Coronary Artery Disease in Women: Lessons Learned from Single-Center SPECT Registry and Future Directions for INOCA Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Methods
2.2.1. Clinical Characteristics
2.2.2. Performed Diagnostic Tests
2.3. Statistical Analysis
3. Results
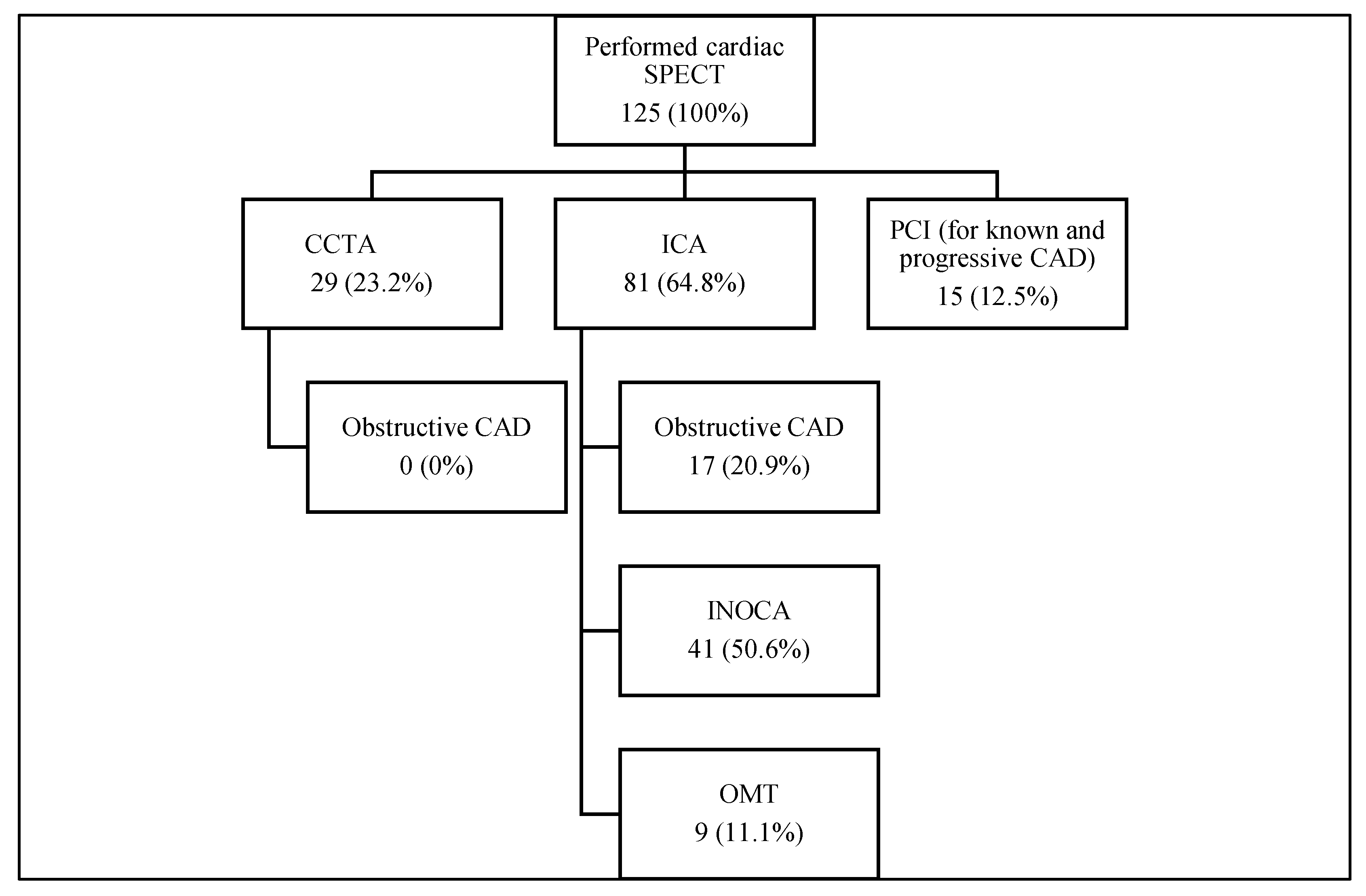
3.1. Diagnostic Approaches
3.2. Clinical Characteristics and Risk Factors
3.3. CAD and INOCA Symptom Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Bajare, I.; Dzerve, V.; Luguzis, A.; Barzdins, J.; Apinis, P.; Jegere, S.; Erglis, A. A nationwide cross-sectional epidemiological study of cardiovascular risk factors as a tool for management of primary and secondary prevention in Latvia. Eur. J. Prev. Cardiol. 2021, 28 (Suppl. S1), zwab061.182. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Nag, D.; Wu, J.C. Sex differences in the diagnostic evaluation of coronary artery disease. J. Nucl. Cardiol. 2011, 18, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Taqueti, V.R.; Dorbala, S.; Wolinsky, D.; Abbott, B.; Heller, G.V.; Bateman, T.M.; Mieres, J.H.; Phillips, L.M.; Wenger, N.K.; Shaw, L.J. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state-of-the-evidence and clinical recommendations. J. Nucl. Cardiol. 2017, 24, 1402–1426. [Google Scholar] [CrossRef] [Green Version]
- Wolk, M.J.; Bailey, S.R.; Doherty, J.U.; Douglas, P.S.; Hendel, R.C.; Kramer, C.M.; Min, J.K.; Patel, M.R.; Rosenbaum, L.; Shaw, L.J.; et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/ SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: A report of the American College of Cardiology Foun- dation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of Amer- ica, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2014, 63, 380–406. [Google Scholar]
- Mieres, J.H.; Gulati, M.; Bairey Merz, N.; Berman, D.S.; Gerber, T.C.; Hayes, S.N.; Kramer, C.M.; Min, J.K.; Newby, L.K.; Nixon, J.V.; et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: A consensus statement from the American Heart Association. Cir. Culation 2014, 130, 350–379. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477, Erratum in Eur. Heart J. 2020, 41, 4242. [Google Scholar] [CrossRef] [Green Version]
