Embryologic Origin of the Primary Tumor and RAS Status Predict Survival after Resection of Colorectal Liver Metastases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of the Patients
2.2. Molecular Diagnosis
2.3. Treatment Allocation
2.4. Long-Term Outcomes
2.5. Prognostic Factors
2.6. Statistical Analysis
3. Results
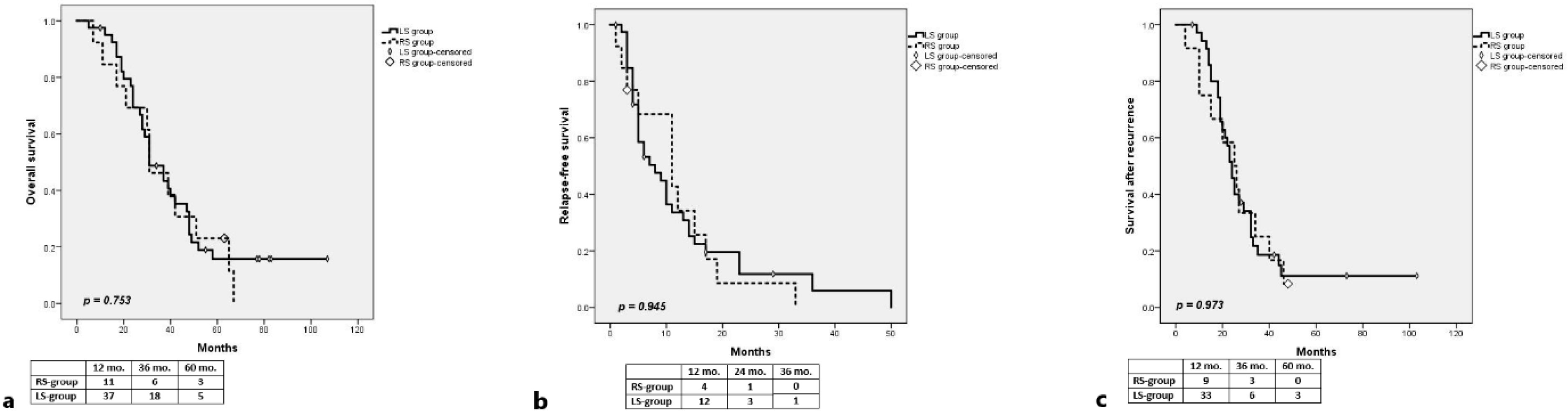
3.1. RAS-Mut
Long-Term Outcomes
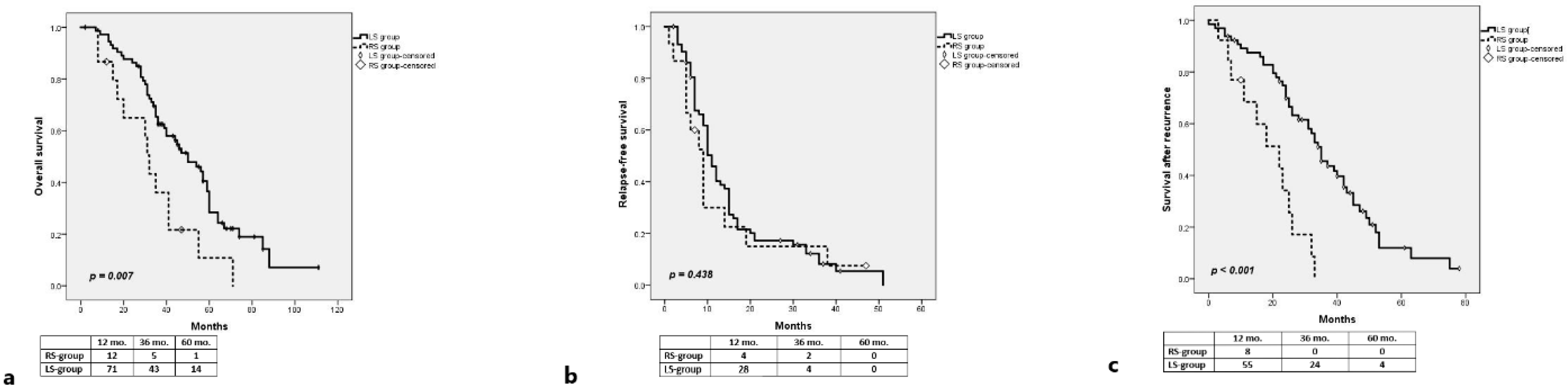
3.2. RAS-Wt
3.2.1. Long-Term Outcomes
3.2.2. Univariate Analysis
3.2.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tejpar, S.; Stintzing, S.; Ciardiello, F.; Tabernero, J.; Van Cutsem, E.; Beier, F.; Esser, R.; Lenz, H.-J.; Heinemann, V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017, 3, 194–201. [Google Scholar] [CrossRef]
- Price, T.J.; Beeke, C.; Ullah, S.; Padbury, R.; Maddern, G.; Roder, D.; Townsend, A.R.; Moore, J.; Roy, A.; Tomita, Y.; et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 2014, 121, 830–835. [Google Scholar] [CrossRef]
- Loupakis, F.; Yang, D.; Yau, L.; Feng, S.; Cremolini, C.; Zhang, W.; Maus, M.K.H.; Antoniotti, C.; Langer, C.; Scherer, S.J.; et al. Primary Tumor Location as a Prognostic Factor in Metastatic Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2015, 107, dju427. [Google Scholar] [CrossRef] [PubMed]
- Scherman, P.; Syk, I.; Holmberg, E.; Naredi, P.; Rizell, M. Influence of primary tumour and patient factors on survival in patients undergoing curative resection and treatment for liver metastases from colorectal cancer. BJS Open 2019, 4, 118–132. [Google Scholar] [CrossRef]
- Wang, K.; Xu, D.; Yan, X.-L.; Poston, G.; Xing, B.-C. The impact of primary tumour location in patients undergoing hepatic resection for colorectal liver metastasis. Eur. J. Surg. Oncol. 2018, 44, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.C.; Ribeiro, H.S.C.; Costa, W.L.; De Jesus, V.H.F.; De Macedo, M.P.; Diniz, A.L.; Godoy, A.; Farias, I.C.; Aguiar, S.; Riechelmann, R.S.P.; et al. Is primary sidedness a prognostic factor in patients with resected colon cancer liver metastases (CLM)? J. Surg. Oncol. 2018, 117, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Saltz, L.B.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; et al. The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann. Surg. Oncol. 2017, 25, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Gasser, E.; Braunwarth, E.; Riedmann, M.; Cardini, B.; Fadinger, N.; Presl, J.; Klieser, E.; Ellmerer, P.; Dupré, A.; Imai, K.; et al. Primary tumour location affects survival after resection of colorectal liver metastases: A two-institutional cohort study with international validation, systematic meta-analysis and a clinical risk score. PLoS ONE 2019, 14, e0217411. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Andreatos, N.; Margonis, G.A.; He, J.; Weiss, M.; Johnston, F.; Wolfgang, C.; Antoniou, E.; Pikoulis, E.; Pawlik, T.M. The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J. Surg. Oncol. 2016, 114, 803–809. [Google Scholar] [CrossRef]
