Abstract
Background and objectives: The effect of beta-blocker use after discharge on patients with acute myocardial infarction (AMI) in the contemporary reperfusion era remains ambiguous. By applying meta-analysis, we sought to assess the role of beta-blockers in the contemporary reperfusion era. Materials and Methods: Randomized controlled trials (RCT) and observational studies using propensity score matching, comparing use of beta-blockers with non-use of beta-blockers, in patients with AMI after discharge. The primary outcome was all-cause mortality. Odds ratios (OR) and associated 95% confidence intervals (CI) were calculated. Results: One RCT and eight observational studies, containing 47,339 patients with AMI, were included. Compared with non-use of beta-blockers, beta-blocker use after discharge may have reduced the risk of all-cause mortality (OR: 0.70, 95% CI: 0.61 to 0.80, I2 = 14.4%), cardiac death (OR: 0.63, 95% CI: 0.44 to 0.91, I2 = 22.8%), myocardial infarction (OR: 0.73, 95% CI: 0.62 to 0.86, I2 = 0), and revascularization (OR: 0.92, 95% CI: 0.85 to 0.99, I2 = 0). No significant differences were found in major adverse cardiovascular events (MACE, OR: 0.88, 95% CI: 0.66 to 1.17, I2 = 78.4%), heart failure (OR: 0.56, 95% CI: 0.29 to 1.08, I2 = 0) or stroke (OR: 1.13, 95% CI: 0.92 to 1.39, I2 = 0). For patients with preserved left ventricular function, beta-blocker use after discharge may have also reduced the risk of all-cause mortality (OR: 0.61, 95% CI: 0.44 to 0.84, I2 = 0). Conclusions: Use of beta-blockers after discharge may still be beneficial for AMI patients in the contemporary reperfusion era, with or without preserved left ventricular function.
1. Introduction
Beta-blockers were among the first-line medications with improved clinical outcomes in patients with acute myocardial infarction (AMI), according to randomized controlled trials (RCT) conducted in the pre-reperfusion or thrombolytic era [,]. However, the last two decades have witnessed substantial evolution in the treatment of AMI, especially in the development and refinement of percutaneous coronary intervention (PCI), which has resulted in a significant decline in deaths []. Correspondingly, the American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines, published in 2004, had already recommended PCI rather than fibrinolytic therapy for treatment of AMI (Class I, Level A) []. In this context, extrapolating the conclusions derived from the pre-perfusion or thrombolytic era to the contemporary PCI era may be not appropriate. However, RCTs investigating the effects of beta-blocker in the contemporary PCI era are limited. Observational studies were influenced by confounding factors, and showed conflicting results [,]. In previously published meta-analyses investigating the effect of beta-blocker use after discharge in the contemporary reperfusion era, only raw data from observational studies were recorded, without considering the impact of confounders [,,]. To investigate the effect of beta-blocker use after discharge in the contemporary reperfusion era, and to minimize the effect of confounding factors, an updated systematic review and meta-analysis which only included RCTs and observational studies, with propensity score matching, was conducted. Moreover, the effect of beta-blocker use after discharge on patients with preserved left ventricular function was also assessed.
2. Methods
The present study was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [], and was registered in the International Prospective Register of Systematic Reviews (CRD42022328398).
2.1. Study Selection
Two reviewers (M.J. Hu and S. Hu) independently searched PubMed, Web of Science, the Cochrane Library, ClinicalTrials.gov, and Google Scholar from publication to February 2022, using the following search terms: ‘acute coronary syndrome’; ‘ACS’; ‘myocardial infarction’; ‘MI’; ‘angiography’; ‘percutaneous coronary intervention’; ‘PCI’; ‘β-blockers’; and ‘beta-blockers’. References in the included articles and meta-analyses to similar topics were also carefully checked. Disagreements were resolved by discussing with the third-party investigator (X.J. Gao). Studies were selected according to the following criteria: RCTs or observational studies, with propensity score matching, that compared use of beta-blockers after discharge to non-use of beta-blockers after discharge, in patients with AMI, in the contemporary reperfusion era. Studies were excluded if they compared the clinical outcomes between different types or dosages of beta-blockers, or if the studies did not focus on patients with AMI.