- Banks, K.; Lo, M.; Khera, A. Angina in Women without Obstructive Coronary Artery Disease. Curr. Cardiol. Rev. 2010, 6, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Ohman, E.M. Chronic stable angina. N. Engl. J. Med. 2016, 374, 1167–1176. [Google Scholar] [CrossRef]
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H. 2014 Williams Harvey Lecture: Importance of coronary vasomotion abnormalities-from bench to bedside. Eur. Heart J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepine, C.J. Multiple causes for ischemia without obstructive coronary artery disease: Not a short list. Circulation 2015, 131, 1044–1046. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734. [Google Scholar] [CrossRef]
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef] [Green Version]
- Suda, A.; Takahashi, J.; Hao, K.; Kikuchi, Y.; Shindo, T.; Ikeda, S.; Sato, K.; Sugisawa, J.; Matsumoto, Y.; Miyata, S.; et al. Coronary functional abnormal- ities in patients with angina and non-obstructive coronary artery disease. J. Am. Coll. Cardiol. 2019, 74, 2350–2360. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Shimokawa, H.; Suda, A.; Takahashi, J.; Berry, C.; Camici, P.G.; Crea, F.; Escaned, J.; Ford, T.; Yii, E.; Kaski, J.C.; et al. Clinical characteristics and prognosis of patients with microvascular angina: An international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur. Heart J. 2021, 42, 4592–4600. [Google Scholar] [CrossRef]
- Mathew, R.C.; Bourque, J.M.; Salerno, M.; Kramer, C.M. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc. Imaging. 2019, 13, 1577–1590. [Google Scholar] [CrossRef]
- Feher, A.; Sinusas, A.J. Quantitative Assessment of Coronary Microvascular Function. Circ. Cardiovasc. Imaging. 2017, 10, e006427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostini, D.; Roule, V.; Nganoa, C.; Roth, N.; Baavour, R.; Parienti, J.-J.; Beygui, F.; Manrique, A. First validation of myocardial flow reserve assessed by dynamic 99mTc-sestamibi CZT-SPECT camera: Head to head comparison with 15O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Slomka, P.J.; Berman, D.S.; Alexanderson-Rosas, E.; Germano, G. The role of PET quantification in cardiovascular imaging. Clin. Transl. Imaging 2014, 2, 343–358. [Google Scholar] [CrossRef]
