Initial Data and a Clinical Diagnosis Transition for the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
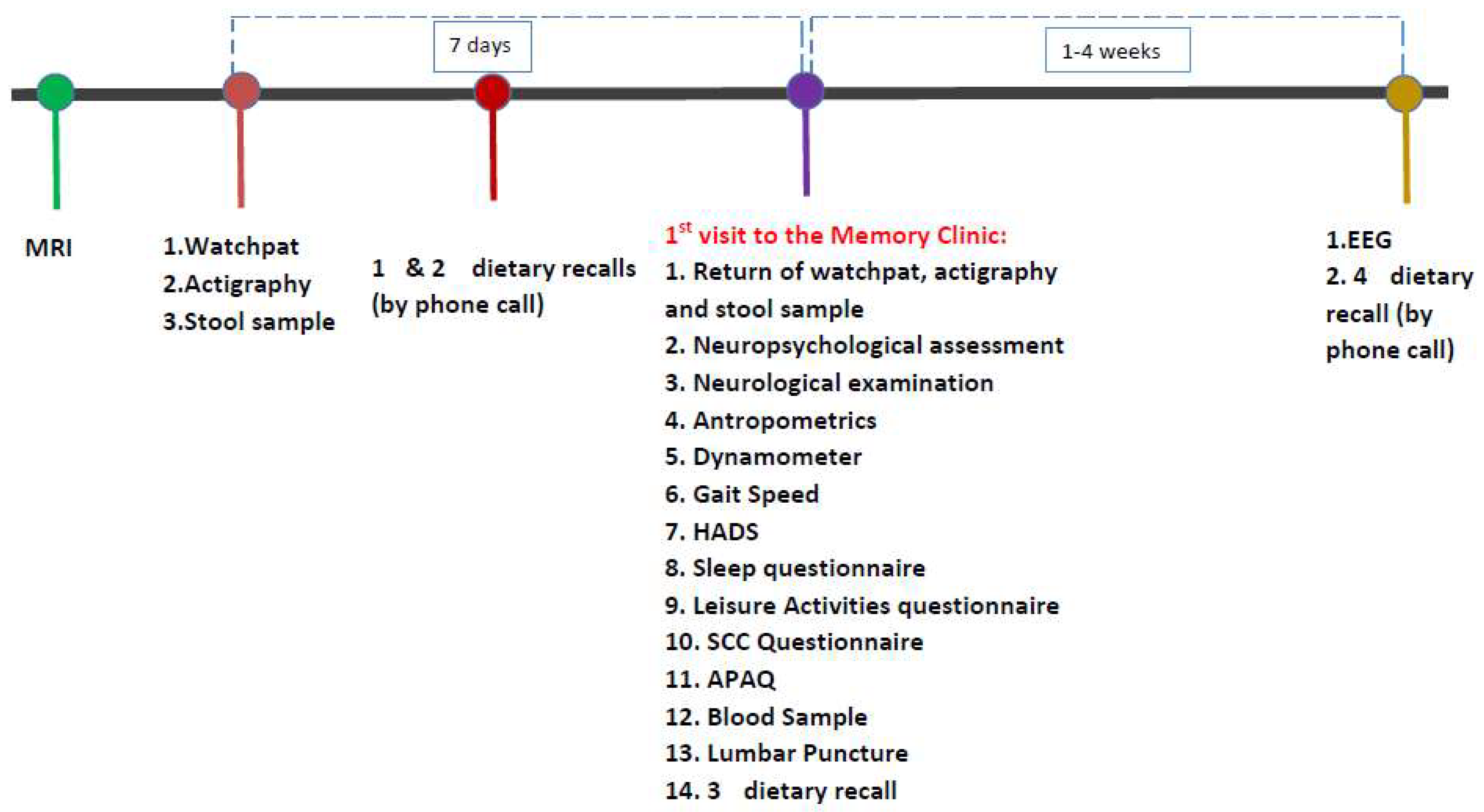
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 5 July 2022).
- Alzheimer Europe. Available online: https://www.alzheimer-europe.org/dementia/prevalence-dementia-europe (accessed on 5 July 2022).
