Association of Parathyroid and Differentiated Thyroid Carcinomas: A Narrative Up-To-Date Review of the Literature
Abstract
1. Background/Introduction
2. Methods
3. Results
4. Discussion
4.1. Etiopathology of PC and DTC
4.2. Clinical Manifestations
4.3. Biological Features
4.4. Imaging
4.5. FNA
4.6. Treatment Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fraser, W.D. Hyperparathyroidism. Lancet 2009, 374, 145–158. [Google Scholar] [CrossRef]
- Cetani, F.; Pardi, E.; Marcocci, C. Update on parathyroid carcinoma. J. Endocrinol. Investig. 2016, 39, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.M.P.; Paiva, C.; Correia, R.; Polónia, J.; Moreira da Costa, A. Parathyroid carcinoma: From a case report to a review of the literature. Int. J. Surg. Case Rep. 2018, 42, 214–217. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, thyroid carcinoma. Nccn.org Web site. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 29 April 2022).
- Lorenz, K.; Schneider, R.; Elwerr, M. Thyroid Carcinoma: Do We Need to Treat Men and Women Differently? Visc. Med. 2020, 36, 10–14. [Google Scholar] [CrossRef]
- Li, M.; Dal Maso, L.; Vaccarella, S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020, 8, 468–470. [Google Scholar] [CrossRef]
- Kilfoy, B.A.; Devesa, S.S.; Ward, M.H.; Zhang, Y.; Rosenberg, P.S.; Holford, T.R.; Anderson, W.F. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1092–1100. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Lam-Chung, C.E.; Rodriguez-Orihuela, D.L.; Anda Gonzalez, J.; Gamboa-Dominguez, A. An Unusual Simultaneous Existence of Parathyroid Carcinoma and Papillary Thyroid Carcinoma: Case Report and Review of Literature. Case Rep. Endocrinol. 2020, 2020, 2128093. [Google Scholar] [CrossRef]
- De Falco, N.; Santangelo, G.; Chirico, F.; Cangiano, A.; Sommella, M.G.; Cosenza, A.; Ronchi, A.; Accardo, M.; Pellino, G.; Parmeggiani, D.; et al. Synchronous intrathyroidal parathyroid carcinoma and thyroid carcinoma: Case report and review of the literature. BMC Endocr. Disord. 2021, 21, 60. [Google Scholar] [CrossRef]
- Song, C.; Wang, J.; Cai, X.; Gao, L. Synchronous parathyroid carcinoma and papillary thyroid carcinoma: A case study and review of literature. Int. J. Clin. Exp. Pathol. 2016, 9, 302–309. [Google Scholar]
- D’cruz, R.T.; Seet, J.E.; Parameswaran, R. Synchronous symptomatic parathyroid carcinoma and parathyroid adenoma with incidental follicular thyroid carcinoma. Ann. R. Coll. Surg. Engl. 2020, 102, e192–e195. [Google Scholar] [CrossRef] [PubMed]
- Preda, C.; Branisteanu, D.; Armasu, I.; Danila, R.; Velicescu, C.; Ciobanu, D.; Covic, A.; Grigorovici, A. Coexistent papillary thyroid carcinoma diagnosed in surgically treated patients for primary versus secondary hyperparathyroidism: Same incidence, different characteristics. BMC Surg. 2019, 19, 94. [Google Scholar] [CrossRef]
- Javadi, H.; Jallalat, S.; Farrokhi, S.; Semnani, S.; Mogharrabi, M.; Riazi, A.; Nabipour, I.; Moshtaghi, D.; Assadi, M. Concurrent papillary thyroid cancer and parathyroid adenoma as a rare condition: A case report. Nucl. Med. Rev. Cent. East. Eur. 2012, 15, 153–155. [Google Scholar] [PubMed]
- Colin, I.M.; Denef, J.F.; Lengele, B.; Many, M.C.; Gerard, A.C. Recent insights into the cell biology of thyroid angiofollicular units. Endocr. Rev. 2013, 34, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.; Howlett, D.; El Teraifi, H.; Kirkland, P. Association of synchronous medullary and papillary thyroid carcinomas with primary hyperparathyroidism: First case report and literature review. J. Laryngol. Otol. 2014, 128, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Koea, J.B.; Shaw, J.H. Parathyroid cancer: Biology and management. Surg. Oncol. 1999, 8, 155–165. [Google Scholar] [CrossRef]
