Mesenteric Ischemia in a Patient with Essential Thrombocythemia: Does COVID-19 Play Any Role? A Case Report and Overview of the Literature
Abstract
1. Introduction
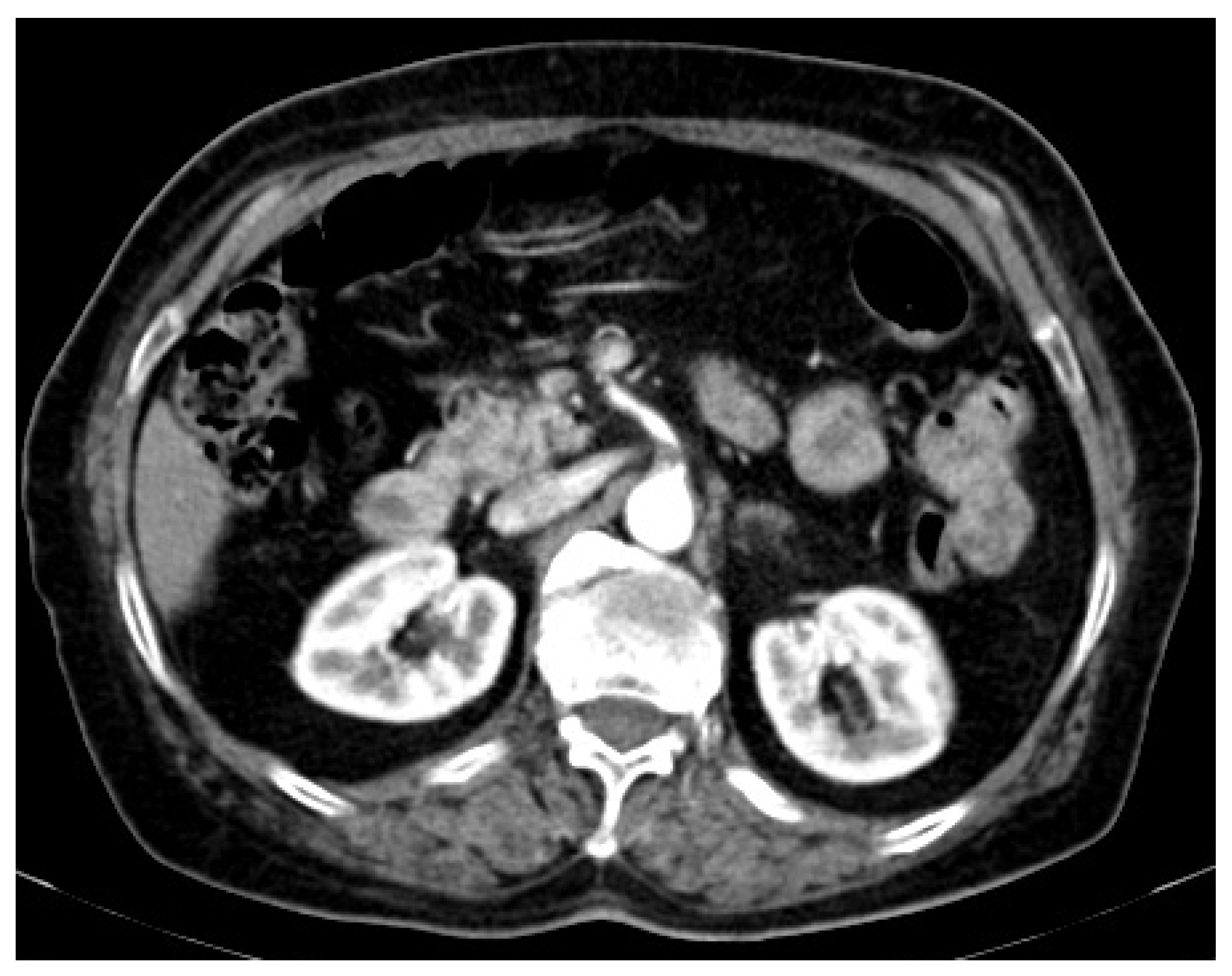
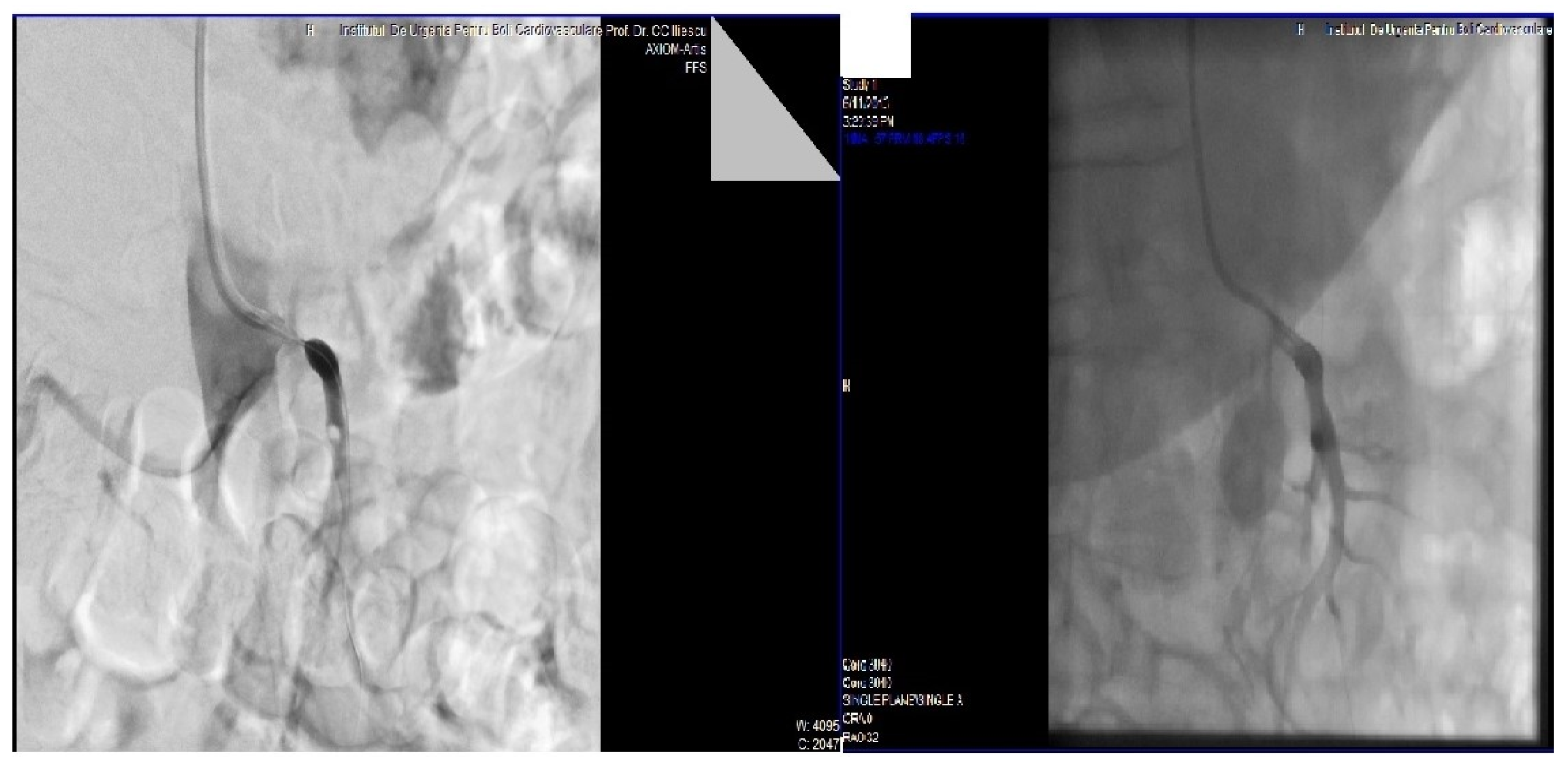
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M. Ischemic bowel disease in 2021. World J. Gastroenterol. 2021, 27, 4746–4762. [Google Scholar] [CrossRef] [PubMed]
- Gnanapandithan, K.; Feuerstadt, P. Review Article: Mesenteric Ischemia. Curr. Gastroenterol. Rep. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Al-Diery, H.; Phillips, A.; Evennett, N.; Pandanaboyana, S.; Gilham, M.; Windsor, J.A. The Pathogenesis of Nonocclusive Mesenteric Ischemia: Implications for Research and Clinical Practice. J. Intensive Care Med. 2018, 34, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Kamath, P.S.; Tefferi, A. Mesenteric Venous Thrombosis. Mayo Clin. Proc. 2013, 88, 285–294. [Google Scholar] [CrossRef]
- Clair, D.G.; Beach, J.M. Mesenteric Ischemia. N. Engl. J. Med. 2016, 374, 959–968. [Google Scholar] [CrossRef]
- Paterno, F.; Longo, W.E. The Etiology and Pathogenesis of Vascular Disorders of the Intestine. Radiol. Clin. N. Am. 2008, 46, 877–885. [Google Scholar] [CrossRef]
- Abu-Daff, S.; Al-Shahed, M. Mesenteric Venous Thrombosis and Factors Associated with Mortality: A Statistical Analysis with Five-Year Follow-Up. J. Gastrointest. Surg. 2009, 13, 1245–1250. [Google Scholar] [CrossRef]
- Sreenarasimhaiah, J. Chronic mesenteric ischemia. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 283–295. [Google Scholar] [CrossRef]
- Wilson, D.B.; Mostafavi, K.; Craven, T.E.; Ayerdi, J.; Edwards, M.S.; Hansen, K.J. Clinical Course of Mesenteric Artery Stenosis in Elderly Americans. Arch. Intern. Med. 2006, 166, 2095–2100. [Google Scholar] [CrossRef]
- Mosele, M.; Cardin, F.; Inelmen, E.M.; Coin, A.; Perissinotto, E.; Sergi, G.; Terranova, O.; Manzato, E. Ischemic colitis in the elderly: Predictors of the disease and prognostic factors to negative outcome. Scand. J. Gastroenterol. 2009, 45, 428–433. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Pirabe, A.; Pereyra, D.; Heber, S.; Hackl, H.; Schmuckenschlager, A.; Brunnthaler, L.; Santol, J.; Kammerer, K.; Oosterlee, J.; et al. Adverse Outcome in COVID-19 Is Associated With an Aggravating Hypo-Responsive Platelet Phenotype. Front. Cardiovasc. Med. 2021, 8, 795624. [Google Scholar] [CrossRef] [PubMed]