- Missiaglia, E.; Jacobs, B.; D’Ario, G.; Di Narzo, A.; Soneson, C.; Budinska, E.; Popovici, V.; Vecchione, L.; Gerster, S.; Yan, P.; et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 2014, 25, 1995–2001. [Google Scholar] [CrossRef]
- Yamashita, S.; Brudvik, K.W.; Kopetz, S.; Maru, D.; Clarke, C.N.; Passot, G.; Conrad, C.; Chun, Y.S.; Aloia, T.A.; Vauthey, J.-N. Embryonic Origin of Primary Colon Cancer Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colon Cancer Liver Metastases. Ann. Surg. 2018, 267, 514–520. [Google Scholar] [CrossRef]
- Goffredo, P.; Utria, A.F.; Beck, A.C.; Chun, Y.S.; Howe, J.R.; Weigel, R.J.; Vauthey, J.-N.; Hassan, I. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J. Gastrointest. Surg. 2018, 23, 1957–1963. [Google Scholar] [CrossRef]
- Margonis, G.A.; Amini, N.; Buettner, S.; Kim, Y.; Wang, J.; Andreatos, N.; Wagner, D.; Sasaki, K.; Beer, A.; Kamphues, C.; et al. The Prognostic Impact of Primary Tumor Site Differs According to the KRAS Mutational Status: A Study By the International Genetic Consortium for Colorectal Liver Metastasis. Ann. Surg. 2019, 273, 1165–1172. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, W.-S.; Jiang, J.-K.; Yang, S.-H.; Wang, H.-S.; Chang, S.-C.; Lan, Y.-T.; Lin, C.-C.; Lin, H.-H.; Huang, S.-C.; et al. Effect of Primary Tumor Location on Postmetastasectomy Survival in Patients with Colorectal Cancer Liver Metastasis. J. Gastrointest. Surg. 2020, 25, 650–661. [Google Scholar] [CrossRef]
- Lieèvre, A.; Bachet, J.-B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.-F.; Coôté, J.-F.; Tomasic, G.; Penna, C.; Ducreux, M.; et al. KRAS Mutation Status Is Predictive of Response to Cetuximab Therapy in Colorectal Cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef]
- Belias, M.; Sasaki, K.; Wang, J.; Andreatos, N.; Kamphues, C.; Kyriakos, G.; Seeliger, H.; Beyer, K.; Kreis, M.E.; Margonis, G.A. Is Laterality Prognostic in Resected KRAS-Mutated Colorectal Liver Metastases? A Systematic Review and Meta-Analysis. Cancers 2022, 14, 799. [Google Scholar] [CrossRef]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Sawada, Y.; Sahara, K.; Endo, I.; Sakamoto, K.; Honda, G.; Beppu, T.; Kotake, K.; Yamamoto, M.; Takahashi, K.; Hasegawa, K.; et al. Long-term outcome of liver resection for colorectal metastases in the presence of extrahepatic disease: A multi-institutional Japanese study. J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 810–818. