Neuropathic Pain Component in Patients with Cervical Radicular Pain: A Single-Center Retrospective Study
Abstract
:1. Introduction
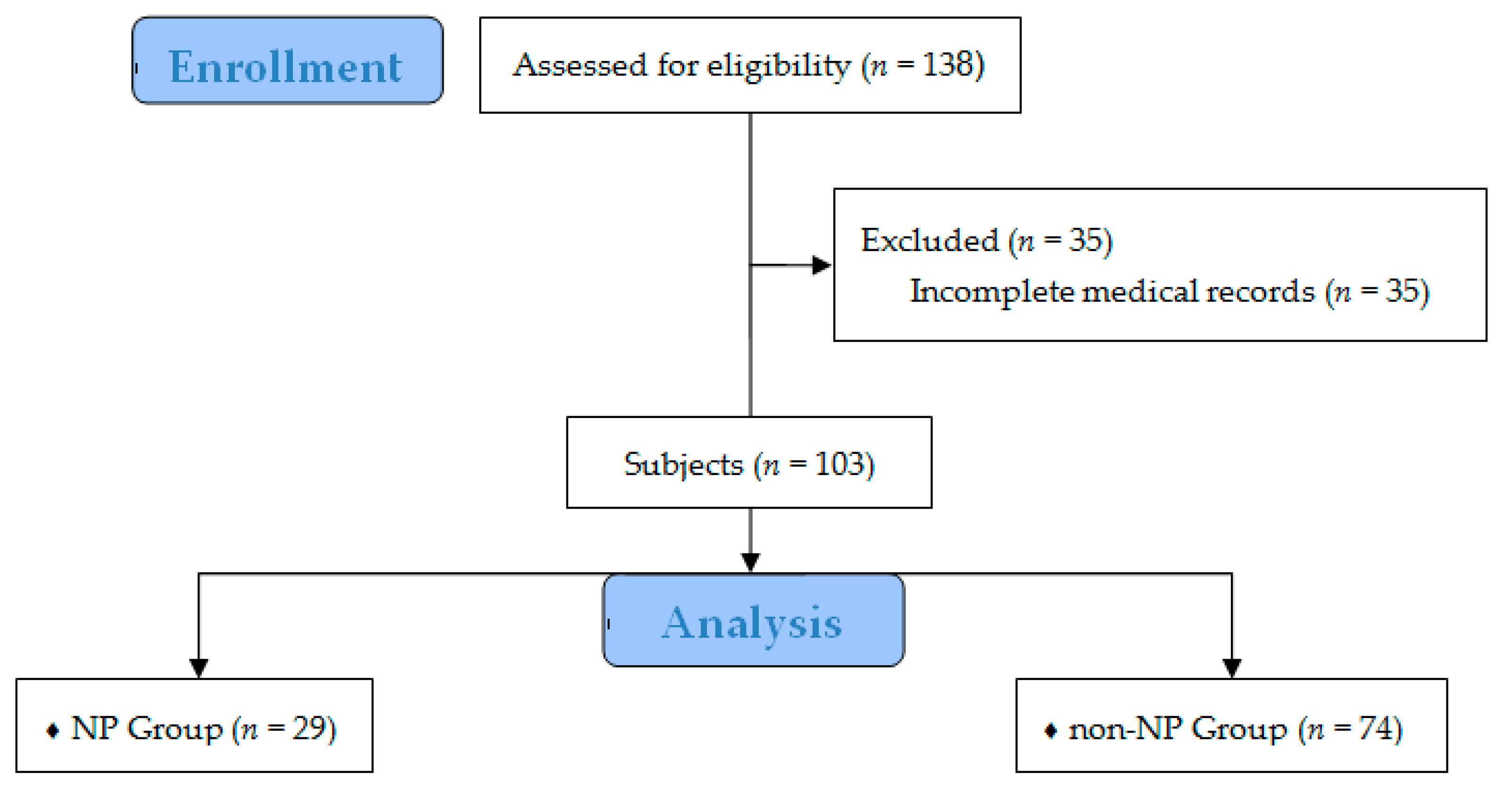
2. Materials and Methods
2.1. Study Population and Data Analysis
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Zundert, J.; Huntoon, M.; Patijn, J.; Lataster, A.; Mekhail, N.; van Kleef, M. 4. Cervical radicular pain. Pain Pract. 2010, 10, 1–17. [Google Scholar] [CrossRef]
- Woods, B.I.; Hilibrand, A.S. Cervical radiculopathy: Epidemiology, etiology, diagnosis, and treatment. J. Spinal Disord. Tech. 2015, 28, E251–E259. [Google Scholar] [CrossRef]
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef]
- Park, S.Y.; An, H.S.; Moon, S.H.; Lee, H.M.; Suh, S.W.; Chen, D.; Jeon, J.H. Neuropathic Pain Components in Patients with Lumbar Spinal Stenosis. Yonsei. Med. J. 2015, 56, 1044–1050. [Google Scholar] [CrossRef]
- Cohen, S.P. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin. Proc. 2015, 90, 284–299. [Google Scholar] [CrossRef]
- Yang, C.W.; Fuh, J.L. Screening tools for neuropathic pain. J. Chin. Med. Assoc. 2018, 81, 1–3. [Google Scholar] [CrossRef]
- Smith, B.H.; Torrance, N. Epidemiology of neuropathic pain and its impact on quality of life. Curr. Pain Headache Rep. 2012, 16, 191–198. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truimi, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Haanpää, M.; Attal, N.; Backonja, M.; Baron, R.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Haythornthwaite, J.A.; DomenicoIannetti, G.; et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011, 152, 14–27. [Google Scholar] [CrossRef]
- Attal, N.; Perrot, S.; Fermanian, J.; Bouhassira, D. The neuropathic components of chronic low back pain: A prospective multicenter study using the DN4 Questionnaire. J. Pain 2011, 12, 1080–1087. [Google Scholar] [CrossRef]
- Epping, R.; Verhagen, A.P.; Hoebink, E.A.; Rooker, S.; Scholten-Peeters, G.G.M. The diagnostic accuracy and test-retest reliability of the Dutch PainDETECT and the DN4 screening tools for neuropathic pain in patients with suspected cervical or lumbar radiculopathy. Musculoskelet. Sci. Pract. 2017, 30, 72–79. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, S.H.; Hwang, C.J.; Cho, Y.E. Prevalence of Neuropathic Pain in Patients Scheduled for Lumbar Spine Surgery: Nationwide, Multicenter, Prospective Study. Pain Physician 2015, 18, E889–E897. [Google Scholar]
- Walsh, J.; Rabey, M.I.; Hall, T.M. Agreement and correlation between the self-report leeds assessment of neuropathic symptoms and signs and Douleur Neuropathique 4 Questions neuropathic pain screening tools in subjects with low back-related leg pain. J. Manip. Physiol. Ther. 2012, 35, 196–202. [Google Scholar] [CrossRef]