- Grzybowski, A.; Puchalski, W.; Zieba, B.; Gruchala, M.; Fijalkowski, M.; Storoniak, K.; Sobiczewski, W.; Ciecwierz, D.; Targonski, R.; Rynkiewicz, A. How to improve noninvasive coronary artery disease diagnostics in premenopausal women? The influence of menstrual cycle on ST depression, left ventricle contractility, and chest pain observed during exercise echocardiography in women with angina and normal coronary angiogram. Am. Heart J. 2008, 156, 964.e1–964.e5. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.W.; Vinke, R.V.; Vos, J.R.; Simoons, M.L. Changes in the electrocardiographic response to exercise in healthy women. Br. Heart J. 1990, 64, 376–380. [Google Scholar] [CrossRef] [Green Version]
- Mieres, J.H.; Shaw, L.J.; Hendel, R.C.; Heller, G.V. The WOMEN study: What is the optimal method for ischemia evaluation in women? A multi-center, prospective, randomized study to establish the optimal method for detection of coronary artery disease (CAD) risk in women at an intermediate-high pretest likelihood of CAD: Study design. J. Nucl. Cardiol. 2009, 16, 105–112. [Google Scholar] [CrossRef]
- Shaw, L.J.; Mieres, J.H.; Hendel, R.H.; Boden, W.E.; Gulati, M.; Veledar, E.; Hachamovitch, R.; Arrighi, J.A.; Merz, C.N.B.; Gibbons, R.J.; et al. WOMEN Trial Investigators. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: Results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation 2011, 124, 1239–1249. [Google Scholar] [CrossRef] [Green Version]
- Kwok, Y.; Kim, C.; Grady, D.; Segal, M.; Redberg, R. Meta-analysis of exercise testing to detect coronary artery disease in women. Am. J. Cardiol. 1999, 83, 660–666. [Google Scholar] [CrossRef]
- Keteepe-Arachi, T.; Sharma, S. Cardiovascular Disease in Women: Understanding Symptoms and Risk Factors. Eur Cardiol. 2017, 12, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Douglas, P.S.; Ginsburg, G.S. The evaluation of chest pain in women. N. Engl. J. Med. 1996, 334, 1311–1315. [Google Scholar] [CrossRef]