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, R.; Wang, Z.; Geng, Y.; Lin, J.; Ma, K.; Zuo, J.-L.; Lu, L.; Zhang, J.-B.; Zhu, W.-W.; et al. Meta-analysis of the association between primary tumour location and prognosis after surgical resection of colorectal liver metastases. Br. J. Surg. 2019, 106, 1747–1760. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Kopetz, S.E.; Li, L.; Conrad, C.; Aloia, T.A.; Vauthey, J. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br. J. Surg. 2015, 102, 1175–1183. [Google Scholar] [CrossRef]
- Dupré, A.; Malik, H.Z.; Jones, R.P.; Diaz-Nieto, R.; Fenwick, S.W.; Poston, G.J. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur. J. Surg. Oncol. 2018, 44, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Dorcaratto, D.; Mazzinari, G.; Fernández-Moreno, M.-C.; Muñoz, E.; Garcés-Albir, M.; Ortega, J.; Sabater, L. Impact of Postoperative Complications on Survival and Recurrence After Resection of Colorectal Liver Metastases: Systematic Review and Meta-analysis. Ann. Surg. 2019, 270, 1018–1027. [Google Scholar] [CrossRef]
- Viganò, L.; Gentile, D.; Galvanin, J.; Corleone, P.; Costa, G.; Cimino, M.; Procopio, F.; Torzilli, G. Very Early Recurrence After Liver Resection for Colorectal Metastases: Incidence, Risk Factors, and Prognostic Impact. J. Gastrointest. Surg. 2021, 26, 570–582. [Google Scholar] [CrossRef]
- Kamran, S.C.; Clark, J.W.; Zheng, H.; Borger, D.R.; Blaszkowsky, L.S.; Allen, J.N.; Kwak, E.L.; Wo, J.Y.; Parikh, A.R.; Nipp, R.D.; et al. Primary tumor sidedness is an independent prognostic marker for survival in metastatic colorectal cancer: Results from a large retrospective cohort with mutational analysis. Cancer Med. 2018, 7, 2934–2942. [Google Scholar] [CrossRef]
- Yin, J.; Cohen, R.; Jin, Z.; Liu, H.; Pederson, L.; Adams, R.; Grothey, A.; Maughan, T.S.; Venook, A.; Van Cutsem, E.; et al. Prognostic and Predictive Impact of Primary Tumor Sidedness for Previously Untreated Advanced Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2021, 113, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Kim, Y.; Wagner, D.; Sasaki, K.; Beer, A.; Schwarz, C.; Loes, I.M.; Smolle, M.; et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018, 153, e180996. [Google Scholar] [CrossRef]
- Schirripa, M.; Bergamo, F.; Cremolini, C.; Casagrande, M.; Lonardi, S.; Aprile, G.; Yang, D.; Marmorino, F.; Pasquini, G.; Sensi, E.; et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br. J. Cancer 2015, 112, 1921–1928. [Google Scholar] [CrossRef]
