Anesthetics and Long Term Cancer Outcomes: May Epigenetics Be the Key for Pancreatic Cancer?
Abstract
1. Introduction
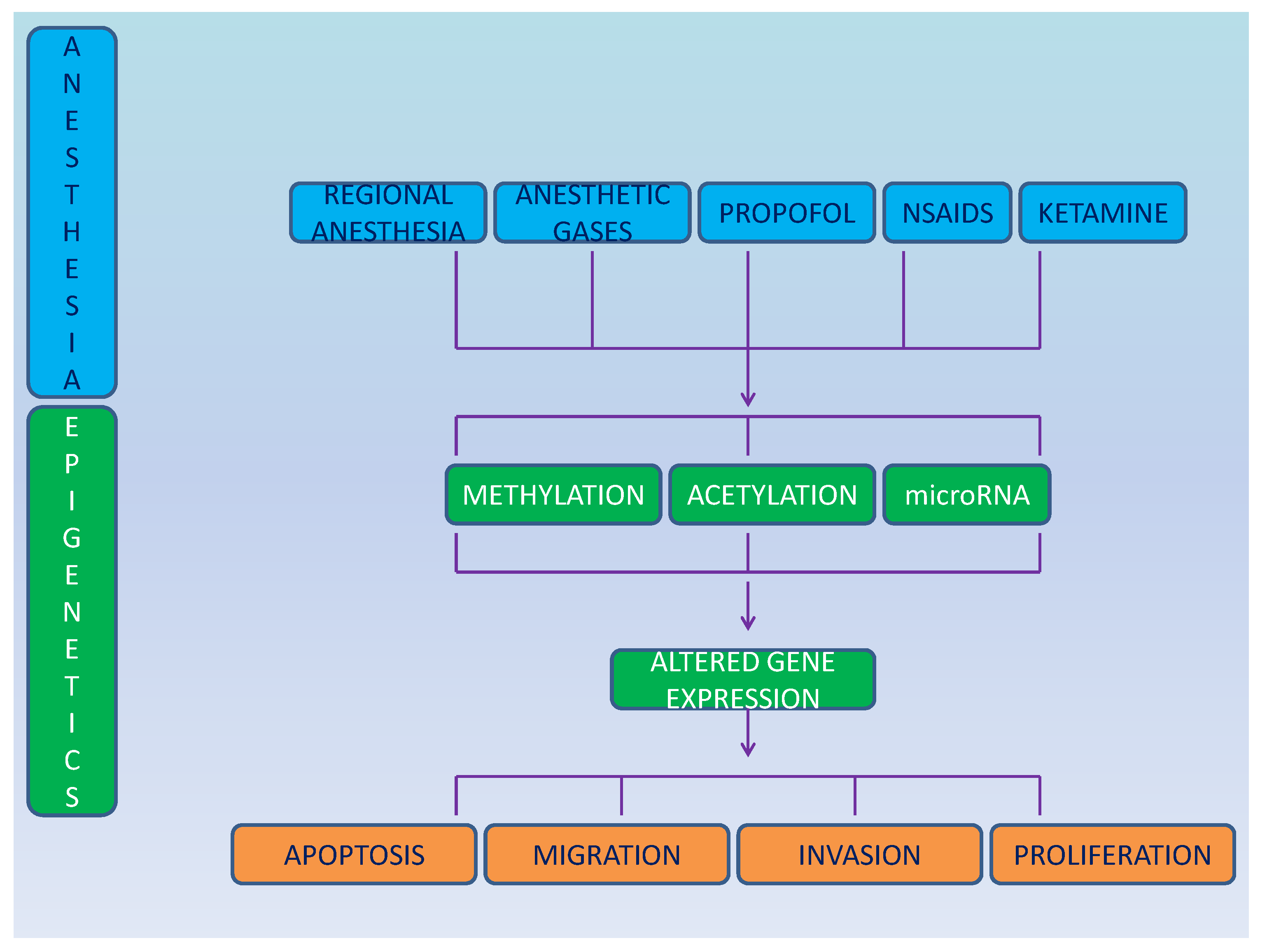
2. Epigenetics
2.1. Epigenetics and Anesthetics
2.2. Post-Translational Chromatin Regulation
2.3. RNA
3. Anesthesia and Cancer Epigenetics
4. Anesthetic Management
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Lellis, L.; Veschi, S.; Tinari, N.; Mokini, Z.; Carradori, S.; Brocco, D.; Florio, R.; Grassadonia, A.; Cama, A. Drug Repurposing, an Attractive Strategy in Pancreatic Cancer Treatment: Preclinical and Clinical Updates. Cancers 2021, 13, 3946. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Sessler, I.D.; Riedel, B. Anesthesia and Cancer Recurrence: Context for Divergent Study Outcomes. Anesthesiology 2019, 130, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Aguirre, J.A.; Bencic, I.; Borgeat, A.; Cama, A.; Condron, C.; Eintrei, C.; Eroles, P.; Gupta, A.; Hales, T.G.; et al. How Anesthetic, Analgesic and Other Non-Surgical Techniques during Cancer Surgery Might Affect Postoperative Oncologic Outcomes: A Summary of Current State of Evidence. Cancers 2019, 11, 592. [Google Scholar] [CrossRef]
- Sessler, I.D.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, A.T.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef]
- Du, Y.-T.; Li, Y.-W.; Zhao, B.-J.; Guo, X.-Y.; Feng, Y.; Zuo, M.-Z.; Fu, C.; Zhou, W.-J.; Li, H.-J.; Liu, Y.-F.; et al. Long-term Survival after Combined Epidural–General Anesthesia or General Anesthesia alone: Follow-up of a Randomized Trial. Anesthesiology 2021, 135, 233–245. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Li, H.-J.; Li, M.-H.; Huang, S.-M.; Li, X.; Liu, Q.-H.; Li, J.; Wang, D.-X.; Sessler, D.I. Epidural Anesthesia–Analgesia and Recurrence-free Survival after Lung Cancer Surgery: A Randomized Trial. Anesthesiology 2021, 135, 419–432. [Google Scholar] [CrossRef]
- Lilley, R.; Chan, E.; Ng, N.; Orr, A.; Szostok, M.; Yeh, G.T.T.; Tulloch, R.; Ramsay, G.; Mokini, Z.; Forget, P. Recurrence Kinetics after Laparoscopic versus Open Surgery in Colon Cancer. A Meta-Analysis. J. Clin. Med. 2021, 10, 4163. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Berger, S.L. The complex language of chromatin regulation during transcription. Nature 2007, 447, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, E.S.; Constancia, M. Mechanisms of Disease: The developmental origins of disease and the role of the epigenotype. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Kangaspeska, S.; Stride, B.; Métivier, R.; Polycarpou-Schwarz, M.; Ibberson, D.; Carmouche, R.P.; Benes, V.; Gannon, F.; Reid, G. Transient cyclical methylation of promoter DNA. Nature 2008, 452, 112–115. [Google Scholar] [CrossRef]
- Métivier, R.; Gallais, R.; Tiffoche, C.; Le Péron, C.; Jurkowska, R.Z.; Carmouche, R.P.; Ibberson, D.; Barath, P.; Demay, F.; Reid, G.; et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature 2008, 452, 45–50. [Google Scholar] [CrossRef]
- Wang, K.; Tian, Y.; Zhang, Y.; Li, X.; Wei, X.; Hu, H.; Xu, S. Toxicity mechanism of sevoflurane in neural stem cells of rats through DNA methylation. Exp. Ther. Med. 2019, 18, 237–241. [Google Scholar] [CrossRef]
- Martynyuk, E.A.; Ju, L.-S.; Morey, E.T.; Zhang, J.-Q. Neuroendocrine, epigenetic, and intergenerational effects of general anesthetics. World J. Psychiatry 2020, 10, 81–94. [Google Scholar] [CrossRef]
- Lin, E.P.; Lee, J.-R.; Lee, C.S.; Deng, M.; Loepke, A.W. Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicology Teratol. 2017, 60, 117–128. [Google Scholar] [CrossRef]
- Sakamoto, A.; Imai, J.-I.; Nishikawa, A.; Honma, R.; Ito, E.; Yanagisawa, Y.; Kawamura, M.; Ogawa, R.; Watanabe, S. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene 2005, 356, 39–48. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takemori, K.; Sakamoto, A. Circadian gene expression is suppressed during sevoflurane anesthesia and the suppression persists after awakening. Brain Res. 2007, 1185, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, K.; Yoshida, Y.; Takemori, K.; Kobayashi, K.; Sakamoto, A. Expressions of genes encoding drug-metabolizing enzymes are altered after sevoflurane, isoflurane, propofol or dexmedetomidine anesthesia. Biomed. Res. 2009, 30, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tsuboko, Y.; Sakamoto, A. Propofol anaesthesia alters the cerebral proteome differently from sevoflurane anaesthesia. Biomed. Res. 2011, 32, 55–65. [Google Scholar] [CrossRef]