- Beith, I.D.; Kemp, A.; Kenyon, J.; Prout, M.; Chestnut, T.J. Identifying neuropathic back and leg pain: A cross-sectional study. Pain 2011, 152, 1511–1516. [Google Scholar] [CrossRef]
- Gudala, K.; Ghai, B.; Bansal, D. Usefulness of four commonly used neuropathic pain screening questionnaires in patients with chronic low back pain: A cross-sectional study. Korean J. Pain 2017, 30, 51–58. [Google Scholar] [CrossRef]
- Abbed, K.M.; Coumans, J.V. Cervical radiculopathy: Pathophysiology, presentation, and clinical evaluation. Neurosurgery 2007, 60, S28–S34. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Song, K.J.; Choi, B.W.; Choi, B.R.; Seo, G.B. Cross-cultural adaptation and validation of the Korean version of the neck disability index. Spine 2010, 35, E1045–E1049. [Google Scholar] [CrossRef]
- Liu, R.; Kurihara, C.; Tsai, H.T.; Silvestri, P.J.; Bennett, M.I.; Pasquina, P.F.; Cohen, S.P. Classification and Treatment of Chronic Neck Pain: A Longitudinal Cohort Study. Reg. Anesth. Pain Med. 2017, 42, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Sanal-Toprak, C.; Ozturk, E.C.; Yucel, F.N.; Sencan, S.; Gunduz, O.H. Does the presence of neuropathic pain affect the outcomes of the interlaminar epidural steroid injection for cervical disc herniation?: A prospective clinical study. Medicine 2021, 100, e25012. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Attal, N.; Casale, R.; Dickenson, A.H.; Treede, R.D. Neuropathic low back pain in clinical practice. Eur. J. Pain 2016, 20, 861–873. [Google Scholar] [CrossRef]
- Tampin, B.; Briffa, N.K.; Goucke, R.; Slater, H. Identification of neuropathic pain in patients with neck/upper limb pain: Application of a grading system and screening tools. Pain 2013, 154, 2813–2822. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Jovicic, M.D.; Konstantinovic, L.M.; Grgurevic, A.D.; Milovanovic, N.D.; Trajkovic, G.; Jovicic, V.Z.; Kostic Dedic, S.I.; Hrkovic, M.K.; Draganac, S.M. Validation of the Neck Disability Index in Serbian Patients With Cervical Radiculopathy. J. Manip. Physiol. Ther. 2018, 41, 496–502. [Google Scholar] [CrossRef]
- Dower, A.; Davies, M.A.; Ghahreman, A. Pathologic Basis of Lumbar Radicular Pain. World Neurosurg. 2019, 128, 114–121. [Google Scholar] [CrossRef]
- Morlion, B. Pharmacotherapy of low back pain: Targeting nociceptive and neuropathic pain components. Curr. Med. Res. Opin. 2011, 27, 11–33. [Google Scholar] [CrossRef]

| Variables | Total (n = 103) | NP Component | p Value | |
|---|---|---|---|---|