- Gagnière, J.; Dupré, A.; Gholami, S.S.; Pezet, D.; Boerner, T.; Gönen, M.; Kingham, T.P.; Allen, P.J.; Balachandran, V.P.; De Matteo, R.P.; et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann. Surg. 2020, 271, 147–154. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Thiam, T.K.; Lu, Y.-J.; Yeh, C.Y.; Tsai, W.-S.; You, J.F.; Hung, H.Y.; Tsai, C.-N.; Hsu, A.; Chen, H.-C.; et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget 2016, 7, 22257–22270. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takahashi, S.; Takahashi, N.; Masuishi, T.; Shoji, H.; Shinozaki, E.; Yamaguchi, T.; Kojima, M.; Gotohda, N.; Nomura, S.; et al. Survival Outcomes of Resected BRAF V600E Mutant Colorectal Liver Metastases: A Multicenter Retrospective Cohort Study in Japan. Ann. Surg. Oncol. 2020, 27, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, K.; Yamamoto, G.; Takenoya, T.; Takahashi, A.; Arai, Y.; Yamada, M.; Kakuta, M.; Yamaguchi, K.; Akagi, Y.; Nishimura, Y.; et al. Metastatic Pattern of Stage IV Colorectal Cancer with High-Frequency Microsatellite Instability as a Prognostic Factor. Anticancer Res. 2017, 37, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.; Timmer, F.; Geboers, B.; Schouten, E.; Opperman, J.; Scheffer, H.; de Vries, J.; Versteeg, K.; et al. Primary Tumor Sidedness, RAS and BRAF Mutations and MSI Status as Prognostic Factors in Patients with Colorectal Liver Metastases Treated with Surgery and Thermal Ablation: Results from the Amsterdam Colorectal Liver Met Registry (AmCORE). Biomedicines 2021, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Schirripa, M.; Cremolini, C.; Loupakis, F.; Morvillo, M.; Bergamo, F.; Zoratto, F.; Salvatore, L.; Antoniotti, C.; Marmorino, F.; Sensi, E.; et al. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer: NRAS mutations as prognostic and predictive markers in mCRC. Int. J. Cancer 2014, 136, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bingmer, K.; Ofshteyn, A.; Bliggenstorfer, J.T.; Kethman, W.; Ammori, J.B.; Charles, R.; Stein, S.L.; Steinhagen, E. Primary tumor location impacts survival in colorectal cancer patients after resection of liver metastases. J. Surg. Oncol. 2020, 122, 745–752. [Google Scholar] [CrossRef] [PubMed]
| Clinico-Pathologic Characteristics | Right-Sided Group | Left-Sided Group | p Value (t test/Fischer’s Exact Test) |
|---|---|---|---|
| N (%) | N (%) | ||
| Age (mean +/− SD) | 58.47 (+/−10.81) | 57.34 (+/−8.87) | 0.6667 |
| Sex | 0.5731 | ||
| Male | 8 (53.3%) | 32 (43.2%) | |
| Female | 7 (46.7%) | 42 (56.8%) | |
| Postoperative complications | 1 | ||
| No | 8 (53.3%) | 42 (56.8%) | |
| Yes | 7 (46.7%) | 32 (43.2%) | |
| Major complications | 1 | ||
| No | 13 (86.7%) | 64 (86.5%) | |
| Yes | 2 (13.3%) | 10 (13.5%) | |
| Extrahepatic metastases | 0.674 | ||