- Heindel, J.J.; Balbus, J.; Birnbaum, L.; Brune-Drisse, M.N.; Grandjean, P.; Gray, K.; Landrigan, P.J.; Sly, P.D.; Suk, W.A.; Cory Slechta, D.; et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology 2015, 156, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, A.M.; Mitchell, M.D. Developmental origins of health and disease: Reducing the burden of chronic disease in the next generation. Genome Med. 2010, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.; Jacob, C.; Barker, M.; Fall, C.H.D.; Hanson, M.; Harvey, N.C.; Inskip, H.M.; Kumaran, K.; Cooper, C. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare 2017, 5, 14. [Google Scholar] [CrossRef]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.R.; Jørgensen, N.; Kristiansen, V.B.; et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef]
- Chastain-Potts, S.E.; Tesic, V.; Tat, Q.L.; Cabrera, O.H.; Quillinan, N.; Jevtovic-Todorovic, V. Sevoflurane Exposure Results in Sex-Specific Transgenerational Upregulation of Target IEGs in the Subiculum. Mol. Neurobiol. 2020, 57, 11–22. [Google Scholar] [CrossRef]
- Bates, S.E. Epigenetic Therapies for Cancer. New Engl. J. Med. 2020, 383, 650–663. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Liu, W.-X.; Yang, J.-J.; Xu, N.; Xie, Z.-M.; Ju, L.-S.; Ji, M.-H.; Martynyuk, A.E.; Yang, J.-J. Role of histone acetylation in long-term neurobehavioral effects of neonatal Exposure to sevoflurane in rats. Neurobiol. Dis. 2016, 91, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.H.; Useinovic, N.; Jevtovic-Todorovic, V. Neonatal anesthesia and dysregulation of the epigenome. Biol. Reprod. 2021, 105, 720–734. [Google Scholar] [CrossRef]
- Sun, Y.; Sahbaie, P.; Liang, D.; Li, W.; Shi, X.; Kingery, P.; Clark, J.D. DNA Methylation Modulates Nociceptive Sensitization after Incision. PLoS ONE 2015, 10, e0142046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caputi, F.F.; Carboni, L.; Rullo, L.; Alessandrini, I.; Balzani, E.; Melotti, R.M.; Romualdi, P.; Candeletti, S.; Fanelli, A. An Exploratory Pilot Study of Changes in Global DNA Methylation in Patients Undergoing Major Breast Surgery under Opioid-Based General Anesthesia. Front. Pharmacol. 2021, 12, 733577. [Google Scholar] [CrossRef]
- Doehring, A.; Oertel, B.G.; Sittl, R.; Lötsch, J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain 2013, 154, 15–23. [Google Scholar] [CrossRef]
- Fragou, D.; Zanos, P.; Kouidou, S.; Njau, S.; Kitchen, I.; Bailey, A.; Kovatsi, L. Effect of chronic heroin and cocaine administration on global DNA methylation in brain and liver. Toxicol. Lett. 2013, 218, 260–265. [Google Scholar] [CrossRef]
- Lirk, P.; Fiegl, H.; Weber, N.C.; Hollmann, M.W. Epigenetics in the perioperative period. Br. J. Pharmacol. 2015, 172, 2748–2755. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, M.; Gao, Q.; He, A.; Liu, Y.; Mei, H. Current Advances on the Important Roles of Enhancer RNAs in Gene Regulation and Cancer. BioMed Res. Int. 2018, 2018, 2405351. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef]
- Amin, N.; McGrath, A.; Chen, Y.-P.P. Author Correction: Evaluation of deep learning in non-coding RNA classification. Nat. Mach. Intell. 2020, 2, 236. [Google Scholar] [CrossRef]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta. Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Tan, J.; Miao, Y.; Zhang, Q. The role of microvesicles containing microRNAs in vascular endothelial dysfunction. J. Cell. Mol. Med. 2019, 23, 7933–7945. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Tanaka, S.; Arai, M.; Genda, Y.; Sakamoto, A. Differences in microRNA Changes of Healthy Rat Liver between Sevoflurane and Propofol Anesthesia. Anesthesiology 2012, 117, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ishikawa, M.; Arai, M.; Genda, Y.; Sakamoto, A. Changes in microRNA expression in rat lungs caused by sevoflurane anesthesia: A TaqMan® low-density array study. Biomed. Res. 2012, 33, 255–263. [Google Scholar] [CrossRef]