2.2. Outcomes
The primary outcome was all-cause mortality. The secondary outcomes included major adverse cardiovascular events (MACE), cardiac death, myocardial infarction, heart failure, revascularization, and stroke. Clinical outcomes were recorded and defined according to per individual study. Information regarding study design, country, inclusion period, age, sex, the percentage of ST-segment elevation myocardial infarction (STEMI) and PCI, left ventricular ejection fractions (LVEF), prior heart failure, type of beta-blockers, clinical outcomes, and follow-up time were carefully extracted by the same two reviewers (M.J. Hu and S. Hu).
2.3. Statistical Analysis
The overall treatment effect was calculated under a random-effects model, expressed as odds ratios (OR) and 95% confidence intervals (CI). The level of heterogeneity was assessed using the I2-statistic test (I2 > 25%, >50%, >75% represented low, moderate, and high heterogeneity, respectively) []. The potential reason for heterogeneity was analyzed by meta-regression. A leave-one-out method was used to identify whether any individual study influenced the overall results. Publication bias was estimated according to the results of funnel plots or Begg’s test. p value < 0.05 represented statistical significance. All analyses were completed using STATA 16.0 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Study Characteristics
Nine studies containing 47,339 patients (24,329 patients received beta-blockers, whereas 23,010 patients did not) were included in the present meta-analysis, including one RCT [] and eight observational studies [,,,,,,,]. Details of the screening process for eligible studies are shown in Figure 1, and the baseline characteristics of the included studies and patients are presented in Table 1.
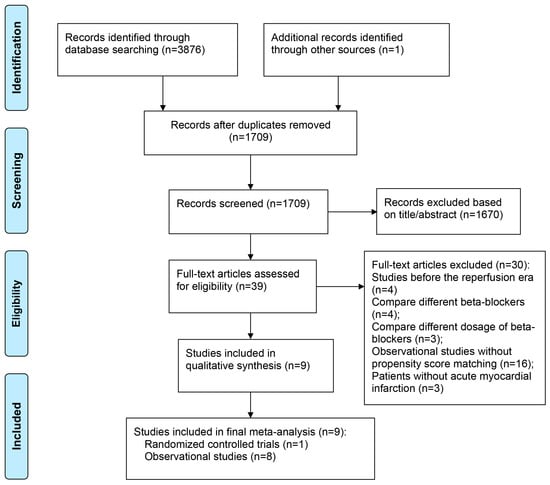
Figure 1.
PRISMA Diagram for Study Inclusion.

Table 1.
Baseline Characteristics of Included Studies and Patients.
3.2. All-Cause Mortality
Seven studies in total reported all-cause mortality. Compared with non-use of beta-blockers, beta-blocker use may have reduced the risk of all-cause mortality (OR: 0.70, 95% CI: 0.61 to 0.80, I2 = 14.4%, Figure 2A). For patients with preserved left ventricular function, a lower risk of all-cause mortality was also observed (OR: 0.61, 95% CI: 0.44 to 0.84, I2 = 0, Figure 2B). Meta-regression analysis of all-cause mortality revealed that age, sex, and the percentage of STEMI did not affect the relationship between beta-blocker use and all-cause mortality (Figure S1).
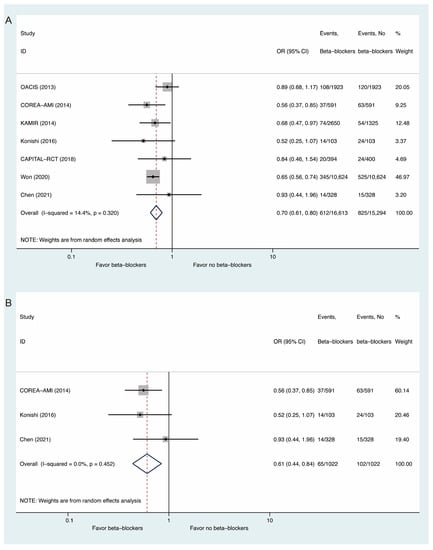
Figure 2.
Comparisons of All-Cause Mortality Between Beta-Blockers and Controls: (A) whole populations; (B) populations with preserved left ventricular function.