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [PubMed]
- Williams, D.R. Tauopathies: Classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J. 2006, 36, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.C.; Sperling, R.A.; Petersen, R.; Sun, C.K.; Weiner, M.W.; Aisen, P.S. Alzheimer’s Disease Neuroimaging Initiative. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017, 317, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629, Erratum in: Lancet Neurol. 2014, 13, 757. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128, Erratum in Arch. Neurol. 1999, 56, 760. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Kalligerou, F.; Ntanasi, E.; Voskou, P.; Velonakis, G.; Karavasilis, E.; Mamalaki, E.; Kyrozis, A.; Sigala, E.; Economou, N.T.; Patas, K.; et al. Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION): Study design, cohort description, and preliminary data. Postgrad Med. 2019, 131, 501–508. [Google Scholar] [CrossRef]
- Vlachos, G.S.; Consentino, S.; Kosmidis, M.; Anastasiou, C.A.; Yannakoulia, M.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Ntanasi, E.; Scarmeas, N. Prevalence and determinants of subjective cognitive decline in a representative Greek elderly population. Int. J. Geriatr. Psychiatry 2019, 34, 846–854. [Google Scholar] [CrossRef]
- Tsapanou, A.; Vlachos, G.; Cosentino, S.; Gu, Y.; Manly, J.J.; Brickman, A.M.; Schupf, N.; Zimmerman, M.E.; Yannakoulia, M.; Kosmidis, M.H.; et al. Sleep and subjective cognitive decline in cognitively healthy elderly: Results from two cohorts. J. Sleep Res. 2018, 28, e12759. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Buchman, A.S.; Boyle, P.A.; Wilson, R.S.; Fleischman, D.A.; Leurgans, S.; Bennett, D.A. Association Between Late-Life Social Activity and Motor Decline in Older Adults. Arch. Intern. Med. 2009, 169, 1139–1146. [Google Scholar] [CrossRef]
- Hays, R.D.; Martin, S.A.; Sesti, A.M.; Spritzer, K.L. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005, 6, 41–44. [Google Scholar] [CrossRef]
- Besson, H.; Harwood, C.A.; Ekelund, U.; Finucane, F.M.; McDermott, C.J.; Shaw, P.; Wareham, N.J. Validation of the historical adulthood physical activity questionnaire (HAPAQ) against objective measurements of physical activity. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 54. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef]
- Vlahou, C.; Kosmidis, M. The Greek Trail Making Test: Preliminary normative data for clinical and research use. Psychol. J. Hell. Psychol. Soc. 2002, 9, 336–352. [Google Scholar]
- Wechsler, D. WechslerAdult Intelligence Scale—Administration and Scoring Manual, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wühr, P. A Stroop Effect for Spatial Orientation. J. Gen. Psychol. 2007, 134, 285–294. [Google Scholar] [CrossRef]
- Vlahou, C.H.; Kosmidis, M.H.; Dardagani, A.; Tsotsi, S.; Giannakou, M.; Giazkoulidou, A.; Zervoudakis, E.; Pontikakis, N. Development of the Greek Verbal Learning Test: Reliability, Construct Validity, and Normative Standards. Arch. Clin. Neuropsychol. 2012, 28, 52–64. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Tumani, H.; Engelborghs, S.; Mollenhauer, B. Biobanking of CSF: International standardization to optimize biomarker development. Clin. Biochem. 2014, 47, 288–292. [Google Scholar] [CrossRef]
- Hansson, O.; Seibyl, J.; Stomrud, E.; Zetterberg, H.; Trojanowski, J.Q.; Bittner, T.; Lifke, V.; Corradini, V.; Eichenlaub, U.; Batrla, R.; et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s Dement. 2018, 14, 1470–1481. [Google Scholar] [CrossRef]
- Doecke, J.D.; The AIBL Research Group; Ward, L.; Burnham, S.C.; Villemagne, V.L.; Li, Q.-X.; Collins, S.; Fowler, C.J.; Manuilova, E.; Widmann, M.; et al. Elecsys CSF biomarker immunoassays demonstrate concordance with amyloid-PET imaging. Alzheimer’s Res. Ther. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Available online: https://www.usa.philips.com/healthcare/product/HC1044809/actiwatch-2-activity-monitor/specifications (accessed on 2 July 2022).
- Available online: https://www.itamar-medical.com/professionals/watchpat-300/ (accessed on 2 July 2022).