- Goto, G.; Hori, Y.; Ishikawa, M.; Tanaka, S.; Sakamoto, A. Changes in the gene expression levels of microRNAs in the rat hippocampus by sevoflurane and propofol anesthesia. Mol. Med. Rep. 2014, 9, 1715–1722. [Google Scholar] [CrossRef]
- Liao, X.; Zhou, S.; Zong, J.; Wang, Z. Sevoflurane exerts protective effects on liver ischemia/reperfusion injury by regulating NFKB3 expression via miR-9-5p. Exp. Ther. Med. 2019, 17, 2632–2640. [Google Scholar] [CrossRef]
- Otsuki, T.; Ishikawa, M.; Hori, Y.; Goto, G.; Sakamoto, A. Volatile anesthetic sevoflurane ameliorates endotoxin-induced acute lung injury via microRNA modulation in rats. Biomed. Rep. 2015, 3, 408–412. [Google Scholar] [CrossRef]
- Lu, Y.; Jian, M.-Y.; Ouyang, Y.-B.; Han, R.-Q. Changes in Rat Brain MicroRNA Expression Profiles Following Sevoflurane and Propofol Anesthesia. Chin. Med J. 2015, 128, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Li, E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 2002, 3, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.H.; van Lohuizen, M. Epigenetics and cancer. Genes Dev. 2004, 18, 2315–2335. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, E.N.; Scaffidi, P. Epigenetics and Cancer Stem Cells: Unleashing, Hijacking, and Restricting Cellular Plasticity. Trends Cancer 2017, 3, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Huitink, J.M.; Heimerikxs, M.; Nieuwland, M.; Loer, S.A.; Brugman, W.; Velds, A.; Sie, D.; Kerkhoven, R.M. Volatile Anesthetics Modulate Gene Expression in Breast and Brain Tumor Cells. Anesth. Analg. 2010, 111, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhao, H.; Liu, X.; Wang, D.; Wang, Y.; Ai, Y.; Yang, J. Sevoflurane suppresses cell viability and invasion and promotes cell apoptosis in colon cancer by modulating exosome-mediated circ-HMGCS1 via the miR-34a-5p/SGPP1 axis. Oncol. Rep. 2020, 44, 2429–2442. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, Y.-T.; Rong, W. Sevoflurane induces apoptosis and inhibits the growth and motility of colon cancer in vitro and in vivo via inactivating Ras/Raf/MEK/ERK signaling. Life Sci. 2019, 239, 116916. [Google Scholar] [CrossRef]
- Fan, L.; Wu, Y.; Wang, J.; He, J.; Han, X. Sevoflurane inhibits the migration and invasion of colorectal cancer cells through regulating ERK/MMP-9 pathway by up-regulating miR-203. Eur. J. Pharmacol. 2019, 850, 43–52. [Google Scholar] [CrossRef]
- Sun, S.Q.; Ren, L.J.; Liu, J.; Wang, P.; Shan, S.M. Sevoflurane inhibits migration and invasion of colorectal cancer cells by regulating microRNA-34a/ADAM10 axis. Neoplasma 2019, 66, 887–895. [Google Scholar] [CrossRef]
- Buschmann, D.; Brandes, F.; Lindemann, A.; Maerte, M.; Ganschow, P.; Chouker, A.; Schelling, G.; Pfaffl, M.W.; Reithmair, M. Propofol and Sevoflurane Differentially Impact MicroRNAs in Circulating Extracellular Vesicles during Colorectal Cancer Resection: A Pilot Study. Anesthesiology 2020, 132, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, D.; Shen, Y.; Liu, B.; Zhou, H.; Tao, H.; Huang, J. The potential combinational effect of miR-34a with celecoxib in osteosarcoma. Anti-Cancer Drugs 2017, 28, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Jiang, H.; Jian, X.; Zhang, W. Change of miRNA expression profiles in patients with knee osteoarthritis before and after celecoxib treatment. J. Clin. Lab. Anal. 2019, 33, e22648. [Google Scholar] [CrossRef]
- Guo, Y.; Kenney, S.R.; Cook, L.; Adams, S.F.; Rutledge, T.; Romero, E.; Oprea, T.I.; Sklar, L.A.; Bedrick, E.; Wiggins, C.L.; et al. A Novel Pharmacologic Activity of Ketorolac for Therapeutic Benefit in Ovarian Cancer Patients. Clin. Cancer Res. 2015, 21, 5064–5072. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kenney, S.R.; Muller, C.Y.; Adams, S.; Rutledge, T.; Romero, E.; Murray-Krezan, C.; Prekeris, R.; Sklar, L.A.; Hudson, L.G.; et al. R-Ketorolac Targets Cdc42 and Rac1 and Alters Ovarian Cancer Cell Behaviors Critical for Invasion and Metastasis. Mol. Cancer Ther. 2015, 14, 2215–2227. [Google Scholar] [CrossRef]
- Lirk, P.; Berger, R.; Hollmann, M.W.; Fiegl, H. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br. J. Anaesth. 2012, 109, 200–207. [Google Scholar] [CrossRef]