- Schneider, A.B.; Recant, W.; Pinsky, S.M.; Ryo, U.Y.; Bekerman, C.; Shore-Freedman, E. Radiation-induced thyroid carcinoma. Clinical course and results of therapy in 296 patients. Ann. Intern. Med. 1986, 105, 405–412. [Google Scholar] [CrossRef]
- Schulte, K.M.; Talat, N.; Galata, G.; Gilbert, J.; Miell, J.; Hofbauer, L.C.; Barthel, A.; Diaz-Cano, S.; Bornstein, S.R. Oncologic resection achieving r0 margins improves disease-free survival in parathyroid cancer. Ann. Surg. Oncol. 2014, 21, 1891–1897. [Google Scholar] [CrossRef]
- Digonnet, A.; Carlier, A.; Willemse, E.; Quiriny, M.; Dekeyser, C.; de Saint Aubain, N.; Lemort, M.; Andry, G. Parathyroid carcinoma: A review with three illustrative cases. J. Cancer 2011, 2, 532–537. [Google Scholar] [CrossRef]
- Dikmen, K.; Bostanci, H.; Gobut, H.; Yildiz, A.; Ertunc, O.; Celik, A.; Akin, M.; Taneri, F. Nonfunctional double parathyroid carcinoma with incidental thyroid micropapillary carcinoma: A rare case. Pan Afr. Med. J. 2017, 27, 241. [Google Scholar] [CrossRef]
- Paschke, R.; Lincke, T.; Müller, S.P.; Kreissl, M.C.; Dralle, H.; Fassnacht, M. The Treatment of Well-Differentiated Thyroid Carcinoma. Dtsch. Ärzteblatt Int. 2015, 112, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.H.; Harari, A. Parathyroid carcinoma: Update and guidelines for management. Curr. Treat. Options Oncol. 2012, 13, 11–23. [Google Scholar] [CrossRef]
- Shane, E.; Bilezikian, J.P. Parathyroid carcinoma: A review of 62 patients. Endocr. Rev. 1982, 3, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Ball, D.W.; Byrd, D.; Dilawari, R.A.; Doherty, G.M.; Duh, Q.Y.; Ehya, H.; Farrar, W.B.; Haddad, R.I.; Kandeel, F.; et al. Thyroid carcinoma. J. Natl. Compr. Cancer Netw. 2010, 8, 1228–1274. [Google Scholar] [CrossRef]
- Long, K.L.; Sippel, R.S. Current and future treatments for parathyroid carcinoma. Int. J. Endocr. Oncol. 2018, 5, IJE06. [Google Scholar] [CrossRef]
- Agarwal, G.; Dhingra, S.; Mishra, S.K.; Krishnani, N. Implantation of parathyroid carcinoma along fine needle aspiration track. Langenbecks Arch. Surg. 2006, 391, 623–626. [Google Scholar] [CrossRef]
- Spinelli, C.; Bonadio, A.G.; Berti, P.; Materazzi, G.; Miccoli, P. Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology. J. Endocrinol. Investig. 2000, 23, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Hoelting, T.; Weber, T.; Werner, J.; Herfarth, C. Surgical treatment of parathyroid carcinoma (Review). Oncol. Rep. 2001, 8, 931–934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ippolito, G.; Palazzo, F.F.; Sebag, F.; De Micco, C.; Henry, J.F. Intraoperative diagnosis and treatment of parathyroid cancer and atypical parathyroid adenoma. Br. J. Surg. 2007, 94, 566–570. [Google Scholar] [CrossRef]
- Lee, P.K.; Jarosek, S.L.; Virnig, B.A.; Evasovich, M.; Tuttle, T.M. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer 2007, 109, 1736–1741. [Google Scholar] [CrossRef]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R.; The American College of Surgeons Commission on Cancer and the American Cancer Society. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985–1995: A National Cancer Data Base Report. Cancer 1999, 86, 538–544. [Google Scholar] [CrossRef]