| No | 13 (86.7%) | 66 (89.2%) | |
| Yes | 2 (13.3%) | 8 (10.8%) | |
| Extension of hepatectomy | 1 | ||
| Minor | 12 (80%) | 56 (78.4%) | |
| Major | 3 (20%) | 16 (21.6%) | |
| Associated ablation | 0.3871 | ||
| No | 12 (80%) | 66 (89.2%) | |
| Yes | 3 (20%) | 8 (10.8%) | |
| Maximum size of CLMs | 0.219 | ||
| ≤5 cm | 9 (60%) | 56 (75.7%) | |
| >5 cm | 6 (40%) | 18 (24.3%) | |
| Maximum size of CLMs | 0.2704 | ||
| ≤3 cm | 5 (33.3%) | 37 (50%) | |
| >3 cm | 10 (66.7%) | 37 (50%) | |
| Number of CLMS | 0.7759 | ||
| Single | 7 (46.7%) | 30 (40.5%) | |
| Multiple | 8 (53.3%) | 44 (59.5%) | |
| Number of CLMS | 1 | ||
| <4 | 11 (73.3%) | 51 (68.9%) | |
| ≥4 | 4 (26.7%) | 23 (31.3%) | |
| TBS | 1 | ||
| ≤4.47 | 7 (46.7%) | 36 (48.6%) | |
| >4.47 | 8 (53.3%) | 38 (51.4%) | |
| CLMs’ distribution | 0.5832 | ||
| Unilobar | 9 (60%) | 38 (51.4%) | |
| Bilobar | 6 (40%) | 36 (48.6%) | |
| T-status | 0.6786 | ||
| T1–T3 | 12 (80%) | 55 (74.3%) | |
| T4 | 1 (6.7%) | 11 (14.9%) | |
| NA | 2 (13.3%) | 8 (10.8%) | |
| N-status | 0.744 | ||
| N− | 3 (20%) | 21 (28.4%) | |
| N+ | 10 (66.7%) | 45 (60.8%) | |
| NA | 2 (13.3%) | 8 (10.8%) | |
| Synchronous vs. Metachronous | 0.5585 | ||
| Synchronous | 11 (73.3%) | 46 (62.2%) | |
| Metachronous | 4 (26.7%) | 28 (37.8%) | |
| Initially resectable CLMs | 0.4933 | ||
| Yes | 11 (73.3%) | 60 (81.1%) | |
| No | 4 (26.7%) | 14 (18.9%) | |
| Preoperative chemotherapy | 0.3958 | ||
| No | 10 (66.7%) | 38 (51.4%) | |
| Yes | 5 (33.3%) | 36 (48.6%) | |
| Adjuvant chemotherapy | 1 | ||
| Yes | 14 (93.3%) | 68 (91.9%) | |
| No | 1 (6.7%) | 4 (5.4%) | |
| NA | 2 (2.7%) | ||
| Resection of recurrence * | 0.1998 | ||
| No | 11 (84.6%) | 40 (61.5%) | |
| Yes | 2 (15.4%) | 25 (38.5%) | |
| Time to recurrence * | 0.526 | ||
| ≤12 months | 10 (76.9%) | 42 (64.6%) | |
| >12 months | 3 (23.1%) | 23 (35.4% |
| Clinico-Pathologic Characteristics | p Value (Univariate, Log-Rank) | HR (Multivariate, Cox Regression) | 95% CI (Multivariate, Cox Regression) | p Value (Multivariate, Cox Regression) |
|---|---|---|---|---|
| Age | 0.095 | 0.002 | ||
| ≤65 y-o | 1 | |||
| >65 y-o | 0.292 | 0.133–0.640 | ||
| Sex | 0.743 | |||
| Female | ||||
| Male | ||||
| Right vs. Left | 0.007 | 0.009 | ||
| Right-sided | 0.398 | 0.199–0.794 | ||
| Left-sided | 1 | |||
| Postoperative complications | 0.672 | |||
| No | ||||
| Yes | ||||
| Major complications | 0.305 | |||
| No | ||||
| Yes | ||||
| Extrahepatic metastases | 0.014 | 0.001 | ||
| No | 1 | |||
| Yes | 0.27 | 0.125–0.684 | ||
| Extension of hepatectomy | 0.109 | |||
| Minor | ||||
| Major | ||||
| Associated ablation | 0.463 | |||
| No | ||||
| Yes | ||||
| Maximum size of CLMs | 0.394 | |||