- Malsy, M.; Graf, B.; Bundscherer, A. The Effects of Analgesics and Local Anesthetics on Gene Transcription Mediated by NFATc2 and Sp1 in Pancreatic Carcinoma. Anticancer Res. 2019, 39, 4721–4728. [Google Scholar] [CrossRef]
- Malsy, M.; Gebhardt, K.; Gruber, M.; Wiese, C.; Graf, B.; Bundscherer, A. Effects of ketamine, s-ketamine, and MK 801 on proliferation, apoptosis, and necrosis in pancreatic cancer cells. BMC Anesthesiol. 2015, 15, 111. [Google Scholar] [CrossRef]
- He, G.-N.; Bao, N.-R.; Wang, S.; Xi, M.; Zhang, T.-H.; Chen, F.-S. Ketamine Induces Ferroptosis of Liver Cancer Cells by Targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des. Dev. Ther. 2021, 15, 3965–3978. [Google Scholar] [CrossRef]
- Li, T.; Yang, J.; Yang, B.; Zhao, G.; Lin, H.; Liu, Q.; Wang, L.; Wan, Y.; Jiang, H. Ketamine Inhibits Ovarian Cancer Cell Growth by Regulating the lncRNA-PVT1/EZH2/p57 Axis. Front. Genet. 2021, 11, 597467. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Huang, L.; Zhang, F.; Kang, R. Effects of propofol on cancer development and chemotherapy: Potential mechanisms. Eur. J. Pharmacol. 2018, 831, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Saleh, S.H.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef]
- Xing, N.; Xing, F.; Li, Y.; Li, P.; Zhang, J.; Wang, D.; Zhang, W.; Yang, J. Dexmedetomidine improves propofol-induced neuronal injury in rat hippocampus with the involvement of miR-34a and the PI3K/Akt signaling pathway. Life Sci. 2020, 247, 117359. [Google Scholar] [CrossRef] [PubMed]
- Li, G.F.; Li, Z.B.; Zhuang, S.J. Inhibition of microRNA-34a protects against propofol anesthesia-induced neurotoxicity and cognitive dysfunction via the MAPK/ERK signaling pathway. Neurosci. Lett. 2018, 675, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiao, H.; Jiang, Z.; Chen, R. Propofol inhibits migration and induces apoptosis of pancreatic cancer PANC-1 cells through miR-34a-mediated E-cadherin and LOC285194 signals. Bioengineered 2020, 11, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gao, Y.; Zhang, F. Propofol inhibits pancreatic cancer proliferation and metastasis by up-regulating miR-328 and down-regulating ADAM8. Basic Clin. Pharmacol. Toxicol. 2019, 125, 271–278. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; You, L.; Chen, S.; Zhu, M.; Miao, C. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur. J. Pharmacol. 2017, 795, 150–159. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, H.; Zheng, F.; Liu, D.; Dong, T. Propofol suppresses proliferation and invasion of pancreatic cancer cells by upregulating microRNA-133a expression. Genet. Mol. Res. 2015, 14, 7529–7537. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Hong, G.; Quan, J.; Zhang, L.; Yu, M. Propofol Inhibits Growth and Invasion of Pancreatic Cancer Cells through Regulation of the MiR-21/Slug Signaling Pathway. Am. J. Transl. Res. 2016, 8, 4120–4133. [Google Scholar]
- Gao, Y.; Yu, X.; Zhang, F.; Dai, J. Propofol inhibits pancreatic cancer progress under hypoxia via ADAM 8. J. Hepato-Biliary-Pancreatic Sci. 2019, 26, 219–226. [Google Scholar] [CrossRef]
- Yu, X.; Shi, J.; Wang, X.; Zhang, F. Propofol affects the growth and metastasis of pancreatic cancer via ADAM8. Pharmacol. Rep. 2019, 72, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.-H. Propofol induces apoptosis and increases gemcitabine sensitivity in pancreatic cancer cells in vitro by inhibition of nuclear factor-κB activity. World J. Gastroenterol. 2013, 19, 5485–5492. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, R.K.; Lee, S.; Abd-Elsayed, A. The Relationship between Regional Anesthesia and Cancer: A Metaanalysis. Ochsner J. 2017, 17, 345–361. [Google Scholar] [PubMed]
- Cakmakkaya, O.S.; Kolodzie, K.; Apfel, C.C.; Pace, N.L. Anaesthetic techniques for risk of malignant tumour recurrence. Cochrane Database Syst. Rev. 2014, 11, CD008877. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Iwasaki, M.; Sakamoto, A.; Ma, D. Anesthetics may modulate cancer surgical outcome: A possible role of miRNAs regulation. BMC Anesthesiol. 2021, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Ponferrada, A.R.; Orriach, J.L.G.; Manso, A.M.; Haro, E.S.; Molina, S.R.; Heredia, A.F.; Lopez, M.B.; Mañas, J.C. Anaesthesia and cancer: Can anaesthetic drugs modify gene expression? Ecancermedicalscience 2020, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Buggy, D.J.; Riedel, B.; Sessler, D.I. Can anaesthetic technique influence cancer outcome? The next step. Br. J. Anaesth. 2021, 127, 5–7. [Google Scholar] [CrossRef]