- Rosengren, A.; Wallentin, L.; Gitt, A.; Behar, S.; Battler, A.; Hasdai, D. Sex, age, and clinical presentation of acute coronary syndromes. Eur. Heart J. 2004, 25, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Canto, J.G.; Rogers, W.J.; Goldberg, R.J.; Peterson, E.D.; Wenger, N.K.; Vaccarino, V.; Kiefe, C.I.; Frederick, P.D.; Sopko, G.; Zheng, Z.J. NRMI Investigators. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012, 307, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Bugiardini, R.; Bairey Merz, C.N. Angina with “normal” coronary arteries: A changing philosophy. JAMA 2005, 293, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.; Rahman, H.; Perera, D. Coronary microvascular disease: Current concepts of pathophysiology, diagnosis and management. Cardiovasc. Endocrinol. Metab. 2020, 10, 22–30. [Google Scholar] [CrossRef]
- Ford, T.J.; Ong, P.; Sechtem, U.; Beltrame, J.; Camici, P.G.; Crea, F.; Kaski, J.; Merz, N.B.; Pepine, C.J.; Shimokawa, H.; et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc. Interv. 2020, 13, 1847–1864. [Google Scholar] [CrossRef]
- Ford, T.J.; Berry, C.; De Bruyne, B.; Yong, A.S.C.; Barlis, P.; Fearon, W.F.; Ng, M.K.C. Physiological Predictors of Acute Coronary Syndromes: Emerging Insights From the Plaque to the Vulnerable Patient. JACC Cardiovasc. Interv. 2017, 10, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Pereyra, V.M.; Seitz, A.; McChord, J.; Hubert, A.; Bekeredjian, R.; Sechtem, U.; Ong, P. Invasive Diagnosis of Coronary Functional Disorders Causing Angina Pectoris. Eur. Cardiol. 2021, 16, e27. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll Cardiol. 2017, 72 Pt 23, 2841–2855. [Google Scholar] [CrossRef]
- Aldiwani, H.; Mahdai, S.; Alhatemi, G.; Bairey Merz, C.N. Microvascular Angina: Diagnosis and Management. Eur. Cardiol. 2021, 16, e46. [Google Scholar] [CrossRef]