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Bin Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Dumurgier, J.; Hanseeuw, B.J.; Hatling, F.B.; Judge, K.A.; Schultz, A.P.; Chhatwal, J.P.; Blacker, D.; Sperling, R.A.; Johnson, K.A.; Hyman, B.T.; et al. Alzheimer’s Disease Biomarkers and Future Decline in Cognitive Normal Older Adults. J. Alzheimer’s Dis. 2017, 60, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef]
- Erickson, C.M.; Chin, N.A.; Johnson, S.C.; Gleason, C.E.; Clark, L.R. Disclosure of preclinical Alzheimer’s disease biomarker results in research and clinical settings: Why, how, and what we still need to know. Alzheimer’s Dement. 2021, 13, e12150. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Minguillón, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s Disease Prevention: From Risk Factors to Early Intervention. Alzheimer’s Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. 2022, 4, e12295. [Google Scholar] [CrossRef]
- Available online: https://www.mayo.edu/research/centers-programs/alzheimers-disease-research-center/research-activities/mayo-clinic-study-aging/overview (accessed on 7 July 2022).
- Available online: https://www.biocard-se.org/ (accessed on 7 July 2022).
- Available online: https://lasa-vu.nl/en/ (accessed on 7 July 2022).
- Mielke, M.M.; Machulda, M.M.; Hagen, C.E.; Christianson, T.J.; Roberts, R.O.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Kremers, W.K.; Clifford, R.H., Jr.; et al. Influence of amyloid and APOE on cognitive performance in a late middle-aged cohort. Alzheimer’s Dement. 2016, 12, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.S.; Palmqvist, S.; Mackin, R.S.; Nosheny, R.L.; Hansson, O.; Weiner, M.W.; Mattsson, N. Assessing risk for preclinical β-amyloid pathology with APOE, cognitive, and demographic information. Alzheimer’s Dement. 2016, 4, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Weston, P.S.; Nicholas, J.M.; Lehmann, M.; Ryan, N.S.; Liang, Y.; Macpherson, K.; Modat, M.; Rossor, M.N.; Schott, J.M.; Ourselin, S.; et al. Presymptomatic cortical thinning in familial Alzheimer disease: A longitudinal MRI study. Neurology 2016, 8, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Arenaza-Urquijo, E.M.; Wirth, M.; Chételat, G. Cognitive reserve and lifestyle: Moving towards preclinical Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 134. [Google Scholar] [CrossRef]
- Brown, B.M.; Peiffer, J.J.; Taddei, K.; Lui, J.K.; Laws, S.M.; Gupta, V.B.; Taddei, T.; Ward, V.K.; Rodrigues, M.A.; Burnham, S.; et al. Physical activity and amyloid-β plasma and brain levels: Results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol. Psychiatry 2013, 18, 875–881. [Google Scholar] [CrossRef] [Green Version]
| Variable | CN (n = 99) | MCI (n = 39) | All (n = 138) | p-Value |
|---|---|---|---|---|
| Sex, female (%) | 67 (67.7) | 24 (61.5) | 91 (65.9) | 0.493 |
| Age, y, mean (SD) | 62.9 (9.2) | 65.9 (8.2) | 63.8 (9.0) | 0.089 |
| Education, y, mean (SD) | 13.6 (3.7) | 12.5 (4.1) | 13.3 (3.8) | 0.144 |
| Family history of dementia, n (%) | 45 (45.5) | 18 (46.2) | 63 (45.7) | 0.941 |
| MMSE, mean (SD) | 28.8 (1.4) | 26.5 (2.0) | 28.2 (1.9) | <0.001 |
| ApoE ε4 carrier, n/N performed (%) | 18/81 (22.2) | 13/33 (33.3) | 31/114 (27.2) | 0.062 |
| Participants with Biomarkers (n = 74) | Participants without Biomarkers (n = 64) | p-Value | |
|---|---|---|---|