- Enlund, M. Is It Definitely Clear That Long-Term Survival after Breast Cancer Surgery Is Not Affected by Anaesthetics? Cancers 2021, 13, 3390. [Google Scholar] [CrossRef]
- Piccart, M.; Veer, L.J.V.; Poncet, C.; Cardozo, J.M.N.L.; Delaloge, S.; Pierga, J.-Y.; Vuylsteke, P.; Brain, E.; Vrijaldenhoven, S.; Neijenhuis, A.P.; et al. 70-gene signature as an aid for treatment decisions in early breast cancer: Updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021, 22, 476–488. [Google Scholar] [CrossRef]
- Wigmore, T.J.; Mohammed, K.; Jhanji, S. Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology 2016, 124, 69–79. [Google Scholar] [CrossRef]
- Lacy, A.M.; García-Valdecasas, J.C.; Delgado, S.; Castells, A.; Taurá, P.; Piqué, J.M.; Visa, J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet 2002, 359, 2224–2229. [Google Scholar] [CrossRef]
- Martin, M.A.; Meyricke, R.; O’Neill, T.; Roberts, S. Breast-Conserving Surgery Versus Mastectomy for Survival from Breast Cancer: The Western Australian Experience. Ann. Surg. Oncol. 2007, 14, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, S.H.; Kim, Y.; Kim, H.-A.; Kim, B.S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: A retrospective study. Korean J. Anesthesiol. 2016, 69, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Malo-Manso, A.; Raigon-Ponferrada, A.; Diaz-Crespo, J.; Escalona-Belmonte, J.J.; Cruz-Mañas, J.; Guerrero-Orriach, J.L. Opioid Free Anaesthesia and Cancer. Curr. Pharm. Des. 2019, 25, 3011–3019. [Google Scholar] [CrossRef]
- Bundscherer, A.; Malsy, M.; Gebhardt, K.; Metterlein, T.; Plank, C.; Wiese, C.; Gruber, M.; Graf, B. Effects of ropivacaine, bupivacaine and sufentanil in colon and pancreatic cancer cells in vitro. Pharmacol. Res. 2015, 95–96, 126–131. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Zhu, X.; Zhu, M.; Sun, Z.; Cata, J.P.; Chen, W.; Miao, C. Association between intraoperative intravenous lidocaine infusion and survival in patients undergoing pancreatectomy for pancreatic cancer: A retrospective study. Br. J. Anaesth. 2020, 125, 141–148. [Google Scholar] [CrossRef]
- Lai, H.-C.; Lee, M.-S.; Liu, Y.-T.; Lin, K.-T.; Hung, K.-C.; Chen, J.-Y.; Wu, Z.-F. Propofol-based intravenous anesthesia is associated with better survival than desflurane anesthesia in pancreatic cancer surgery. PLoS ONE 2020, 15, e0233598. [Google Scholar] [CrossRef]
- Call, T.R.; Pace, N.L.; Thorup, D.B.; Maxfield, D.; Chortkoff, B.; Christensen, J.; Mulvihill, S.J. Factors Associated with Improved Survival after Resection of Pancreatic Adenocarcinoma. Anesthesiology 2015, 122, 317–324. [Google Scholar] [CrossRef]
- Soliz, J.M.; Ifeanyi, I.C.; Katz, M.H.; Wilks, J.; Cata, J.P.; McHugh, T.; Fleming, J.B.; Feng, L.; Rahlfs, T.; Bruno, M.; et al. Comparing Postoperative Complications and Inflammatory Markers Using Total Intravenous Anesthesia Versus Volatile Gas Anesthesia for Pancreatic Cancer Surgery. Anesthesiol. Pain Med. 2017, 7, e13879. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Pei, L.-J.; Sun, C.; Zhao, M.-Y.; Che, L.; Huang, Y.-G. Regional anesthesia and cancer recurrence in patients with late-stage cancer: A systematic review and meta-analysis. Chin. Med J. 2021, 134, 2403–2411. [Google Scholar] [CrossRef]
- Pusztai, L.; Hess, K.R. Clinical trial design for microarray predictive marker discovery and assessment. Ann. Oncol. 2004, 15, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Piccart-Gebhart, M.; van’t Veer, L.; Rutgers, E. The MINDACT trial: The first prospective clinical validation of a genomic tool. Mol. Oncol. 2007, 1, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Vassy, J.L.; Christensen, K.; Schonman, E.F.; Blout, C.L.; Robinson, J.O.; Krier, J.B.; Diamond, P.M.; Lebo, M.; Machini, K.; Azzariti, D.R.; et al. The Impact of Whole-Genome Sequencing on the Primary Care and Outcomes of Healthy Adult Patients: A Pilot Randomized Trial. Ann. Intern. Med. 2017, 167, 159–169. [Google Scholar] [CrossRef]