| ≤5 cm | ||||
| >5 cm | ||||
| Maximum size of CLMs | 0.88 | |||
| ≤3 cm | ||||
| >3 cm | ||||
| Number of CLMS | 0.324 | |||
| Single | ||||
| Multiple | ||||
| Number of CLMS | 0.628 | |||
| <4 | ||||
| ≥4 | ||||
| TBS | 0.105 | |||
| ≤4.47 | ||||
| >4.47 | ||||
| CLMs’ distribution | 0.765 | |||
| Unilobar | ||||
| Bilobar | ||||
| T-status | 0.274 | |||
| T1-T3 | ||||
| T4 | ||||
| N-status | 0.004 | 0.014 | ||
| N− | 1 | |||
| N+ | 0.426 | 0.216–0.841 | ||
| Synchronous vs. Metachronous | 0.366 | |||
| Synchronous | ||||
| Metachronous | ||||
| Initially resectable CLMs | 0.396 | |||
| Yes | ||||
| No | ||||
| Preoperative chemotherapy | 0.084 | 0.004 | ||
| No | 1 | |||
| Yes | 0.44 | 0.251–0.774 | ||
| Adjuvant chemotherapy | 0.939 | |||
| Yes | ||||
| No |
| Clinico-Pathologic Characteristics | p Value (Univariate, Log-Rank) | HR (Multivariate, Cox Regression) | 95% CI (Multivariate, Cox Regression) | p Value (Multivariate, Cox Regression) |
|---|---|---|---|---|
| Age | 0.937 | |||
| ≤65 y-o | ||||
| >65 y-o | ||||
| Sex | 0.902 | |||
| Female | ||||
| Male | ||||
| Right vs. Left | 0.438 | |||
| Right-sided | ||||
| Left-sided | ||||
| Postoperative complications | 0.024 | 0.024 | ||
| No | 1 | |||
| Yes | 0.587 | 0.370–0.932 | ||
| Major complications | 0.441 | |||
| No | ||||
| Yes | ||||
| Extrahepatic metastases | 0.003 | 0.015 | ||
| No | 1 | |||
| Yes | 0.407 | 0.197–0.839 | ||
| Extension of hepatectomy | 0.481 | |||
| Minor | ||||
| Major | ||||
| Associated ablation | 0.918 | |||
| No | ||||
| Yes | ||||
| Maximum size of CLMs | 0.636 | |||
| ≤5 cm | ||||
| >5 cm | ||||
| Maximum size of CLMs | 0.87 | |||
| ≤3 cm | ||||
| >3 cm | ||||
| Number of CLMS | 0.026 | 0.057 | ||
| Single | 1 | |||
| Multiple | 0.627 | 0.387–1.015 | ||
| Number of CLMS | 0.971 | |||
| <4 | ||||
| ≥4 | ||||
| TBS | 0.472 | |||
| ≤4.47 | ||||
| >4.47 | ||||
| CLMs’ distribution | 0.126 | |||
| Unilobar | ||||
| Bilobar | ||||
| T-status | 0.903 | |||
| T1–T3 | ||||
| T4 | ||||
| N-status | 0.188 | |||
| N− | ||||
| N+ | ||||
| Synchronous vs. Metachronous | 0.315 | |||
| Synchronous | ||||
| Metachronous | ||||
| Initially resectable CLMs | 0.716 | |||
| Yes | ||||
| No | ||||
| Preoperative chemotherapy | 0.123 | |||
| No | ||||
| Yes | ||||
| Adjuvant chemotherapy | 0.383 | |||
| Yes | ||||
| No |
| Clinico-Pathologic Characteristics | p Value (Univariate, Log-Rank) | HR (Multivariate, Cox Regression) | 95% CI (Multivariate, Cox Regression) | p Value (Multivariate, Cox Regression) |
|---|---|---|---|---|
| Age | 0.248 | |||
| ≤65 y-o | ||||
| >65 y-o | ||||
| Sex | 0.796 | |||
| Female | ||||
| Male | ||||
| Right vs. Left | <0.001 | <0.001 | ||