- Martyanov, A.A.; Boldova, A.E.; Stepanyan, M.G.; An, O.I.; Gur’Ev, A.S.; Kassina, D.V.; Volkov, A.Y.; Balatskiy, A.V.; Butylin, A.A.; Karamzin, S.S.; et al. Longitudinal multiparametric characterization of platelet dysfunction in COVID-19: Effects of disease severity, anticoagulation therapy and inflammatory status. Thromb. Res. 2022, 211, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Accurso, V.; Santoro, M.; Mancuso, S.; Napolitano, M.; Carlisi, M.; Mattana, M.; Russo, C.; Di Stefano, A.; Sirocchi, D.; Siragusa, S. The Essential Thrombocythemia in 2020: What We Know and Where We Still Have to Dig Deep. Clin. Med. Insights Blood Disord. 2020, 13, 2634853520978210. [Google Scholar] [CrossRef] [PubMed]
- Langabeer, S.E. Double-mutant myeloproliferative neoplasms. Med. Oncol. 2018, 35, 137. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Terlouw, L.G.; Moelker, A.; Abrahamsen, J.; Acosta, S.; Bakker, O.J.; Baumgartner, I.; Boyer, L.; Corcos, O.; Van Dijk, L.J.; Duran, M.; et al. European guidelines on chronic mesenteric ischaemia—Joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepatogastroenterologists, Hellenic Society of Gastroenterology, Cardiovascular and Interventional Radiological Society of Europe, and Dutch Mesenteric Ischemia Study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia. United Eur. Gastroenterol. J. 2020, 8, 371–395. [Google Scholar] [CrossRef]
- Tilsed, J.V.T.; Casamassima, A.; Kurihara, H.; Mariani, D.; Martinez, I.; Pereira, J.; Ponchietti, L.; Shamiyeh, A.; Al-Ayoubi, F.; Barco, L.A.B.; et al. ESTES guidelines: Acute mesenteric ischaemia. Eur. J. Trauma Emerg. Surg. 2016, 42, 253–270. [Google Scholar] [CrossRef]