3.3. Secondary Outcomes
For secondary outcomes, beta-blocker use may have decreased the risks of cardiac death (OR: 0.63, 95% CI: 0.44 to 0.91, I2 = 22.8%, Figure 3B), myocardial infarction (OR: 0.73, 95% CI: 0.62 to 0.86, I2 = 0, Figure 3C), and revascularization (OR: 0.92, 95% CI: 0.85 to 0.99, I2 = 0, Figure 3E), without significant influences on MACE (OR: 0.88, 95% CI: 0.66 to 1.17, I2 = 78.4%, Figure 3A), heart failure (OR: 0.56, 95% CI: 0.29 to 1.08, I2 = 0, Figure 3D) or stroke (OR: 1.13, 95% CI: 0.92 to 1.39, I2 = 0, Figure 3F).
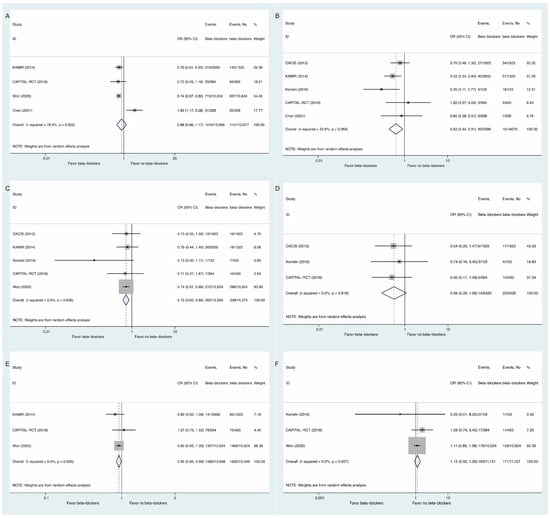
Figure 3.
Comparisons of Secondary Outcomes Between Beta-Blockers and Controls: (A) major adverse cardiovascular events; (B) cardiac death; (C) myocardial infarction; (D) heart failure; (E) revascularization; (F) stroke.
3.4. Publication Bias and Sensitivity Analysis
4. Discussion
In this updated meta-analysis, including both RCT and observational studies with propensity score matching, we found that beta-blocker use after discharge may have reduced the risks of all-cause mortality, cardiac death, myocardial infarction, and revascularization without influence on MACE, heart failure or stroke. For patients with preserved left ventricular function, a lower risk of all-cause mortality was also found.
The evidence for beta-blocker use after AMI originates from the First International Study of Infarct Survival (ISIS-1) trial, published in 1986, where atenolol significantly reduced vascular death compared with non-use of beta-blockers (3.87% versus 4.57%, p < 0.05) []. However, in the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT), published in 2005, the investigators found no significant differences in the co-primary endpoints of 30-day death, myocardial infarction, or cardiac arrest, between metoprolol and non-use of beta-blockers (p = 0.1) []. It is noteworthy that in the COMMIT trial, 54% of patients received fibrinolytic agents, 100% of patients received aspirin, and 50% of patients received dual antiplatelet therapy. However, in the ISIS-1 trial, only 5% of patients received antiplatelet agents. Deficiency in reperfusion and current medical treatment likely led to extensive myocardial scarring and subsequent fatal ventricular arrhythmias. Improved PCI techniques and increased use of aspirin, clopidogrel, and statins have substantially decreased all-cause mortality. Timely PCI can rescue more viable myocardium from necrosis, and prevent scar formation and left ventricular dysfunction, thereby further reducing the influence of beta-blockers []. As a result, it is hypothesized that the effectiveness of beta-blockers would be diminished or diluted by the modern treatment modality. The level of recommendation for use of beta-blockers has been downgraded in the recent ACC/AHA and European Society of Cardiology (ESC) Guidelines. The 2014 ACC/AHA Guidelines gave a Class IIa (Level C) recommendation in non-ST-segment elevation myocardial infarction patients with normal left ventricular function []. The 2020 ESC Guidelines also gave a Class IIa (Level B) recommendation in patients with prior myocardial infarction to reduce all-cause mortality, cardiovascular death, and cardiovascular morbidity [].