- Marinescu, M.A.; Löffler, A.I.; Ouellette, M.; Smith, L.; Kramer, C.M.; Bourque, J.M. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc. Imaging. 2015, 8, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layland, J.; Carrick, D.; McEntegart, M.; Ahmed, N.; Payne, A.; McClure, J.; Sood, A.; McGeoch, R.; MacIsaac, A.; Whitbourn, R.; et al. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non-ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2013, 6, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, H.G.; Kronmal, R.A.; Vlietstra, R.E.; Frye, R.L. Seven year survival of patients with normal or near normal coronary arteriograms: A CASS registry study. J. Am. Coll Cardiol. 1986, 7, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Kenkre, T.S.; Malhotra, P.; Johnson, B.D.; Handberg, E.M.; Thompson, D.V.; Marroquin, O.C.; Rogers, W.J.; Pepine, C.J.; Bairey Merz, C.N.; Kelsey, S.F. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003863. [Google Scholar] [CrossRef]
- Sedlak, T.L.; Lee, M.; Izadnegahdar, M.; Merz, C.N.; Gao, M.; Humphries, K.H. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am. Heart J. 2013, 166, 38–44. [Google Scholar] [CrossRef]
- Handberg, E.M.; Merz, C.N.B.; Cooper-Dehoff, R.M.; Wei, J.; Conlon, M.; Lo, M.C.; Boden, W.; Frayne, S.M.; Villines, T.; Spertus, J.A. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am. Heart J. 2021, 237, 90–103. [Google Scholar] [CrossRef]
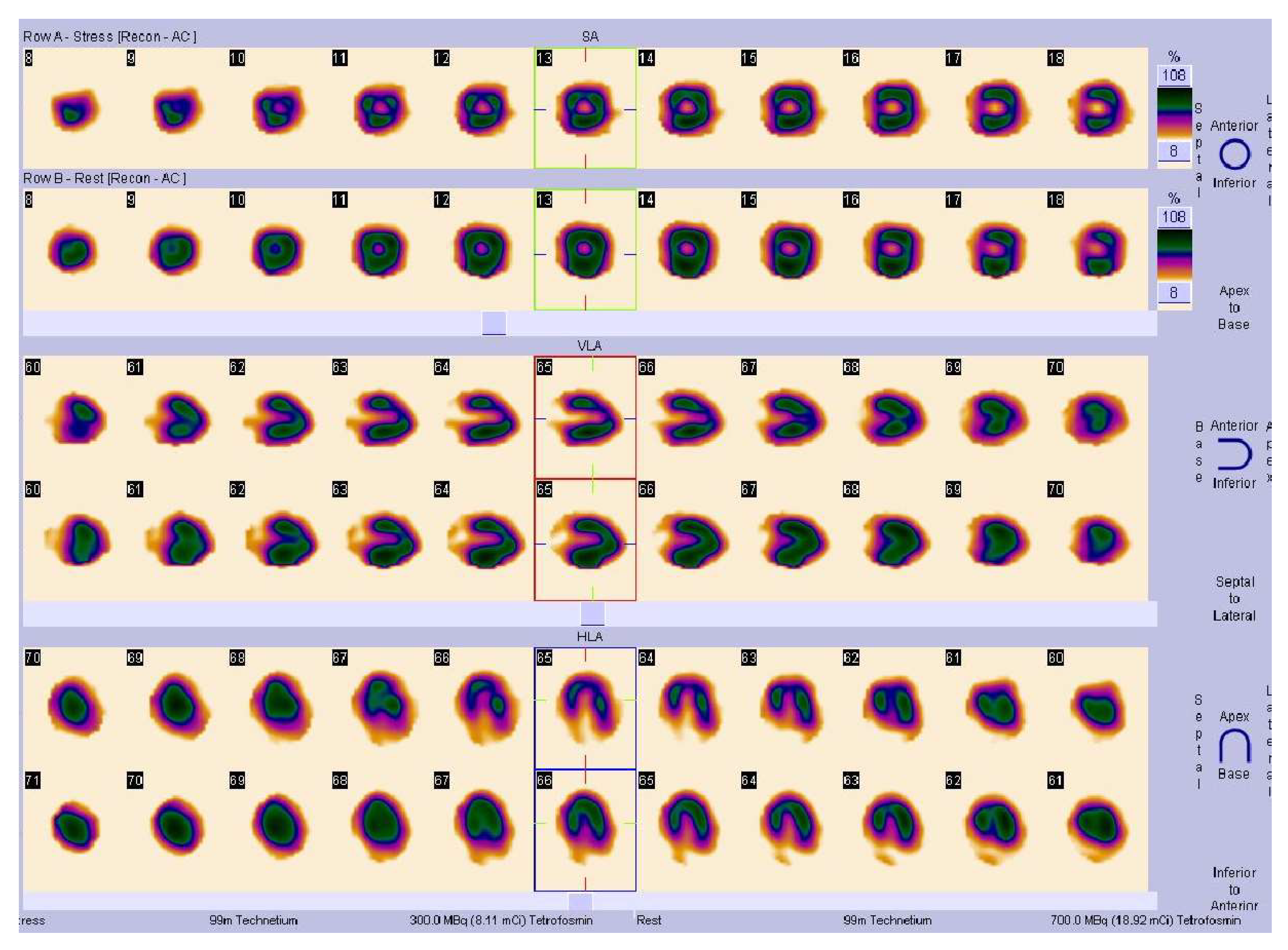
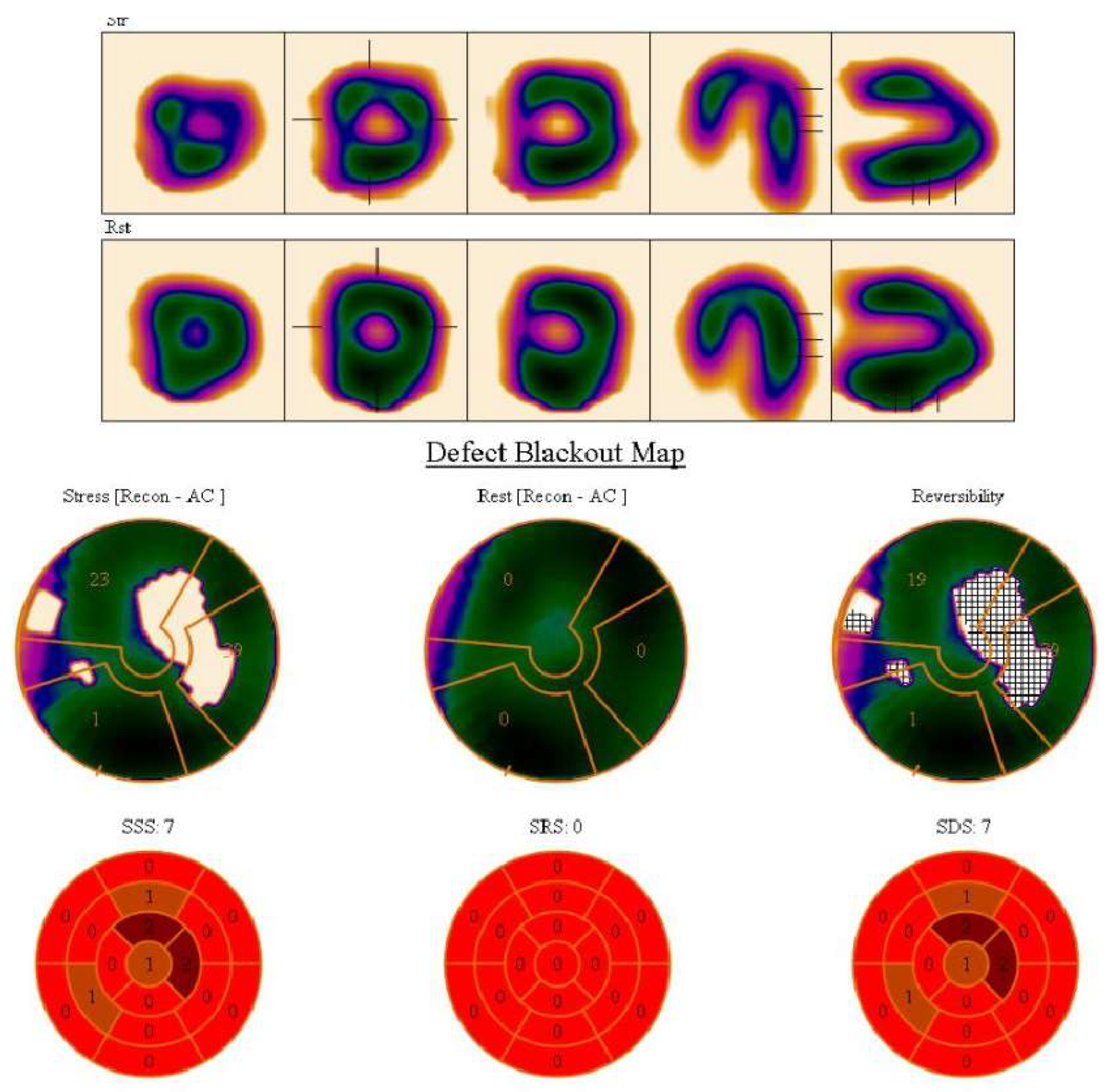
| 1. | Symptoms of myocardial ischemia: |
| Effort and/or rest angina | |
| Angina equivalents (i.e., shortness of breath) | |
| 2. | Absence of obstructive CAD by: |
| Computed coronary angiography | |
| Invasive coronary angiography | |
| 3. | Objective evidence of myocardial ischemia: |
| Ischemic ECG changes during an episode of chest pain | |
| Stress induced chest pain and/or ischemic ECG changes in the presence of absence of transient/reversible abnormal myocardial perfusion and/or wall motion abnormality | |
| 4. | Evidence of impaired coronary microvascular function: |
| Impaired coronary flow reserve (cutoff values depending on methodology use between <2.0 and <2.5) | |
| Coronary microvascular spasm, defined as reproduction of symptoms, ischemic ECG shifts but no epicardial spasm during acetylcholine testing | |