- Kalthoum, M.; Nacef, I.B.; Mekni, S.; Rojbi, I.; Mechirgui, N.; Khiari, K. Synchronous parathyroid carcinoma and papillary thyroid carcinoma: A case report. Endocr. Abstr. 2020, 70, EP95. [Google Scholar] [CrossRef]
- Edafe, O.; Debono, M.; Tahir, F.; Balasubramanian, S.P. Simultaneous presentation of parathyroid carcinoma, papillary thyroid cancer and ACTH-independent hypercortisolism due to benign cortical adenoma. BMJ Case Rep. 2019, 12, e230438. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, F.; Cinkaya, A.; Ekici, M.; Kodaz, H.; Deger, A. Synchronous parathyroid carcinoma and multifocal papillary thyroid carcinoma: A case report. Eurasian J. Med. Oncol. 2017, 1, 49–52. [Google Scholar]
- Baek, C.-O.; Kim, K.H.; Song, S.K. Synchronous parathyroid carcinoma and papillary thyroid carcinoma in a patient with long-standing schizophrenia. Korean J. Intern. Med. (Korean Assoc. Intern. Med.) 2017, 32, 1104–1107. [Google Scholar] [CrossRef]
- Demir, D. A very rare case, coexistence of invasive thyroid papillary carcinoma and parathyroid carcinoma in hypercalcemic 29 year old woman how is treated for urolithiasis. Diabetes Metab. Disord. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- Aljabri, K.S.; Bokhari, S.A.; Alshareef, M.A.; Khan, P.M.; Abdulhafez, D.A.; Aljabri, B.K. Coexistence of parathyroid cancer and papillary thyroid cancer: A case report with a review of the literature. EC Endocrinol. Metab. Res. 2017, 81, 98–102. [Google Scholar]
- Neslihan, S.A.; Sibel, G.; Ebru, T.; Hüseyin, Ç.; Atakan, S.; Nermin, T.; Armağan, T. A Rare Co-Occurrence of Parathyroid and Papillary Thyroid Carcinoma. ARC J. Clin. Case Rep. 2016, 2, 1–4. [Google Scholar]
- Lee, H.W.; Kim, H.J.; Moon, J.S. A case of recurred parathyroid carcinoma with multiple lymph node metastasis: Consurrent with papillary thyroid cancer. In Proceedings of the 18th European Congress of Endocrinology 2016, Munich, Germany, 28–31 May 2016. [Google Scholar]
- Al-Sulami, S.S. Parathyroid carcinoma: Atypical presentation and coexistence with papillary thyroid cancer. Saudi J. Med. Med. Sci. 2015, 3, 245–246. [Google Scholar] [CrossRef]
- Zakerkish, M.; Rajaei, E.; Dargahi, M.; Bahadoram, M. A Rare Constellation of Hürthle Cell Thyroid Carcinoma and Parathyroid Carcinoma. J. Clin. Diagn. Res. 2015, 9, OD08–OD10. [Google Scholar] [CrossRef]
- Chaychi, L.; Belbruno, K.; Golding, A.; Memoli, V. Unusual manifestation of parathyroid carcinoma in the setting of papillary thyroid cancer. Endocr. Pract. 2010, 16, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Marcy, P.Y.; Thariat, J.; Sudaka, A.; Poissonnet, G. Synchronous parathyroid and papillary thyroid carcinomas. Thyroid 2009, 19, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, M.; O’Neal, P.; Shih, J.L.; Hartzband, P.; Connolly, J.; Hasselgren, P.O. Synchronous parathyroid carcinoma, parathyroid adenoma, and papillary thyroid carcinoma in a patient with severe and long-standing hyperparathyroidism. Endocr. Pract. 2009, 15, 463–468. [Google Scholar] [CrossRef]
- Mazeh, H.; Kouniavsky, G.; Schneider, D.F.; Makris, K.I.; Sippel, R.S.; Dackiw, A.P.; Chen, H.; Zeiger, M.A. Intrathyroidal parathyroid glands: Small, but mighty (a Napoleon phenomenon). Surgery 2012, 152, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.D.; Tu, S.T.; Hsu, S.R.; Chang, J.H.; Yang, K.T.; Yang, L.H. Synchronous parathyroid and papillary thyroid carcinoma. J. Chin. Med. Assoc. 2005, 68, 87–91. [Google Scholar] [CrossRef]