- Dubowitz, J.; Hiller, J.; Riedel, B. Anesthetic technique and cancer surgery outcomes. Curr. Opin. Anaesthesiol. 2021, 34, 317–325. [Google Scholar] [CrossRef]
- Buggy, D.; Freeman, J.; Johnson, M.; Leslie, K.; Riedel, B.; Sessler, D.; Kurz, A.; Gottumukkala, V.; Short, T.; Pace, N.; et al. Systematic review and consensus definitions for standardised endpoints in perioperative medicine: Postoperative cancer outcomes. Br. J. Anaesth. 2018, 121, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Aguado, G.; de la Mata, D.M.-A.; Valenciano, C.M.-L.; Sainz, I.F.-U. Endoscopic ultrasonography-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer: An update. World J. Gastrointest. Endosc. 2021, 13, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Citterio, D.; Pusceddu, S.; Facciorusso, A.; Coppa, J.; Milione, M.; Buzzoni, R.; Bongini, M.; Debraud, F.; Mazzaferro, V. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur. J. Surg. Oncol. (EJSO) 2016, 43, 380–387. [Google Scholar] [CrossRef]
- Cardoso, T.A.A.M.; Kunst, G.; Neto, C.N.; Júnior, J.D.R.C.; Silva, C.G.S.; Bastos, G.M.; Borges, J.B.; Hirata, M.H. Effect of sevoflurane on the inflammatory response during cardiopulmonary bypass in cardiac surgery: The study protocol for a randomized controlled trial. Trials 2021, 22, 25. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Wang, G.; Wei, P.; Gao, S. Identification of anesthetic-induced expression changes using DNA microarray. Mol. Med. Rep. 2014, 11, 589–596. [Google Scholar] [CrossRef]
- Fokas, E.; Engenhart-Cabillic, R.; Daniilidis, K.; Rose, F.; An, H.-X. Metastasis: The seed and soil theory gains identity. Cancer Metastasis Rev. 2007, 26, 705–715. [Google Scholar] [CrossRef]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef]
- Nepogodiev, D.; Martin, J.; Biccard, B.; Makupe, A.; Bhangu, A.; Ademuyiwa, A.; Adisa, A.O.; Aguilera, M.-L.; Chakrabortee, S.; Fitzgerald, J.; et al. Global burden of postoperative death. Lancet 2019, 393, 401. [Google Scholar] [CrossRef]
- Lucas, A.L.; Malvezzi, M.; Carioli, G.; Negri, E.; La Vecchia, C.; Boffetta, P.; Bosetti, C. Global Trends in Pancreatic Cancer Mortality From 1980 Through 2013 and Predictions for 2017. Clin. Gastroenterol. Hepatol. 2016, 14, 1452–1462.e4. [Google Scholar] [CrossRef] [PubMed]
- Lomberk, G.; Dusetti, N.; Iovanna, J.; Urrutia, R. Emerging epigenomic landscapes of pancreatic cancer in the era of precision medicine. Nat. Commun. 2019, 10, 3875. [Google Scholar] [CrossRef] [PubMed]
| Identifier | Status | Intervention | Type | Condition | Outcome | Title |
|---|---|---|---|---|---|---|
| NCT02335151 | C | Desflurane Propofol | Randomized Parallel assignment Double blind | 86 patients Adenocarcinoma Circulating Tumor Cells Pancreatic Neoplasms Surgery | Peak of CTC in the postoperative phase after curative tumor removal Kinetics of CTC after surgery up to day 7 Month to Tumor recurrence Number of surviving patients | CTC Pancreatic Adenocarcinoma |
| NCT04048278 | R | Intravenous (IV) lidocaine Saline Solution for Injection | Randomized Parallel Assignment Double blind | 46 patients Pancreatic Cancer Surgery | Enzymatic activity of SCR TK on CTC Cytokine levels Chemokine levels Gene expression CTC number | Lidocaine Infusion in Pancreatic Cancer |
| NCT04449289 | NYR | Intravenous (IV) lidocaine Epidural ropivacaine | Randomized Parallel Assignment Open label | 100 patients Pancreatic Cancer Surgery | 1- and 3-years recurrence rate after surgery Lidocaine and ropivacaine concentration Complication rate after surgery | Influence of Local Anesthetic Administration on the Cancer Recurrence Rate After Pancreatic Oncologic Surgery |
