M.; Seelam, S. Updates in Treatment of Recurrent Clostridium difficile Infection. J. Clin. Med. Res. 2019, 11, 465–471. [Google Scholar] [CrossRef]
- Cha, M.H.; Regueiro, M.; Sandhu, D.S. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J. Gastroenterol. 2020, 26, 2323–2331. [Google Scholar] [CrossRef]
- Sultan, S.; Altayar, O.; Siddique, S.M.; Davitkov, P.; Feuerstein, J.D.; Lim, J.K.; Falck-Ytter, Y.; El-Serag, H.B. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020, 159, 320–334.e27. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e948. [Google Scholar] [CrossRef]
- Lakkasani, S.; Chan, K.H.; Shaaban, H.S. Clostridiodes difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020. Emerg. Infect. Dis. 2020, 26, 2299–2300. [Google Scholar] [CrossRef]
- Han, C.; Duan, C.; Zhang, S.; Spiegel, B.; Shi, H.; Wang, W.; Zhang, L.; Lin, R.; Liu, J.; Ding, Z.; et al. Digestive Symptoms in COVID-19 Patients with Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am. J. Gastroenterol. 2020, 115, 916–923. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Living Guidance for Clinical Management of COVID-19: Living Guidance, 23 November 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/349321 (accessed on 4 December 2021).
- Lee, H.S.; Plechot, K.; Gohil, S.; Le, J. Clostridium difficile: Diagnosis and the Consequence of Over Diagnosis. Infect. Dis. Ther. 2021, 10, 687–697. [Google Scholar] [CrossRef]