- Kern, M.; Lee, G.; Robbins, P.; Bynevelt, M.; Watson, P. Intracranial metastatic parathyroid carcinoma. Case report and review of the literature. J. Neurosurg. 2004, 101, 1065–1069. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; Melissas, J.; Kafousi, M.; Karkavitsas, N.; Tsiftsis, D.D. Synchronous parathyroid and papillary thyroid carcinoma: A case report. Am. J. Otolaryngol. 2002, 23, 382–385. [Google Scholar] [CrossRef]
- Bednarek-Tupikowska, G.; Tołłoczko, T.; Tupikowski, W.; Bogdańska, M.; Karwacki, J.; Medraś, M.; Milewicz, A. Coexistence of parathyroid carcinoma and non-medullary carcinoma of the thyroid. Med. Sci. Monit. 2001, 7, 448–456. [Google Scholar]
- Savli, H.; Sevinc, A.; Sari, R.; Ozen, S.; Buyukberber, S.; Ertas, E. Occult parathyroid carcinoma in a patient with papillary thyroid carcinoma and Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2001, 24, 42–44. [Google Scholar] [CrossRef]
- Kurita, S.; Mihashi, S.; Hirano, M.; Nakashima, T.; Tanimura, A. [Hyperfunctioning parathyroid carcinoma combined with papillary carcinoma of the thyroid gland--report of a case (author’s transl)]. Nihon Gan Chiryo Gakkai Shi 1979, 14, 1127–1135. [Google Scholar]
- Basceken, S.I.; Genc, V.; Ersoz, S.; Sevim, Y.; Celik, S.U.; Bayram, I.K. Is local resection sufficient for parathyroid carcinoma? Clinics 2015, 70, 247–249. [Google Scholar] [CrossRef]
- Tseleni-Balafouta, S.; Gakiopoulou, H.; Kavantzas, N.; Agrogiannis, G.; Givalos, N.; Patsouris, E. Parathyroid proliferations: A source of diagnostic pitfalls in FNA of thyroid. Cancer 2007, 111, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Christmas, T.J.; Chapple, C.R.; Noble, J.G.; Milroy, E.J.; Cowie, A.G. Hyperparathyroidism after neck irradiation. Br. J. Surg. 1988, 75, 873–874. [Google Scholar] [CrossRef]
- Amoodi, H.A.; Makki, F.M.; Mark Taylor, S.; Bullock, M.J.; Hart, R.D.; Trites, J.R. Synchronous Thyroid/parathyroid Carcinomas. J. Otolaryngol. Head Neck Surg. 2010, 39, E42–E47. [Google Scholar] [PubMed]
- Torresan, F.; Iacobone, M. Clinical Features, Treatment, and Surveillance of Hyperparathyroidism-Jaw Tumor Syndrome: An Up-to-Date and Review of the Literature. Int. J. Endocrinol. 2019, 2019, 1761030. [Google Scholar] [CrossRef]
- Kebebew, E. Parathyroid carcinoma. Curr. Treat. Options Oncol. 2001, 2, 347–354. [Google Scholar] [CrossRef]
- Sharretts, J.M.; Simonds, W.F. Clinical and molecular genetics of parathyroid neoplasms. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 491–502. [Google Scholar] [CrossRef]
- McMullen, T.; Bodie, G.; Gill, A.; Ihre-Lundgren, C.; Shun, A.; Bergin, M.; Stevens, G.; Delbridge, L. Hyperparathyroidism after irradiation for childhood malignancy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1164–1168. [Google Scholar] [CrossRef]
- Rasmuson, T.; Damber, L.; Johansson, L.; Johansson, R.; Larsson, L.G. Increased incidence of parathyroid adenomas following X-ray treatment of benign diseases in the cervical spine in adult patients. Clin. Endocrinol. 2002, 57, 731–734. [Google Scholar] [CrossRef]
- Ferraro, V.; Sgaramella, L.I.; Di Meo, G.; Prete, F.P.; Logoluso, F.; Minerva, F.; Noviello, M.; Renzulli, G.; Gurrado, A.; Testini, M. Current concepts in parathyroid carcinoma: A single centre experience. BMC Endocr. Disord. 2019, 19 (Suppl. S1), 46. [Google Scholar] [CrossRef]
- Dudney, W.C.; Bodenner, D.; Stack, B.C., Jr. Parathyroid carcinoma. Otolaryngol. Clin. North Am. 2010, 43, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Givi, B.; Shah, J.P. Parathyroid carcinoma. Clin. Oncol. (R. Coll. Radiol.) 2010, 22, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.N.; Wilhelm, S.M. Parathyroid Cancer: A Review. Cancers 2019, 11, 1676. [Google Scholar] [CrossRef]