Our results indicated that beta-blocker use after discharge may still reduce the risks of all-cause mortality, cardiac death, myocardial infarction, and revascularization in the contemporary reperfusion era. For patients with preserved left ventricular function, a lower risk of all-cause mortality was also found. Concordant with our results, the meta-analysis conducted by Maqsood, et al. [], also suggested that beta-blocker treatment was associated with a reduced risk of all-cause mortality in patients with STEMI and preserved LVEF who underwent PCI. In the meta-analysis conducted by Dahl Aarvik and colleagues [], beta-blocker treatment after discharge also reduced the risk of all-cause mortality [rate ratio (RR): 0.74, 95% CI: 0.64 to 0.85]. However, in the aforementioned meta-analyses, only raw data from observational studies were included, without considering the impact of confounders on the association. Therefore, in our meta-analysis, only RCTs and observational studies with propensity score matching were included, which enabled us to limit the influence of confounders as much as possible. Moreover, the overall heterogeneity was low in our meta-analysis, except for MACE, which may be explained by the different definitions of MACE.
The beneficial effects of beta-blockers in patients with AMI may be explained by multiple actions of beta-blockers on the heart. Firstly, monocyte recruitment to atherosclerotic plaques is significantly increased after AMI, resulting in the development of larger atherosclerotic lesions and more advanced morphology. Moreover, pain and anxiety alert the sympathetic nervous system, and activate neuroimmune synapses in the bone marrow, amplifying extramedullary myelopoiesis. However, these processes can be ameliorated by beta-blockers []. Secondly, adverse remodeling after AMI is associated with poor prognosis []. Beta-blockers have a beneficial effect on ventricular remodeling []. Thirdly, ventricular arrhythmia may increase the incidence of 90-day all-cause mortality []. Beta-blocker treatment could decrease the incidences of both short- and long-term ventricular arrhythmia [,]. Fourthly, oxygen supply to the affected portion of the heart is reduced in the context of AMI. The blockade of beta receptors results in slow heart rate, reduced myocardial contractility, and low systemic blood pressure, ultimately reducing myocardial workload and oxygen demand.
While beta-blockers are relatively safe and inexpensive, they also have adverse effects []. For example, the incidence of coronary spasm was more common in patients receiving beta-blockers than in those receiving calcium antagonist (1.2% versus 0.2%, p = 0.02) [], which requires our attention.
5. Limitations
Some limitations associated with the meta-analysis deserve attention. Firstly, only one RCT was included in our meta-analysis. The inclusion of observational studies may have biased our pooled estimates, because of the effect of confounding factors. We tried to limit this bias by only including data with propensity score matching. Secondly, statistical heterogeneity was high in MACE, which may be related to the different definitions of MACE. Thirdly, the types of beta-blockers were varied, and the dosages of beta-blockers were not available, which may have affected clinical outcomes. However, according to the randomized Carvedilol Acute Myocardial Infarction Study, different types of beta-blockers (carvedilol versus atenolol) had no effect on composite cardiovascular events (p = 0.99) []. In addition, there was no significant benefit from high-dose (≥25% of target dose) compared to low-dose (<25% of target dose) beta-blockers, for cardiac death [].
6. Conclusions
Use of beta-blockers after discharge may still be beneficial for AMI patients in the contemporary reperfusion era, even for those with preserved left ventricular function.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina58091177/s1, Figure S1: Meta-regression for All-Cause Mortality; Figure S2: Funnel Plot of Publication Bias for Primary and Secondary Outcomes; Figure S3: Begg’s Test for Primary and Secondary Outcomes; Figure S4: Leave-One-Out Analyses for Primary and Secondary Outcomes.