| Yes (n = 29) | No (n = 74) | |||
| Sex | ||||
| male | 68 (66.0) | 20 (69.0) | 48 (64.9) | 0.693 1 |
| female | 35 (34.0) | 9 (31.0) | 26 (35.1) | |
| Age | 56.55 ± 10.14 | 57.03 ± 9.36 | 56.36 ± 10.48 | 0.765 3 |
| Height | 167.01 ± 7.28 | 168.48 ± 8.17 | 166.43 ± 6.88 | 0.200 3 |
| Weight | 66.33 ± 10.01 | 67.66 ± 10.17 | 65.81 ± 9.97 | 0.308 4 |
| BMI | 23.85 ± 3.07 | 24.26 ± 3.70 | 23.70 ± 2.80 | 0.521 4 |
| DM | 15 (14.6) | 2 (6.9) | 13 (17.6) | 0.223 2 |
| HTN | 29 (28.2) | 8 (27.6) | 21 (28.4) | 0.936 1 |
| OP history | 9 (8.7) | 4 (13.8) | 5 (6.8) | 0.265 2 |
| Duration | ||||
| acute | 63 (61.2) | 16 (55.2) | 47 (63.5) | 0.435 1 |
| chronic | 40 (38.8) | 13 (44.8) | 27 (36.5) | |
| DN4 | 3.30 ± 2.04 | 5.66 ± 1.59 | 2.38 ± 1.33 | <0.001 4 |
| PD-Q | 10.17 ± 5.28 | 16.14 ± 4.29 | 7.84 ± 3.49 | <0.001 4 |
| NDI (45) | 19.94 ± 7.69 | 23.79 ± 6.35 | 18.43 ± 7.68 | <0.001 4 |
| NRS neck | 56.12 ± 27.45 | 61.38 ± 22.16 | 54.05 ± 29.14 | 0.252 4 |
| NRS arm | 68.54 ± 20.60 | 70.00 ± 18.71 | 67.97 ± 21.39 | 0.651 4 |
| Dominant site | ||||
| arm | 48 (46.6) | 13 (44.8) | 35 (47.3) | 0.795 1 |
| neck | 14 (13.6) | 5 (17.2) | 9 (12.2) | |
| non-dominant | 41 (39.8) | 11 (37.9) | 30 (40.5) | |
| NRS dominant site | 71.07 ± 20.04 | 73.10 ± 18.34 | 70.27 ± 20.74 | 0.474 4 |
| r | p Value | |
|---|---|---|
| DN4 | 0.221 | 0.025 |
| PD-Q | 0.368 | <0.001 |
| DN4 | |||
|---|---|---|---|
| PD-Q | Positive | Negative | Total |
| Positive | 29 | 5 | 34 |
| Negative | 13 | 56 | 69 |
| Total | 42 | 61 | 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Oh, D.; Lee, B.; Lee, H.; Ko, M.; Moon, S.; Park, Y.; Kim, S.; Kim, S. Neuropathic Pain Component in Patients with Cervical Radicular Pain: A Single-Center Retrospective Study. Medicina 2022, 58, 1191. https://doi.org/10.3390/medicina58091191
Kwon J, Oh D, Lee B, Lee H, Ko M, Moon S, Park Y, Kim S, Kim S. Neuropathic Pain Component in Patients with Cervical Radicular Pain: A Single-Center Retrospective Study. Medicina. 2022; 58(9):1191. https://doi.org/10.3390/medicina58091191
Chicago/Turabian StyleKwon, Jiyeon, Daeseok Oh, Byeongcheol Lee, Hyunseong Lee, Myoungjin Ko, Sungho Moon, Yeiheum Park, Sehun Kim, and Sunyoung Kim. 2022. "Neuropathic Pain Component in Patients with Cervical Radicular Pain: A Single-Center Retrospective Study" Medicina 58, no. 9: 1191. https://doi.org/10.3390/medicina58091191
APA StyleKwon, J., Oh, D., Lee, B., Lee, H., Ko, M., Moon, S., Park, Y., Kim, S., & Kim, S. (2022). Neuropathic Pain Component in Patients with Cervical Radicular Pain: A Single-Center Retrospective Study. Medicina, 58(9), 1191. https://doi.org/10.3390/medicina58091191