| Right-sided | 0.222 | 0.102–0.483 | ||
| Left-sided | 1 | |||
| Postoperative complications | 0.415 | |||
| No | ||||
| Yes | ||||
| Major complications | 0.343 | |||
| No | ||||
| Yes | ||||
| Extrahepatic metastases | 0.237 | |||
| No | ||||
| Yes | ||||
| Extension of hepatectomy | 0.187 | |||
| Minor | ||||
| Major | ||||
| Associated ablation | 0.161 | |||
| No | ||||
| Yes | ||||
| Maximum size of CLMs | 0.738 | |||
| ≤5 cm | ||||
| >5 cm | ||||
| Maximum size of CLMs | 0.419 | |||
| ≤3 cm | ||||
| >3 cm | ||||
| Number of CLMS | 0.784 | |||
| Single | ||||
| Multiple | ||||
| Number of CLMS | 0.464 | |||
| <4 | ||||
| ≥4 | ||||
| TBS | 0.389 | |||
| ≤4.47 | ||||
| >4.47 | ||||
| CLMs’ distribution | 0.496 | |||
| Unilobar | ||||
| Bilobar | ||||
| T-status | 0.321 | |||
| T1-T3 | ||||
| T4 | ||||
| N-status | 0.011 | 0.007 | ||
| N− | ||||
| N+ | 0.407 | 0.211–0.786 | ||
| Synchronous vs. Metachronous | 0.447 | |||
| Synchronous | ||||
| Metachronous | ||||
| Initially resectable CLMs | 0.192 | |||
| Yes | ||||
| No | ||||
| Preoperative chemotherapy | 0.746 | |||
| No | ||||
| Yes | ||||
| Adjuvant chemotherapy | 0.285 | |||
| Yes | ||||
| No | ||||
| Resection of recurrence | 0.007 | 0.123 | ||
| No | 0.621 | 0.339–1.138 | ||
| Yes | 1 | |||
| Time to recurrence | 0.048 | 0.25 | ||
| ≤12 months | 0.677 | 0.349–1.315 | ||
| >12 months | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrescu, S.T.; Dinu, I.M.; Diaconescu, A.S.; Micu, A.; Pasare, E.; Durdu, C.; Dorobantu, B.M.; Popescu, I. Embryologic Origin of the Primary Tumor and RAS Status Predict Survival after Resection of Colorectal Liver Metastases. Medicina 2022, 58, 1100. https://doi.org/10.3390/medicina58081100
Alexandrescu ST, Dinu IM, Diaconescu AS, Micu A, Pasare E, Durdu C, Dorobantu BM, Popescu I. Embryologic Origin of the Primary Tumor and RAS Status Predict Survival after Resection of Colorectal Liver Metastases. Medicina. 2022; 58(8):1100. https://doi.org/10.3390/medicina58081100
Chicago/Turabian StyleAlexandrescu, Sorin Tiberiu, Ioana Mihaela Dinu, Andrei Sebastian Diaconescu, Alexandru Micu, Evelina Pasare, Cristiana Durdu, Bogdan Mihail Dorobantu, and Irinel Popescu. 2022. "Embryologic Origin of the Primary Tumor and RAS Status Predict Survival after Resection of Colorectal Liver Metastases" Medicina 58, no. 8: 1100. https://doi.org/10.3390/medicina58081100
APA StyleAlexandrescu, S. T., Dinu, I. M., Diaconescu, A. S., Micu, A., Pasare, E., Durdu, C., Dorobantu, B. M., & Popescu, I. (2022). Embryologic Origin of the Primary Tumor and RAS Status Predict Survival after Resection of Colorectal Liver Metastases. Medicina, 58(8), 1100. https://doi.org/10.3390/medicina58081100