Author Contributions
Conceptualization: M.H. and Y.Y. Methodology: M.H. and S.H. Software: M.H. and S.H. Validation: X.G. and Y.Y. Formal analysis: M.H. and S.H. Investigation: M.H. Resources: Y.Y. Data curation: X.G. and Y.Y. Writing—original draft preparation: M.H. and S.H. Writing—review and editing: Y.Y. Visualization: M.H. Supervision: Y.Y. Project administration: X.G. and Y.Y. Funding acquisition: Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFC1700503), CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-009), and the Twelfth Five-Year Planning Project of the Scientific and Technological Department of China (2011BAI11B02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anonymous. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982, 247, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N. Engl. J. Med. 1981, 304, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.A.; Steg, P.G.; Eagle, K.A.; Goodman, S.G.; Anderson, F.A.; Granger, C.B.; Flather, M.D.; Budaj, A.; Quill, A.; Gore, J.M.; et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA 2007, 297, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Anbe, D.T.; Armstrong, P.W.; Bates, E.R.; Green, L.A.; Hand, M.; Hochman, J.; Krumholz, H.M.; Kushner, F.G.; Lamas, G.A.; et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction, A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J. Am. Coll. Cardiol. 2004, 44, E1–E211. [Google Scholar] [PubMed]
- Peck, K.Y.; Andrianopoulos, N.; Dinh, D.; Roberts, L.; Duffy, S.J.; Sebastian, M.; Clark, D.; Brennan, A.; Oqueli, E.; Ajani, A.E.; et al. Role of beta blockers following percutaneous coronary intervention for acute coronary syndrome. Heart 2021, 107, 728–733. [Google Scholar] [CrossRef]
- Ozasa, N.; Kimura, T.; Morimoto, T.; Hou, H.; Tamura, T.; Shizuta, S.; Nakagawa, Y.; Furukawa, Y.; Hayashi, Y.; Nakao, K.; et al. Lack of effect of oral beta-blocker therapy at discharge on long-term clinical outcomes of ST-segment elevation acute myocardial infarction after primary percutaneous coronary intervention. Am. J. Cardiol. 2010, 106, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Dahl Aarvik, M.; Sandven, I.; Dondo, T.B.; Gale, C.P.; Ruddox, V.; Munkhaugen, J.; Atar, D.; Otterstad, J.E. Effect of oral β-blocker treatment on mortality in contemporary post-myocardial infarction patients: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 12–20. [Google Scholar] [CrossRef]
- Hong, J.; Barry, A.R. Long-Term Beta-Blocker Therapy after Myocardial Infarction in the Reperfusion Era: A Systematic Review. Pharmacotherapy 2018, 38, 546–554. [Google Scholar] [CrossRef]
- Sterling, L.H.; Filion, K.B.; Atallah, R.; Reynier, P.; Eisenberg, M.J. Intravenous beta-blockers in ST-segment elevation myocardial infarction: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 228, 295–302. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ozasa, N.; Morimoto, T.; Shiomi, H.; Bingyuan, B.; Suwa, S.; Nakagawa, Y.; Izumi, C.; Kadota, K.; Ikeguchi, S.; et al. Long-term use of carvedilol in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. PLoS ONE 2018, 13, e0199347. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, D.; Sakata, Y.; Suna, S.; Usami, M.; Matsumoto, S.; Shimizu, M.; Hara, M.; Uematsu, M.; Fukunami, M.; Hamasaki, T.; et al. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am. J. Cardiol. 2013, 111, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.H.; Chang, K.; Ahn, Y.; Jeon, D.S.; Lee, J.M.; Bin Kim, D.; Her, S.-H.; Park, C.S.; Kim, H.Y.; Yoo, K.-D.; et al. Benefit of β-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart 2014, 100, 492–499. [Google Scholar] [CrossRef]
- Yang, J.H.; Hahn, J.-Y.; Bin Song, Y.; Choi, S.-H.; Choi, J.-H.; Lee, S.H.; Kim, J.H.; Ahn, Y.-K.; Jeong, M.-H.; Choi, D.-J.; et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc. Interv. 2014, 7, 592–601. [Google Scholar] [CrossRef]
- Konishi, H.; Miyauchi, K.; Kasai, T.; Tsuboi, S.; Ogita, M.; Naito, R.; Nishizaki, Y.; Okai, I.; Tamura, H.; Okazaki, S.; et al. Long-term effect of β-blocker in ST-segment elevation myocardial infarction in patients with preserved left ventricular systolic function: A propensity analysis. Heart Vessel. 2016, 31, 441–448. [Google Scholar] [CrossRef]