- Busaidy, N.L.; Jimenez, C.; Habra, M.A.; Schultz, P.N.; El-Naggar, A.K.; Clayman, G.L.; Asper, J.A.; Diaz, E.M., Jr.; Evans, D.B.; Gagel, R.F.; et al. Parathyroid carcinoma: A 22-year experience. Head Neck 2004, 26, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Piciu, D.; Irimie, A.; Kontogeorgos, G.; Piciu, A.; Buiga, R. Highly aggressive pathology of non-functional parathyroid carcinoma. Orphanet J. Rare Dis. 2013, 8, 115. [Google Scholar] [CrossRef]
- Wilkins, B.J.; Lewis, J.S., Jr. Non-functional parathyroid carcinoma: A review of the literature and report of a case requiring extensive surgery. Head Neck Pathol. 2009, 3, 140–149. [Google Scholar] [CrossRef]
- Poppe, K.; Pipeleers-Marichal, M.; Flamen, P.; Bossuyt, A.; Lamote, J.; Vanhaelst, L.; Velkeniers, B. Non-secreting atypical parathyroid adenoma. J. Endocrinol. Investig. 2001, 24, 107–110. [Google Scholar] [CrossRef]
- Sen, M.; Nagaoka, R.; Kazusaka, H.; Matsui, M.; Saitou, M.; Sugitani, I.; Sakatani, T.; Kameyama, K. Non-functioning oxyphilic parathyroid carcinoma: A case report. Surg. Case Rep. 2021, 7, 119. [Google Scholar] [CrossRef]
- Wang, L.; Han, D.; Chen, W.; Zhang, S.; Wang, Z.; Li, K.; Gao, Y.; Zou, S.; Yang, A. Non-functional parathyroid carcinoma: A case report and review of the literature. Cancer Biol. Ther. 2015, 16, 1569–1576. [Google Scholar] [CrossRef]
- Gao, W.C.; Ruan, C.P.; Zhang, J.C.; Liu, H.M.; Xu, X.Y.; Sun, Y.P.; Wang, Q. Nonfunctional parathyroid carcinoma. J. Cancer Res. Clin. Oncol. 2010, 136, 969–974. [Google Scholar] [CrossRef]
- Harari, A.; Clark, O.H.; Duh, Q.-Y.; Kebebew, E.; Gosnell, J.E. Parathyroid carcinoma. In Textbook of Endocrine Surgery, 3rd ed.; Shen, W.T., Ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2016; pp. 927–935. [Google Scholar]
- Nam, M.; Jeong, H.S.; Shin, J.H. Differentiation of parathyroid carcinoma and adenoma by preoperative ultrasonography. Acta Radiol. 2017, 58, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Jabiev, A.A.; Lew, J.I.; Solorzano, C.C. Surgeon-performed ultrasound: A single institution experience in parathyroid localization. Surgery 2009, 146, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Balash, P.R.; Yoo, J.; Smith, G.S.; Prinz, R.A. Benefits of surgeon-performed ultrasound for primary hyperparathyroidism. Langenbecks Arch. Surg. 2009, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.P.; Gionfriddo, M.R.; Al Nofal, A.; Boehmer, K.R.; Leppin, A.L.; Reading, C.; Callstrom, M.; Elraiyah, T.A.; Prokop, L.J.; Stan, M.N.; et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 1253–1263. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Lebda, P.; Feldstein, V.A.; Sellami, D.; Goldstein, R.B.; Brasic, N.; Jin, C.; Kornak, J. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: Results of a population-based study. JAMA Intern. Med. 2013, 173, 1788–1796. [Google Scholar] [CrossRef]
- Lee, J.Y.; Baek, J.H.; Ha, E.J.; Sung, J.Y.; Shin, J.H.; Kim, J.H.; Lee, M.K.; Jung, S.L.; Lee, Y.H.; Ahn, H.S.; et al. 2020 Imaging Guidelines for Thyroid Nodules and Differentiated Thyroid Cancer: Korean Society of Thyroid Radiology. Korean J. Radiol. 2021, 22, 840–860. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Lavely, W.C.; Goetze, S.; Friedman, K.P.; Leal, J.P.; Zhang, Z.; Garret-Mayer, E.; Dackiw, A.P.; Tufano, R.P.; Zeiger, M.A.; Ziessman, H.A. Comparison of SPECT/CT, SPECT, and planar imaging with single- and dual-phase (99m)Tc-sestamibi parathyroid scintigraphy. J. Nucl. Med. 2007, 48, 1084–1089. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, R.; Malhotra, A. Brown tumor of the sternum: A potential source of false-positive Tl-201 and Tc-99m subtraction imaging in the mediastinum. Clin. Nucl. Med. 2000, 25, 44–47. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Zhang, M.; Zhang, M.; Meng, H.; Zhang, Y.; Li, B. Value of 99mTc-MIBI SPECT/CT parathyroid imaging and ultrasonography for concomitant thyroid carcinoma. Nucl. Med. Commun. 2017, 38, 676–682. [Google Scholar] [CrossRef]
- Shrestha, M.; Crothers, B.A.; Burch, H.B. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine-needle aspiration: A 10-year study from a single institution. Thyroid 2012, 22, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.B.; Abele, J.; Ali, S.Z.; Duick, D.; Elsheikh, T.M.; Jeffrey, R.B.; Powers, C.N.; Randolph, G.; Renshaw, A.; Scoutt, L. Techniques for thyroid FNA: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, D.C.; Canberk, S.; Poller, D.N.; Schmitt, F. Thyroid FNAC: Causes of false-positive results. Cytopathology 2018, 29, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.A.; Sturgeon, C.; Winchester, D.J.; Liu, L.; Palis, B.; Perrier, N.D.; Evans, D.B.; Winchester, D.P.; Wang, T.S. Parathyroid Carcinoma: An Update on Treatment Outcomes and Prognostic Factors from the National Cancer Data Base (NCDB). Ann. Surg. Oncol. 2015, 22, 3990–3995. [Google Scholar] [CrossRef]
- Sandelin, K.; Tullgren, O.; Farnebo, L.O. Clinical course of metastatic parathyroid cancer. World J. Surg. 1994, 18, 594–598, discussion 599. [Google Scholar] [CrossRef]
- Fernandez-Ranvier, G.G.; Khanafshar, E.; Jensen, K.; Zarnegar, R.; Lee, J.; Kebebew, E.; Duh, Q.Y.; Clark, O.H. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer 2007, 110, 255–264. [Google Scholar] [CrossRef]
- Al-Kurd, A.; Mekel, M.; Mazeh, H. Parathyroid carcinoma. Surg. Oncol. 2014, 23, 107–114. [Google Scholar] [CrossRef]
- Schantz, A.; Castleman, B. Parathyroid carcinoma. A study of 70 cases. Cancer 1973, 31, 600–605. [Google Scholar] [CrossRef]
- Shattuck, T.M.; Välimäki, S.; Obara, T.; Gaz, R.D.; Clark, O.H.; Shoback, D.; Wierman, M.E.; Tojo, K.; Robbins, C.M.; Carpten, J.D.; et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N. Engl. J. Med. 2003, 349, 1722–1729. [Google Scholar] [CrossRef]
- Hosny Mohammed, K.; Siddiqui, M.T.; Willis, B.C.; Zaharieva Tsvetkova, D.; Mohamed, A.; Patel, S.; Sharma, J.; Weber, C.; Cohen, C. Parafibromin, APC and MIB-1 are useful markers for distinguishing parathyroid carcinomas from adenomas. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 731–735. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, Q.; Cao, S.; Gao, X.; Zhao, Y. Diagnostic performance of parafibromin immunohistochemical staining for sporadic parathyroid carcinoma: A meta-analysis. Endocrine 2016, 54, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Bergero, N.; De Pompa, R.; Sacerdote, C.; Gasparri, G.; Volante, M.; Bussolati, G.; Papotti, M. Galectin-3 expression in parathyroid carcinoma: Immunohistochemical study of 26 cases. Hum. Pathol. 2005, 36, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Vasef, M.A.; Brynes, R.K.; Sturm, M.; Bromley, C.; Robinson, R.A. Expression of cyclin D1 in parathyroid carcinomas, adenomas, and hyperplasias: A paraffin immunohistochemical study. Mod. Pathol. 1999, 12, 412–416. [Google Scholar] [PubMed]