- Van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27, S1–S21. [Google Scholar] [CrossRef] [PubMed]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.; Rosołowski, M.; Kaniewska, M.; Kucha, P.; Meler, A.; Wierzba, W.; Rydzewska, G. Clostridioides difficile infection in coronavirus disease 2019 (COVID-19): An underestimated problem? Pol. Arch. Intern. Med. 2021, 131, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gavrielatou, E.; Temperikidis, P.; Tsimaras, M.; Magira, E. 707. Hospital-Onset Clostridioides difficile Infection Rates during COVID-19 Pandemic in the ICU Patients. Open Forum Infect. Dis. 2021, 8, S453. [Google Scholar] [CrossRef]
- Davies, K.; Davis, G.; Barbut, F.; Eckert, C.; Petrosillo, N.; Pisapia, R.; Gärtner, B.; Berger, F.K.; Reigadas-Ramirez, E.; Bouza, E.; et al. Factors affecting reported Clostridioides difficile infection rates; the more you look the more you find, but should you believe what you see? Anaerobe 2020, 62, 102178. [Google Scholar] [CrossRef]
- Granata, G.; Bartoloni, A.; Codeluppi, M.; Contadini, I.; Cristini, F.; Fantoni, M.; Ferraresi, A.; Fornabaio, C.; Grasselli, S.; Lagi, F.; et al. The Burden of Clostridioides Difficile Infection during the COVID-19 Pandemic: A Retrospective Case-Control Study in Italian Hospitals (CloVid). J. Clin. Med. 2020, 9, 3855. [Google Scholar] [CrossRef]
- Marinescu, A.R.; Laza, R.; Musta, V.F.; Cut, T.G.; Dumache, R.; Tudor, A.; Porosnicu, M.; Lazureanu, V.E.; Licker, M. Clostridium Difficile and COVID-19: General Data, Ribotype, Clinical Form, Treatment-Our Experience from the Largest Infectious Diseases Hospital in Western Romania. Medicina 2021, 57, 1099. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Spernovasilis, N.A.; Kofteridis, D.P. COVID-19 and antimicrobial stewardship: What is the interplay? Infect. Control Hosp. Epidemiol. 