- Tefferi, A.; Thiele, J.; Orazi, A.; Kvasnicka, H.M.; Barbui, T.; Hanson, C.A.; Barosi, G.; Verstovsek, S.; Birgegard, G.; Mesa, R.; et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: Recommendations from an ad hoc international expert panel. Blood 2007, 110, 1092–1097. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 92, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Isac, S.; Jelea, D.; Martac, C.; Stefan, M.-G.; Cotorogea-Simion, M.; Buzatu, C.G.S.; Ingustu, D.; Abdulkareem, I.; Vasilescu, C.; et al. COVID-19 Pandemic Was Associated with Lower Activity but Not Higher Perioperative Mortality in a Large Eastern European Center. Med. Sci. Monit. 2022, 28, e935809. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Isac, S.; Carstea, M.; Martac, C.; Mihalcea, L.; Buzatu, C.; Ionescu, D.; Georgescu, D.E.; Droc, G. Isolated liver trauma: A clinical perspective in a non-emergency center for liver surgery. Exp. Ther. Med. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Sreenarasimhaiah, J. Diagnosis and management of intestinal ischaemic disorders. BMJ 2003, 326, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Kashuk, J.; Moore, E.E.; Kluger, Y.; Biffl, W.; Gomes, C.A.; Ben-Ishay, O.; Rubinstein, C.; Balogh, Z.J.; Civil, I.; et al. Acute mesenteric ischemia: Guidelines of the World Society of Emergency Surgery. World J. Emerg. Surg. 2017, 12, 38. [Google Scholar] [CrossRef]
- Oldenburg, W.A.; Lau, L.L.; Rodenberg, T.J.; Edmonds, H.J.; Burger, C.D. Acute Mesenteric Ischemia. Arch. Intern. Med. 2004, 164, 1054–1062. [Google Scholar] [CrossRef]
- Atkins, M.D.; Kwolek, C.J.; LaMuraglia, G.M.; Brewster, D.C.; Chung, T.; Cambria, R.P. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: A comparative experience. J. Vasc. Surg. 2007, 45, 1162–1171. [Google Scholar] [CrossRef]
- Cervantes, F. Management of Essential Thrombocythemia. Hematology 2011, 2011, 215–221. [Google Scholar] [CrossRef][Green Version]
- Serban, D.; Tribus, L.C.; Vancea, G.; Stoian, A.P.; Dascalu, A.M.; Suceveanu, A.I.; Tanasescu, C.; Costea, A.C.; Tudosie, M.S.; Tudor, C.; et al. Acute Mesenteric Ischemia in COVID-19 Patients. J. Clin. Med. 2021, 11, 200. [Google Scholar] [CrossRef]
- Azouz, E.; Yang, S.; Monnier-Cholley, L.; Arrivé, L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med. 2020, 46, 1464–1465. [Google Scholar] [CrossRef]