- Salcuni, A.S.; Cetani, F.; Guarnieri, V.; Nicastro, V.; Romagnoli, E.; de Martino, D.; Scillitani, A.; Cole, D.E.C. Parathyroid carcinoma. Clin. Endocrinol. Metab. 2018, 32, 877–889. [Google Scholar] [CrossRef] [PubMed]
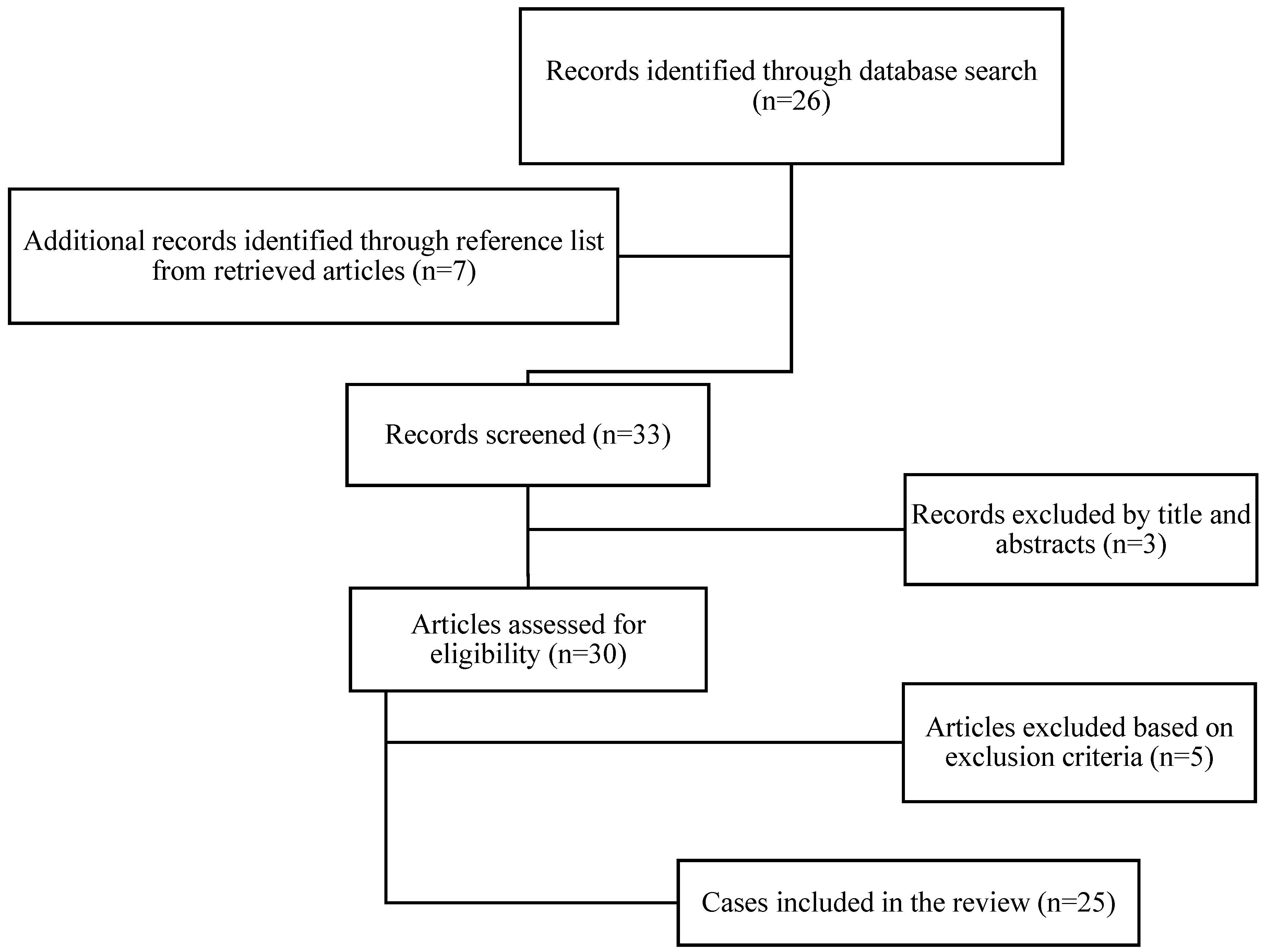
| No. | Authors Year (Reference) | Age Sex | Suspicious Clinical Data EBRT+ Fh+ hPHCa+ | Ca > 14 mg/dL | PTH > 5x NV | Suspicious US Parathyroid/FNA + Thyroid/FNA | 99mTC MIBI. SPECT/CT Uptake | Possible Preop. PC Suspicion | Stated Indication for Surgery | PC Location | Intraop. PC Suspicion | En Bloc Resection PC+Thyroid | PC (cm) (g) IHC | NMTC (cm) Bilateral/Multifocal pTMN | Assoc. PA/PH | Outcome N/P/R | FLL-U (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | De Falco et al., 2021 [10] | 63, M | No | No | No | No + Yes (FNA − benign) | 99mTC MIBI. Yes | NO | PA NG | Left inferior | No | Yes | 1.2 No | 2x mPTC (0.8, 0.6) pT1a(m)NxMx | No | N | 84 |
| 2. | Lam-Chung et al., 2020 [9] | 50, F | Yes hPHCa+ | Yes | Yes | No + No | SPECT/CT Yes | Yes | PC suspicion – | Left superior | N/A | Yes | 2.4 Yes | PTC (1.3) pT1bNxMx | No | N | 1 ½ |
| 3. | Kalthoum et al., 2020 [33] | 60, F | Yes | No | Yes | No + Yes | 99mTC MIBI. Yes | Yes | PHPT – | Left superior | Yes | Yes | 4 No | 2x mPTC (NS) pT1a(m)NxMx | No | N | N/A |
| 4. | D’cruz et al., 2020 [12] | 89, F | Yes | No | No | Yes + No | 99mTC MIBI. Yes | Yes | PA NG | Right inferior | Yes | Yes | 1.7 Yes | FTC (3.5) pT2NxMx | PA | N | N/A |
| 5. | Edafe et al., 2019 [34] | 46, F | Yes hPHCa+ | No | Yes | No + Yes (FNA − PTC) | 99mTC MIBI. Yes | Yes | PC suspicion PTC | Right ? | Yes | Yes | 3.3 No | PTC + mPTC (>4, NS) pT4(m)NxMx | No | N | 12 |
| 6. | Kuzu et al., 2017 [35] | 52, F | Yes hPHCa+ | No | No | No (FNA wash-out) + Yes (FNA − benign) | 99mTC MIBI. No | Yes | PA NG | Right inferior | Yes | Yes | 1.8 No | PTC + mPTC (1, NS) pT1b(m)NxMx | No | N | 12 |
| 7. | Baek et al., 2017 [36] | 68, F | Yes hPHCa+ | No | Yes | Yes + Yes (FNA − AUS/FLUS) | 99mTC MIBI. Yes | Yes | PA NG | Left inferior | Yes | N/S | 4.2 No | mPTC (NS) pT1a(m)NxMx | No | N | 6 |
| 8. | Demir et al., 2017 [37] | 29, F | Yes hPHCa+ | Yes | Yes | Yes + Yes | 99mTC MIBI. Yes | Yes | PA NG | Right ? | Yes | NS | 2.8 No | PTC (1.6) pT1bNxMx | No | N | N/A |
| 9. | Aljabri et al., 2017 [38] | 72, F | Yes hPHCa+ | No | Yes | ∅ + Yes (FNA –benign) | 99mTC MIBI. No | Yes | – NG | Right Inferior | Yes | Yes | 4.5 Yes | mPTC (0.2) pT1a(m)NxMx | No | N | 1 |
| 10. | Dikmen et al., 2017 [21] | 57, M | No | No | No | Yes+ ∅ | 99mTC MIBI. Yes | Yes | Persistent elevated Ca + PTH after ePC removal | Mediastinal + left inferior | No | No | 30 + 21 (2xPC) Yes | mPTC (0.2) pT1a(m)NxMx | ePC | N | N/A |
| 11. | Neslihan et al., 2016 [39] | 65, F | No | No | Yes | No + Yes (FNA − benign) | 99mTC MIBI. No | Yes | PC suspicion – | Left inferior | N/A | Yes | 2.5 Positive surgical margins Yes | 2x mPTC (0.5, 0.2) pT1a(m)NxMx | No | N | N/A |
| 12. | Lee et al., 2016 [40] | 57, F | No | Yes | Yes | ∅ + No | 99mTC MIBI. Yes | Yes | PA NG | Left inferior | Yes | N/S | 4.5 | PTC (NS) pT? | No | -N -2xR (PC) -N | 72 |
| 13. | Song et al., 2016 [11] | 45, F | Yes | Yes | Yes | Yes + Yes | 99mTC MIBI. Yes | Yes | N/S | Left inferior | Yes | No | 4.3 | mPTC (0.5) pT1a(m)NxMx | NA | -N -2xR (PC) -N | 6mo after III surgery |
| 14. | Al-Sulami, 2015 [41] | 75, F | Yes | No | Yes | ∅ + No | 99mTC MIBI. Yes | Yes | N/S | Left ? | N/A | N/S | 3.5 Positive surgical margins | 3x mPTC (all < 0.5) pT1a(m)NxMx | N/A | P | 24 |