2020, 42, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Fadel, H.J.; Tande, A.J.; Pardi, D.S.; Khanna, S. Outcomes in Patients with SARS-CoV-2 and Clostridioides difficile Coinfection. Infect. Drug Resist. 2021, ume 14, 1645–1648. [Google Scholar] [CrossRef]
- Sehgal, K.; Yadav, D.; Khanna, S. The interplay of Clostridioides difficile infection and inflammatory bowel disease. Ther. Adv. Gastroenterol. 2021, 14, 17562848211020285. [Google Scholar] [CrossRef]
- Rotramel, A.; Poritz, L.S.; Messaris, E.; Berg, A.; Stewart, D.B. PPI Therapy and Albumin are Better Predictors of Recurrent Clostridium difficile Colitis than Choice of Antibiotics. J. Gastrointest. Surg. 2012, 16, 2267–2273. [Google Scholar] [CrossRef]
- Kelly, C.P.; Chen, X.; Williams, D.; Xu, H.; A Cuddemi, C.; Daugherty, K.; Barrett, C.; Miller, M.; Foussadier, A.; Lantz, A.; et al. Host Immune Markers Distinguish Clostridioides difficile Infection From Asymptomatic Carriage and Non–C. difficile Diarrhea. Clin. Infect. Dis. 2019, 70, 1083–1093. [Google Scholar] [CrossRef]
- Spigaglia, P. Clostridioides difficile infection in the COVID-19 era: Old and new problems. Pol. Arch. Intern. Med. 2021, 131, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Spigaglia, P. Clostridioides difficile infection (CDI) during the COVID-19 pandemic. Anaerobe 2022, 74, 102518. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Tillotson, G.; Polistico, J.; Salimnia, H.; Cranis, M.; Moshos, J.; Cullen, L.; Jabbo, L.; Diebel, L.; Chopra, T. Clostridioides difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020. Emerg. Infect. Dis. 2020, 26, 2272–2274. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Ufimtseva, A.; Poluektova, E.; Ulyanin, A. Clostridioides difficile co-infection in patients with COVID-19. Futur. Microbiol. 2022, 17, 653–663. [Google Scholar] [CrossRef]
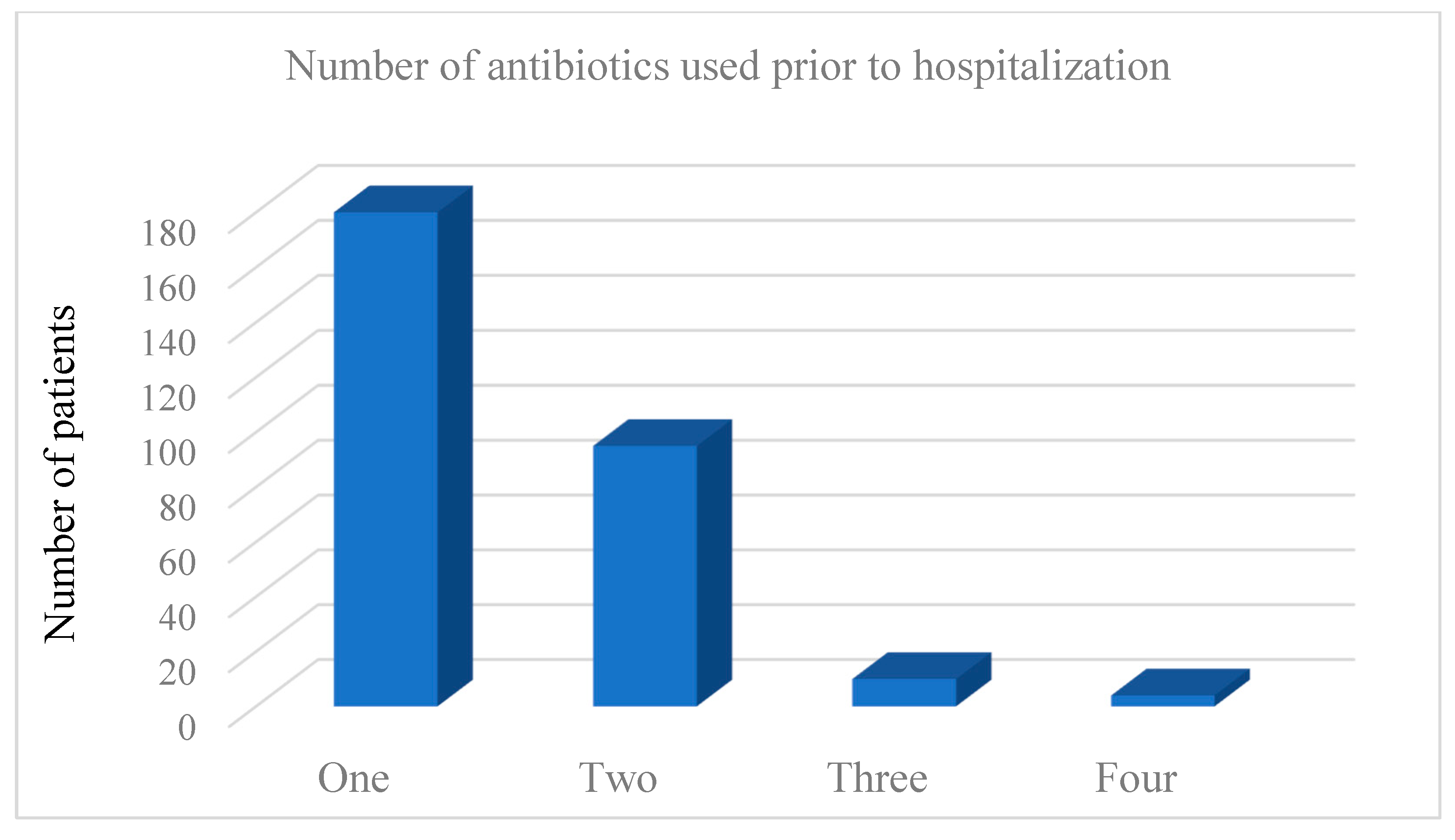
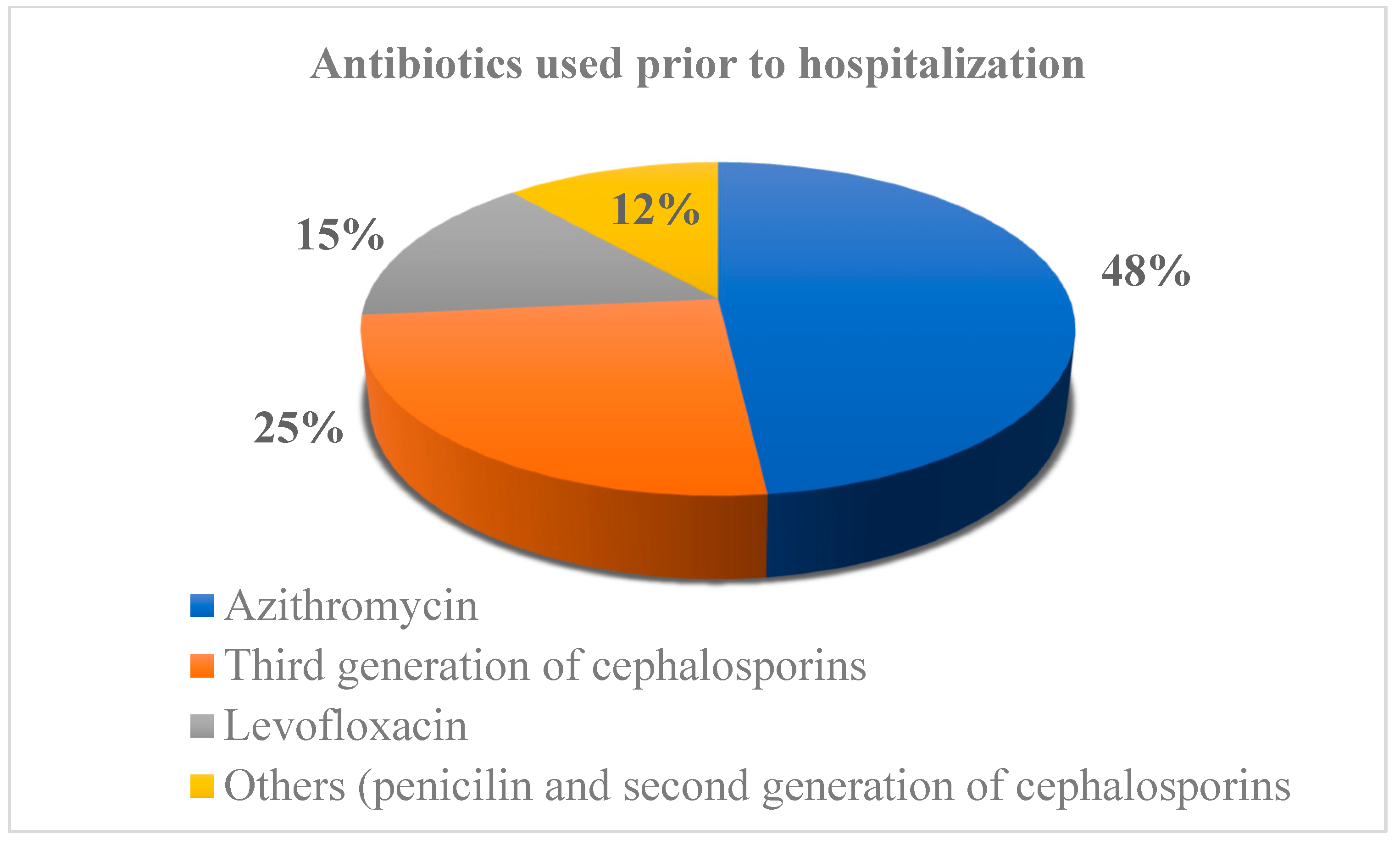
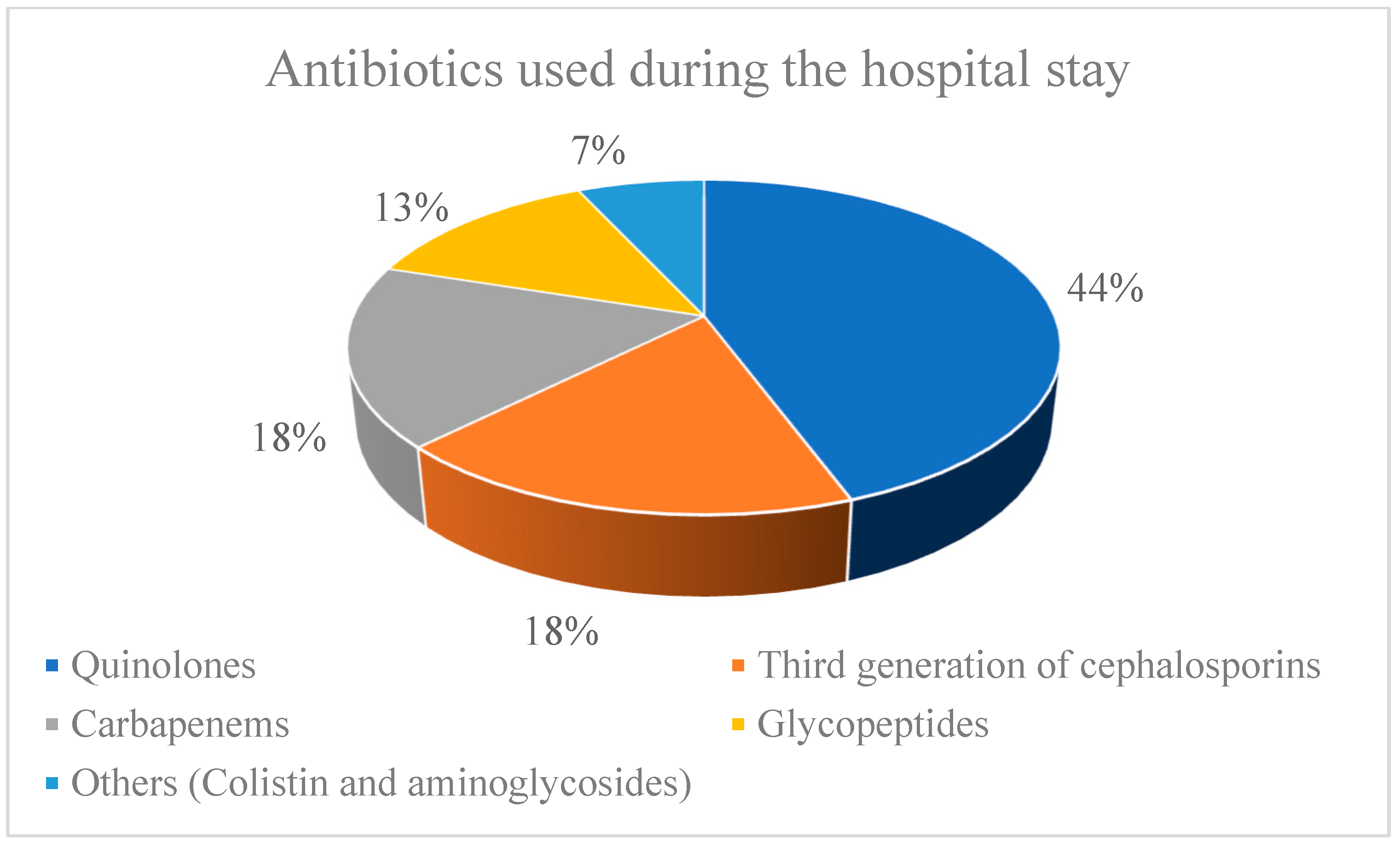
| CDI (n = 326) | Non-CDI Diarrhea (n = 152) | p-Value | |
|---|---|---|---|
| Gender (M) | 197 (60.42%) | 89 (58.55%) | 0.469 |
| Age > 65 years | 289 (88.65%) | 114 (75.0%) | 0.421 |
| Hospitalization in the previous two months | 228 (69.93%) | 59 (38.81%) | 0.029 |
| Diarrhea onset (days) | 12.88 ± 3.51 | 5.61 ± 2.95 | 0.028 |
| Comorbidities | |||
| No comorbidities | 27 (8.28%) | 28 (18.42%) | 0.037 |
| Cardiovascular disease | 39 (11.96%) | 25 (16.44%) | 0.541 |
| Diabetes | 99 (30.37%) | 50 (32.89%) | 0.912 |
| Chronic renal failure | 50 (15.33%) | 19 (12.50%) | 0.769 |
| Malignancies | 87 (26.68%) | 27 (17.76%) | 0.063 |
| Neurological disease | 62 (19.01%) | 26 (17.11%) | 0.841 |
| Chronic liver failure | 43 (13.19%) | 13 (8.55%) | 0.443 |
| Chronic pulmonary disease | 125 (38.34%) | 40 (26.31%) | 0.296 |
| Concomitant bacterial infections at admission | 120 (36.81%) | 39 (25.65%) | 0.078 |
| Medication before hospital admission | |||
| Proton pump inhibitors | 173 (53.06%) | 84 (55.26%) | 0.902 |
| Antibiotics | 286 (87.73%) | 96 (63.16%) | 0.042 |
| Steroids + | 112 (34.35%) | 57 (37.50%) | 0.759 |
| Laboratory results | |||
| White blood cell count ≥ 15 × 103/uL | 143 (43.86%) | 44 (28.94%) | 0.021 |