| NCT03245346 | R | GEA PCEA GA PCIA | Randomized Parallel Assignment Open label | 540 patients Cancer of Pancreas Surgery | Overall survival (OS) Disease-free survival (DFS) Postoperative pain score and side effects of patient-controlled Incidence of delirium Incidence of persistent post-surgical pain (PPSP) after surgery Length of stay in hospital after surgery and total costs after surgery Return of bowel function Start of enteral tube feeding Removal of Perianastomotic drains Removal of Urinary drainage Removal of nasogastric tube Removal of enteral feeding tube Blood level of neuroendocrine, stress and inflammatory response (blood epinephrine, norepinephrine, cortisol, VEGF, interleukin-6 (IL-6), interleukin-8 (IL-8), peripheral blood NLR (neutrophil-lymphocyte ratio)) Serum CA19-9, CA125, CEA, CA72-4, CA242, AFP, CA15-3, CA50 levels Plasma levels of ropivacaine and sufentanil | Effects of Epidural Anesthesia and Analgesia on the Prognosis in Patients Undergoing Pancreatic Cancer Surgery |
| NCT04025840 | R | Epidural Block Dexamethasone | Randomized Intervention Factorial Assignment Intervention Model Single blind | 260 patients Pancreatic Cancer Surgery | 2-year overall survival Postoperative gastrointestinal complications Overall postoperative complications Length of stay in hospital after surgery All-cause 30-day mortality Quality of life in 1- and 2-year survivors Hospital readmission within 2 years after surgery 2-year progression-free survival Subjective sleep quality: Pain severity Time to ambulation after surgery Time to oral intake after surgery | Perioperative Epidural Block and Dexamethasone in Pancreatic Cancer Surgery |
| NCT03447691 | R | Desflurane Total Intravenous Anesthesia with Propofol and Remifentanil | Randomized Parallel Assignment Triple blind | 132 patients Pancreatic Cancer Distal CBD Cancer Surgery | Score of QoR40 Score of QoR40 | Comparison Between Volatile Anesthetic-desflurane and Total Intravenous Anesthesia With Propofol and Remifentanil on Early Recovery Quality and Long Term Prognosis of Patients Undergoing Pancreatic Cancer and Common Bile Duct Cancer Surgery |
| NCT03838029 | R | Propranolol Etodolac Other: Placebo | Randomized Intervention Parallel Assignment Quadruple blind | 210 patients Pancreatic Neoplasms Surgery | Rate of cancer recurrence Biomarkers in extracted tumor tissue samples Number of patients with treatment related adverse events Depression, Anxiety, Global distress Fatigue | Perioperative Intervention to Reduce Metastatic Processes in Pancreatic Cancer Patients Undergoing Curative Surgery (BC-PC) |
| NCT03034096 | R | Propofol Volatile Agent (sevoflurane, isoflurane, or desflurane) | Randomized Parallel assignment Double blind | 2000 patients Patients with known or suspected cancer and scheduled to undergo any of the following oncologic surgical procedures: Lobectomy or pneumonectomy Esophagectomy Radical (total) cystectomy Pancreatectomy Partial hepatectomy Hyperthermic intraperitoneal chemotherapy (HIPEC) Gastrectomy (subtotal or total) Cholecystectomy or bile duct resection | All-cause mortality (Time Frame: 2 year minimum) Time to event Secondary Outcome Measures: Recurrence free survival (Time Frame: Minimum 2 years) Time to event All-cause mortality as a binary outcome (Time Frame: 2 years) | General Anesthetics in CAncer REsection Surgery (GA-CARES) Trial (GA-CARES) |
| NCT02660411 | C | Sevoflurane Propofol | Randomized Parallel Assignment Triple blind | 1228 patients Age ≥ 65 years and < 90 years; Primary malignant tumor; Do not receive radiation therapy or chemotherapy before surgery; Scheduled to undergo surgery for the treatment of tumors, with an expected duration of 2 h or more, under general anesthesia; | Over survival after surgery. (Time Frame: Up to 5 years after surgery.) Time from surgery to the date of all-cause death. Secondary Outcome Measures: Recurrence-free survival after surgery (Time Frame: Up to 5 years after surgery) Time from surgery to the date of cancer recurrence/metastasis or all-cause death, whichever occurs first. Event-free survival after surgery (Time Frame: Up to 5 years after surgery) Time from surgery to the date of cancer recurrence/metastasis, new cancer, new serious non-cancer disease, or all-cause death, whichever occurs first. Quality of life in 3-year survivors after surgery. (Time Frame: Assessed at the end of the 3rd year after surgery.) Quality of life is assessed with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30). Cognitive function in 3-year survivors after surgery. (Time Frame: Assessed at the end of the 3rd year after surgery.) Cognitive function is assessed with the Telephone Interview for Cognitive Status-Modified (TICS-m). | Impact of Inhalational Versus Intravenous Anesthesia Maintenance Methods on Long-term Survival in Elderly Patients After Cancer Surgery: a Randomized Controlled Trial |