| 15. | Zakerkish et al., 2015 [42] | 21, M | Yes | No | Yes | No + ∅ (previous TT) | 99mTC MIBI. No | Yes | PC suspected – | Right ? | N/A | No (2 years previous TT) | N/A | mHHC (0.6) pT1a(m)NxMx | No | P -3w later death | <1 |
| 16. | Chaychi et al., 2010 [43] | 79, F | No hPHCa+, 6y | No | No | Yes + Yes (FNA − PTC) | 99mTC MIBI. Yes | Yes | PHPT 2xPTC | Left superior, | N/A | Yes | 5 Yes | 2x PTC (2.4, 1.7) pT2(m)NxMx | No | N | 6 |
| 17. | Marcy et al., 2009 [44] | 42, F | Yes hEBRT+ | Yes | Yes | Yes (FNA: unconclusive) + Yes (FNA − inconclusive) | 99mTC MIBI. Yes | Yes | PC suspected – | Right inferior | N/A | N/S | 1.3 | 2x mPTC (0.8, 0.5) (m)T1aNxMx | No | N | 14 |
| 18. | Goldfarb et al., 2009 [45] | 59, M | Yes hPHCa+, 6y | Yes | Yes | Yes + Yes | 99mTC MIBI. Yes | Yes | PHPT – | Left ? | Yes | Yes | 3.9 17 g | PTC + mPTC (3.2, 0.4) pT2(m)NxMx | PA | -h -R (PA) -N | 14 |
| 19. | Mazeh et al., 2008 [46] | 44, F | No | No | N/A | No + Yes (FNA − inconclusive) | N/A | NO | – NG | Left ? | N/A | Yes (non-intended) | 1.5 | PTC (NS) pT? | No | N | 60 |
| 20. | Lin et al., 2005 [47] | 38, M | Yes hPHCa+, 6y | Yes | Yes | No + Yes (FNA − PC) | 99mTC MIBI. 201TI Scinti Yes | Yes | PC NG | Left inferior | Yes (frozen section!) | No | N/A | PTC (4) pT2(m)N + Mx | No | N | 72 |
| 21. | Kern et al., 2004 [48] | 54, F | No Fh+ | N/A | Yes | N/A | N/A | Yes | PHPT | Right inferior | Yes | No (although PC very adherent) | 2.5 7 g | 2x mPT + mFTC; (0.3, 0.4, 0.3) pT1a(m)N + Mx | No | N − R + 2y later (PC distant meta) − 3y death | 36 |
| 22. | Schoretsanitis et al., 2002 [49] | 55, F | Yes hPHCa+ | Yes | Yes | Ø + No | +99mTC MIBI. Yes | Yes | PHPT NG | Left inferior | Yes | Yes | 3 | mPTC (0.7) pT1a(m)NxMx | PH | N | 24 |
| 23. | Bednarek-Tupikowska et al., 2001 [50] | 42, F | Yes hPHCa+ | Yes | Yes | Yes (FNA − no cells) + Ø (previous TT) | 99mTC MIBI. Yes 99mTC MIBI for persistent disease No | Yes | PHPT – | Left ? | Yes | No (6 years previous TT) | 5 Yes | FC (NS) pT? | No | P | N/A |
| 24. | Savil et al., 2001 [51] | 47, F | No | No | N/A | Ø + Yes (FNA − PTC) | N/A | NO | – PTC | Left ? | No | No | 3 | PTC (3) pT2(m)NxMx | PH | N | 12 |
| 25. | Kurita et al., 1979 [52] | 68, M | No | No | Yes | N/A | N/A | Yes | PHPT – | Left superior | Yes | Yes | 4.2 21 g | PTC (NS) pT? | No | N | 24 |
| Parathyroid Disease | PA | PH | Ectopic PC |
|---|---|---|---|
| No./total (%) | 2/5 (40) | 2/5 (40) | 1/5 (20) |
| Calcium levels mg/dL | 13.8; 14.7 | 11.3; 12.1 | 16.2 |
| PTH levels pg/mL | 441; 318 | 197; 249 | 4211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simescu, R.; Pop, M.; Piciu, A.; Muntean, V.; Piciu, D. Association of Parathyroid and Differentiated Thyroid Carcinomas: A Narrative Up-To-Date Review of the Literature. Medicina 2022, 58, 1184. https://doi.org/10.3390/medicina58091184
Simescu R, Pop M, Piciu A, Muntean V, Piciu D. Association of Parathyroid and Differentiated Thyroid Carcinomas: A Narrative Up-To-Date Review of the Literature. Medicina. 2022; 58(9):1184. https://doi.org/10.3390/medicina58091184
Chicago/Turabian StyleSimescu, Razvan, Miana Pop, Andra Piciu, Valentin Muntean, and Doina Piciu. 2022. "Association of Parathyroid and Differentiated Thyroid Carcinomas: A Narrative Up-To-Date Review of the Literature" Medicina 58, no. 9: 1184. https://doi.org/10.3390/medicina58091184
APA StyleSimescu, R., Pop, M., Piciu, A., Muntean, V., & Piciu, D. (2022). Association of Parathyroid and Differentiated Thyroid Carcinomas: A Narrative Up-To-Date Review of the Literature. Medicina, 58(9), 1184. https://doi.org/10.3390/medicina58091184