| Albumin ≤ 25 g/L | 129 (39.57%) | 33 (21.71%) | 0.019 |
| Creatinine ≥ 1.5 mg/dL | 162 (49.69%) | 71 (46.71%) | 0.699 |
| C-reactive protein mean (± SD) | 129.44 (±48.69) | 94.70 (±38.52) | 0.076 |
| COVID-19 severity | |||
| Mild pneumonia | 68 (20.86%) | 59 (38.81%) | 0.288 |
| Severe pneumonia | 258 (79.14%) | 96 (63.15%) | 0.193 |
| Medication during hospital stay | |||
| Proton pump inhibitors | 262 (80.37%) | 110 (72.36%) | 0.764 |
| Antibiotics | 289 (88.65%) | 104 (68.42%) | 0.037 |
| Steroids + | 217 (66.56%) | 105 (69.07%) | 0.828 |
| CDI severity | |||
| Mild | 87 (26.69%) | ||
| Severe | 207 (63.49%) | ||
| Severe complicated | 32 (9.81%) | ||
| Total length of hospital stay (days) | 25.68 (±13.52) | 14.34 (±8.74) | 0.044 |
| Patient outcomes | |||
| Recovered | 258 (79.14%) | 129 (84.87%) | |
| Deceased | 68 (20.86%) | 23 (15.13%) | 0.097 |
| OR Odds Ratio | 95% CI Confidence Interval | p-Value | |
|---|---|---|---|
| Hospitalization in the preceding two months | 2.364 | 1.328–4.786 | 0.021 |
| Antibiotics before hospital admission | 1.203 | 1.042–1.969 | 0.193 |
| Antibiotics during hospital stay | 1.496 | 1.039–1.961 | 0.025 |
| Diarrhea onset after COVID-19 diagnosis | 0.759 | 0.284–1.128 | 0.367 |
| White blood cell count of ≥ 15 × 103/μL | 0.187 | 0.053–0.654 | 0.084 |
| Albumin level of ≤ 25 g/L | 4.153 | 2.368–6.412 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, N.; Lendak, D.; Popović, M.; Plećaš Đuric, A.; Pete, M.; Petrić, V.; Sević, S.; Tomić, S.; Alargić, J.; Damjanov, D.; et al. Clinical Presentations, Predictive Factors, and Outcomes of Clostridioides difficile Infection among COVID-19 Hospitalized Patients—A Single Center Experience from the COVID Hospital of the University Clinical Center of Vojvodina, Serbia. Medicina 2022, 58, 1262. https://doi.org/10.3390/medicina58091262