- Puymirat, E.; Riant, E.; Aissaoui, N.; Soria, A.; Ducrocq, G.; Coste, P.; Cottin, Y.; Aupetit, J.F.; Bonnefoy, E.; Blanchard, D.; et al. β blockers and mortality after myocardial infarction in patients without heart failure: Multicentre prospective cohort study. BMJ 2016, 354, i4801. [Google Scholar] [CrossRef]
- Lee, P.H.; Park, G.-M.; Han, S.; Kim, Y.-G.; Lee, J.-Y.; Roh, J.-H.; Lee, J.-H.; Kim, Y.-H.; Lee, S.-W. Beta-blockers provide a differential survival benefit in patients with coronary artery disease undergoing contemporary post-percutaneous coronary intervention management. Sci. Rep. 2020, 10, 22121. [Google Scholar] [CrossRef]
- Won, H.; Suh, Y.; Kim, G.S.; Ko, Y.-G.; Hong, M.-K. Clinical Impact of Beta Blockers in Patients with Myocardial Infarction from the Korean National Health Insurance Database. Korean Circ. J. 2020, 50, 499–508. [Google Scholar] [CrossRef]
- Chen, R.-Z.; Liu, C.; Zhou, P.; Li, J.-N.; Zhou, J.-Y.; Wang, Y.; Zhao, X.-X.; Chen, Y.; Song, L.; Zhao, H.-J.; et al. Prognostic impacts of β-blockers in acute coronary syndrome patients without heart failure treated by percutaneous coronary intervention. Pharmacol. Res. 2021, 169, 105614. [Google Scholar] [CrossRef]
- Anonymous. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS—1. First International Study of Infarct Survival Collaborative Group. Lancet 1986, 2, 57–66. [Google Scholar]
- Chen, Z.M.; Pan, H.C.; Chen, Y.P.; Peto, R.; Collins, R.; Jiang, L.X.; Xie, J.X.; Liu, L.S.; COMMIT Collaborative Group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005, 366, 1622–1632. [Google Scholar] [PubMed]
- Exner, D.V.; Reiffel, J.A.; Epstein, A.E.; Ledingham, R.; Reiter, M.J.; Yao, Q.; Duff, H.; Follmann, D.; Schron, E.; Greene, H.; et al. Beta-blocker use and survival in patients with ventricular fibrillation or symptomatic ventricular tachycardia: The Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. J. Am. Coll. Cardiol. 1999, 34, 325–333. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Maqsood, M.H.; Alam, M.; Atar, D.; Birnbaum, Y. Efficacy of Long-Term Oral Beta-Blocker Therapy in Patients Who Underwent Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction with Preserved Left Ventricular Ejection Fraction: A Systematic Review and Meta-analysis. J. Cardiovasc. Pharmacol. 2021, 77, 87–93. [Google Scholar] [CrossRef]
- Dutta, P.; Courties, G.; Wei, Y.; Leuschner, F.; Gorbatov, R.; Robbins, C.S.; Iwamoto, Y.; Thompson, B.; Carlson, A.L.; Heidt, T.; et al. Myocardial infarction accelerates atherosclerosis. Nature 2012, 487, 325–329. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef]
- Doughty, R.N.; Whalley, G.A.; Walsh, H.A.; Gamble, G.D.; López-Sendón, J.; Sharpe, N. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: The CAPRICORN Echo Substudy. Circulation 2004, 109, 201–206. [Google Scholar] [CrossRef]
- O’gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Ko, D.; Hebert, P.; Coffey, C.; Sedrakyan, A.; Curtis, J.; Krumholz, H. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002, 288, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Japanese beta-Blockers and Calcium Antagonists Myocardial Infarction (JBCMI) Investigators. Comparison of the effects of beta blockers and calcium antagonists on cardiovascular events after acute myocardial infarction in Japanese subjects. Am. J. Cardiol. 2004, 93, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, G.; Abdelnoor, M.; Müller, C.; Kjeldsen, S.E.; Os, I.; Westheim, A. A comparison of the two beta-blockers carvedilol and atenolol on left ventricular ejection fraction and clinical endpoints after myocardial infarction. A single-centre, randomized study of 232 patients. Cardiology 2005, 103, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lee, J.M.; Kim, H.K.; Choi, K.H.; Rhee, T.-M.; Park, J.; Park, T.K.; Yang, J.H.; Bin Song, Y.; Choi, J.-H.; et al. Prognostic Impact of β-Blocker Dose After Acute Myocardial Infarction. Circ. J. 2019, 83, 410–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).