| NCT03838029 | R | Propranolol Etodolac Placebo | Randomized Parallel Assignment Quadruple blind | 210 patients Pancreatic Neoplasms | Primary: -Rate of cancer recurrence (Time Frame: From the date of surgery until malignant disease is identified, assessed up to 60 months post-surgery) -Data regarding post-surgical recurrence will be recorded at 1, 3, 6, 12, 18, 24, 36, 48, and 60 following surgery -Biomarkers in extracted tumor tissue samples (Time Frame: An average of one year following surgery) Epithelial-to-mesenchymal-transition (EMT) status and natural-killer cell, macrophage, T-cell, and B-cell infiltration levels into tumor tissue (as assessed by messenger RNA profiling of tissue samples. -Biomarkers in blood samples (Time Frame: An average of one year following surgery) Cytokine levels in blood samples (interleukin-6, interleukin-10, C-reactive protein, interferon-gamma, and vascular endothelial growth factor and additional exploratory analysis of other cytokines) | Perioperative Intervention to Reduce Metastatic Processes in Pancreatic Cancer Patients Undergoing Curative Surgery |
| NCT05451043 | NYR | Biological: Durvalumab Drug: Gemcitabine Drug: Nab paclitaxel Biological: Tremelimumab Drug: Propranolol Drug: Cisplatin | Single group Single Group Assignment Open label | 62 patients Pancreatic Cancer Hepatocellular Cancer Biliary Tract Cancer Cholangiocarcinoma | Primary: -Investigating and establishing the efficacy of propranolol in boosting the effects of immunotherapy in pancreatic adenocarcinoma (Time Frame: Assessed one year after enrollment of last participant) combination of gemcitabine+nab-paclitaxel+propranolol+durvalumab+tremelimumab’s objective response rate is greater than or equal to 50% -Investigating and establishing the efficacy of propranolol in boosting the effects of immunotherapy in hepatocellular carcinoma (Time Frame: Assessed one year after enrollment of last participant) propranolol + durvalumab + tremelimumab objective response rate is greater than 45% Investigating and establishing the efficacy of propranolol in boosting the effects of immunotherapy in biliary tract tumors (Time Frame: Assessed one year after enrollment of last participant) -To demonstrate in unresectable BTC (gallbladder, cholangiocarcinoma of the biliary tracts including ampullary carcinomas) that the combination of gemcitabine + cisplatin + propranolol + durvalumab + tremelimumab’s objective response rate is greater than 50%. | Durvalumab and Tremelimumab in Combination With Propranolol and Chemotherapy for Treatment of Advanced Hepato-pancreabiliary Tumors (BLOCKED) |
| EudraCT number: 2018-000415-25 | Propranolol Etodolac Placebo | Randomized Parallel Assignment Single blind | 100 patients Non-metastatic head PC of the head undergoing elective pancreatoduodenectomy. | Primary: serious adverse events and reactions within 3 months. Secondary: utility of the two drugs in improving survival. | Pancreatic resection with perioperative drug repurposing of propranolol and etodolac: trial protocol of the phase-II randomized placebo controlled PROSPER trial. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokini, Z.; Cama, A.; Forget, P. Anesthetics and Long Term Cancer Outcomes: May Epigenetics Be the Key for Pancreatic Cancer? Medicina 2022, 58, 1102. https://doi.org/10.3390/medicina58081102
Mokini Z, Cama A, Forget P. Anesthetics and Long Term Cancer Outcomes: May Epigenetics Be the Key for Pancreatic Cancer? Medicina. 2022; 58(8):1102. https://doi.org/10.3390/medicina58081102
Chicago/Turabian StyleMokini, Zhirajr, Alessandro Cama, and Patrice Forget. 2022. "Anesthetics and Long Term Cancer Outcomes: May Epigenetics Be the Key for Pancreatic Cancer?" Medicina 58, no. 8: 1102. https://doi.org/10.3390/medicina58081102
APA StyleMokini, Z., Cama, A., & Forget, P. (2022). Anesthetics and Long Term Cancer Outcomes: May Epigenetics Be the Key for Pancreatic Cancer? Medicina, 58(8), 1102. https://doi.org/10.3390/medicina58081102