| Sex, female (%) | 47 (34.1) | 44 (31.9) | 0.517 |
| Age, y, mean (SD) | 64.5 (9.0) | 63.0 (9.0) | 0.329 |
| Education, y, mean (SD) | 13.0 (3.7) | 13.6 (3.9) | 0.332 |
| MMSE, mean (SD) | 28.13 (1.81) | 28.25 (2.1) | 0.717 |
| Clinical diagnosis | |||
| CN | 49 | 50 | 0.121 |
| MCI | 25 | 14 |
| Normal AD Biomarkers | AD Continuum | Non-AD Pathologic Change | p-Value | ||
|---|---|---|---|---|---|
| AD Pathologic Change | AD Disease | ||||
| Participants, N (%) | 30 (40.5) | 29 (39.2) | 12 (16.2) | 3 (4.1) | |
| Clinical diagnosis, N (%) | |||||
| CN | 19 (38.8) | 21 (42.9) | 6 (12.2) | 3 (6.1) | 0.371 |
| MCI | 11 (44.0) | 8 (32.0) | 6 (24.0) | 0 | |
| Sex, female (%) male (%) | 21 (44.7) 9 (33.3) | 17 (36.2) 12 (44.4) | 8 (17.0) 4 (10.9) | 1 (2.1) 2 (7.4) | 0.563 |
| Age, y, mean (SD) | 62.2 (7.9) | 64.8 (9.7) | 67.7 (8.5) | 71.7 (11.0) | 0.148 |
| Education, y, mean (SD) | 12.8 (4.0) | 13.5 (3.3) | 11.2 (4.4) | 13.3 (3.2) | 0.407 |
| Family history of dementia, n (%) | 14 (46.7) | 16 (55.2) | 7 (58.3) | 2 (66.7) | 0.843 |
| MMSE, mean (SD) | 28.4 (1.8) | 28.1 (1.9) | 27.2 (1.8) | 28.5 (0.7) | 0.274 |
| ApoE status | |||||
| ε4 carrier, n (%) | 8 (38.1) | 5 (23.8) | 8 (38.1) | 0 | 0.005 |
| non-carriers | 22 (42.3) | 24 (46.2) | 3 (5.8) | 3 (5.8) | |
| Diagnosis Conversion | |||||
|---|---|---|---|---|---|
| Total (45) | CN | MCI | Dementia | ||
| Biomarker category | Normal AD biomarkers | 16 | 16 (100%) | 0 | 0 |
| AD pathologic change | 20 | 19 (95%) | 1 (5%) | 0 | |
| AD disease | 6 | 2 (33.3%) | 2 (33.3%) | 2 (33.3) | |
| Non-AD pathologic change | 3 | 2 (66.7%) | 1 (33.3%) | 0 | |
| Diagnosis Conversion | |||||
|---|---|---|---|---|---|
| Total (21) | CN | MCI | Dementia | ||
| Biomarker category | Normal AD biomarkers | 9 | 2 (22.2%) | 5 (55.6%) | 2 (22.2%) |
| AD pathologic change | 6 | 1 (16.7%) | 2 (33.3%) | 3 (50%) | |
| AD disease | 6 | 0 | 2 (33.3%) | 4 (66.7%) | |
| Non-AD pathologic change | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarmeas, N.; Daskalaki, A.; Kalligerou, F.; Ntanasi, E.; Mamalaki, E.; Gargalionis, A.N.; Patas, K.; Chatzipanagiotou, S.; Yannakoulia, M.; Constantinides, V.C. Initial Data and a Clinical Diagnosis Transition for the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Medicina 2022, 58, 1179. https://doi.org/10.3390/medicina58091179
Scarmeas N, Daskalaki A, Kalligerou F, Ntanasi E, Mamalaki E, Gargalionis AN, Patas K, Chatzipanagiotou S, Yannakoulia M, Constantinides VC. Initial Data and a Clinical Diagnosis Transition for the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Medicina. 2022; 58(9):1179. https://doi.org/10.3390/medicina58091179
Chicago/Turabian StyleScarmeas, Nikolaos, Argyro Daskalaki, Faidra Kalligerou, Eva Ntanasi, Eirini Mamalaki, Antonios N. Gargalionis, Kostas Patas, Stylianos Chatzipanagiotou, Mary Yannakoulia, and Vasilios C. Constantinides. 2022. "Initial Data and a Clinical Diagnosis Transition for the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study" Medicina 58, no. 9: 1179. https://doi.org/10.3390/medicina58091179
APA StyleScarmeas, N., Daskalaki, A., Kalligerou, F., Ntanasi, E., Mamalaki, E., Gargalionis, A. N., Patas, K., Chatzipanagiotou, S., Yannakoulia, M., & Constantinides, V. C. (2022). Initial Data and a Clinical Diagnosis Transition for the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Medicina, 58(9), 1179. https://doi.org/10.3390/medicina58091179