- Al Mahruqi, G.; Stephen, E.; Abdelhedy, I.; Al Wahaibi, K. Our early experience with mesenteric ischemia in COVID-19 positive patients. Ann. Vasc. Surg. 2021, 73, 129.e1–129.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.-B.; Lyu, J.-R.; Lei, X.-M.; Li, W.; Wu, G.; Lyu, J.; Dai, Z.-M. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020, 96, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hajifathalian, K.; Krisko, T.; Mehta, A.; Kumar, S.; Schwartz, R.; Fortune, B.; Sharaiha, R.Z.; Kaplan, A.; Gonzalez, S.; Skaf, D.; et al. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology 2020, 159, 1137–1140.e2. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotorogea-Simion, M.; Isac, S.; Tita, A.; Toma, L.; Iliescu, L.E.; Mercan-Stanciu, A.; Isac, T.; Bobirca, A.; Bobirca, F.; Cobilinschi, C.; et al. Mesenteric Ischemia in a Patient with Essential Thrombocythemia: Does COVID-19 Play Any Role? A Case Report and Overview of the Literature. Medicina 2022, 58, 1147. https://doi.org/10.3390/medicina58091147
Cotorogea-Simion M, Isac S, Tita A, Toma L, Iliescu LE, Mercan-Stanciu A, Isac T, Bobirca A, Bobirca F, Cobilinschi C, et al. Mesenteric Ischemia in a Patient with Essential Thrombocythemia: Does COVID-19 Play Any Role? A Case Report and Overview of the Literature. Medicina. 2022; 58(9):1147. https://doi.org/10.3390/medicina58091147
Chicago/Turabian StyleCotorogea-Simion, Mihail, Sebastian Isac, Alina Tita, Letitia Toma, Laura Elena Iliescu, Adriana Mercan-Stanciu, Teodora Isac, Anca Bobirca, Florin Bobirca, Cristian Cobilinschi, and et al. 2022. "Mesenteric Ischemia in a Patient with Essential Thrombocythemia: Does COVID-19 Play Any Role? A Case Report and Overview of the Literature" Medicina 58, no. 9: 1147. https://doi.org/10.3390/medicina58091147
APA StyleCotorogea-Simion, M., Isac, S., Tita, A., Toma, L., Iliescu, L. E., Mercan-Stanciu, A., Isac, T., Bobirca, A., Bobirca, F., Cobilinschi, C., Tanasescu, M. D., & Droc, G. (2022). Mesenteric Ischemia in a Patient with Essential Thrombocythemia: Does COVID-19 Play Any Role? A Case Report and Overview of the Literature. Medicina, 58(9), 1147. https://doi.org/10.3390/medicina58091147