| Abnormal coronary microvascular resistance indices (e.g., IMR > 25) | |
| Coronary slow flow phenomenon, defined as TIMI frame count > 25 |
| N, % from All SPECTs | N, % from SPECTs with Perfusion Defect | 95% CI | p Value | |
|---|---|---|---|---|
| Age, years ± SD | 62.6 ± 10.6 | 60.1 ± 10.4 | (58.26–61.94) | 0.004 |
| Body mass index (BMI), kg/m2 ± SD | 28.1 ± 5.5 | 27.5 ± 5.6 | (26.51–28.49) | 0.361 |
| - Overweight (BMI > 25.0 kg/m2) | 486 (66.9%) | 72 (57.6%) | (0.48–0.66) | 0.015 |
| - Obese (BMI > 30.0 kg/m2) | 242 (33.3%) | 33 (26.4%) | (0.18–0.35) | 0.003 |
| Current or ex-smokers | 87 (11.9%) | 19 (15.2%) | (0.09–0.22) | 0.286 |
| Dyslipidemia | 614 (84.6%) | 114 (91.2%) | (0.84–0.95) | 0.029 |
| Arterial hypertension | 535 (73.7%) | 81 (64.8%) | (0.55–0.73) | 0.013 |
| Type 2 diabetes | 60 (8.3%) | 10 (8.0%) | (0.03–0.14) | 0.951 |
| Known CAD | 298 (41.0%) | 43 (34.4%) | (0.26–0.43) | 0.119 |
| Positive exercise test | 306 (42.1%) | 62 (49.6%) | (0.40–0.58) | 0.063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitola, B.; Trusinskis, K.; Mintale, I.; Kalnina, M.; Erglis, A. Coronary Artery Disease in Women: Lessons Learned from Single-Center SPECT Registry and Future Directions for INOCA Patients. Medicina 2022, 58, 1139. https://doi.org/10.3390/medicina58091139
Vitola B, Trusinskis K, Mintale I, Kalnina M, Erglis A. Coronary Artery Disease in Women: Lessons Learned from Single-Center SPECT Registry and Future Directions for INOCA Patients. Medicina. 2022; 58(9):1139. https://doi.org/10.3390/medicina58091139
Chicago/Turabian StyleVitola, Barbara, Karlis Trusinskis, Iveta Mintale, Marika Kalnina, and Andrejs Erglis. 2022. "Coronary Artery Disease in Women: Lessons Learned from Single-Center SPECT Registry and Future Directions for INOCA Patients" Medicina 58, no. 9: 1139. https://doi.org/10.3390/medicina58091139
APA StyleVitola, B., Trusinskis, K., Mintale, I., Kalnina, M., & Erglis, A. (2022). Coronary Artery Disease in Women: Lessons Learned from Single-Center SPECT Registry and Future Directions for INOCA Patients. Medicina, 58(9), 1139. https://doi.org/10.3390/medicina58091139