Kovačević N, Lendak D, Popović M, Plećaš Đuric A, Pete M, Petrić V, Sević S, Tomić S, Alargić J, Damjanov D, et al. Clinical Presentations, Predictive Factors, and Outcomes of Clostridioides difficile Infection among COVID-19 Hospitalized Patients—A Single Center Experience from the COVID Hospital of the University Clinical Center of Vojvodina, Serbia. Medicina. 2022; 58(9):1262. https://doi.org/10.3390/medicina58091262
Chicago/Turabian StyleKovačević, Nadica, Dajana Lendak, Milica Popović, Aleksandra Plećaš Đuric, Maria Pete, Vedrana Petrić, Siniša Sević, Slavica Tomić, Jelica Alargić, Dimitrije Damjanov, and et al. 2022. "Clinical Presentations, Predictive Factors, and Outcomes of Clostridioides difficile Infection among COVID-19 Hospitalized Patients—A Single Center Experience from the COVID Hospital of the University Clinical Center of Vojvodina, Serbia" Medicina 58, no. 9: 1262. https://doi.org/10.3390/medicina58091262
APA StyleKovačević, N., Lendak, D., Popović, M., Plećaš Đuric, A., Pete, M., Petrić, V., Sević, S., Tomić, S., Alargić, J., Damjanov, D., Kosjer, D., & Lekin, M. (2022). Clinical Presentations, Predictive Factors, and Outcomes of Clostridioides difficile Infection among COVID-19 Hospitalized Patients—A Single Center Experience from the COVID Hospital of the University Clinical Center of Vojvodina, Serbia. Medicina, 58(9), 1262. https://doi.org/10.3390/medicina58091262








