Utility of PIVKA-II and AFP in Differentiating Hepatocellular Carcinoma from Non-Malignant High-Risk Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design & Patient Selection
2.2. Sample Size Calculation
- FP False Positive
- TN True Negative
- Z Value from the standard normal distribution reflecting the confidence level that will be used (e.g., Z = 1.96 for 95%)
- SP Specificity
- W Accuracy (0.05)
- N Sample size (sample population)
- P Prevalence
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Comparison of the Clinical Features and Baseline Characteristics between the HCC and NMHR Groups
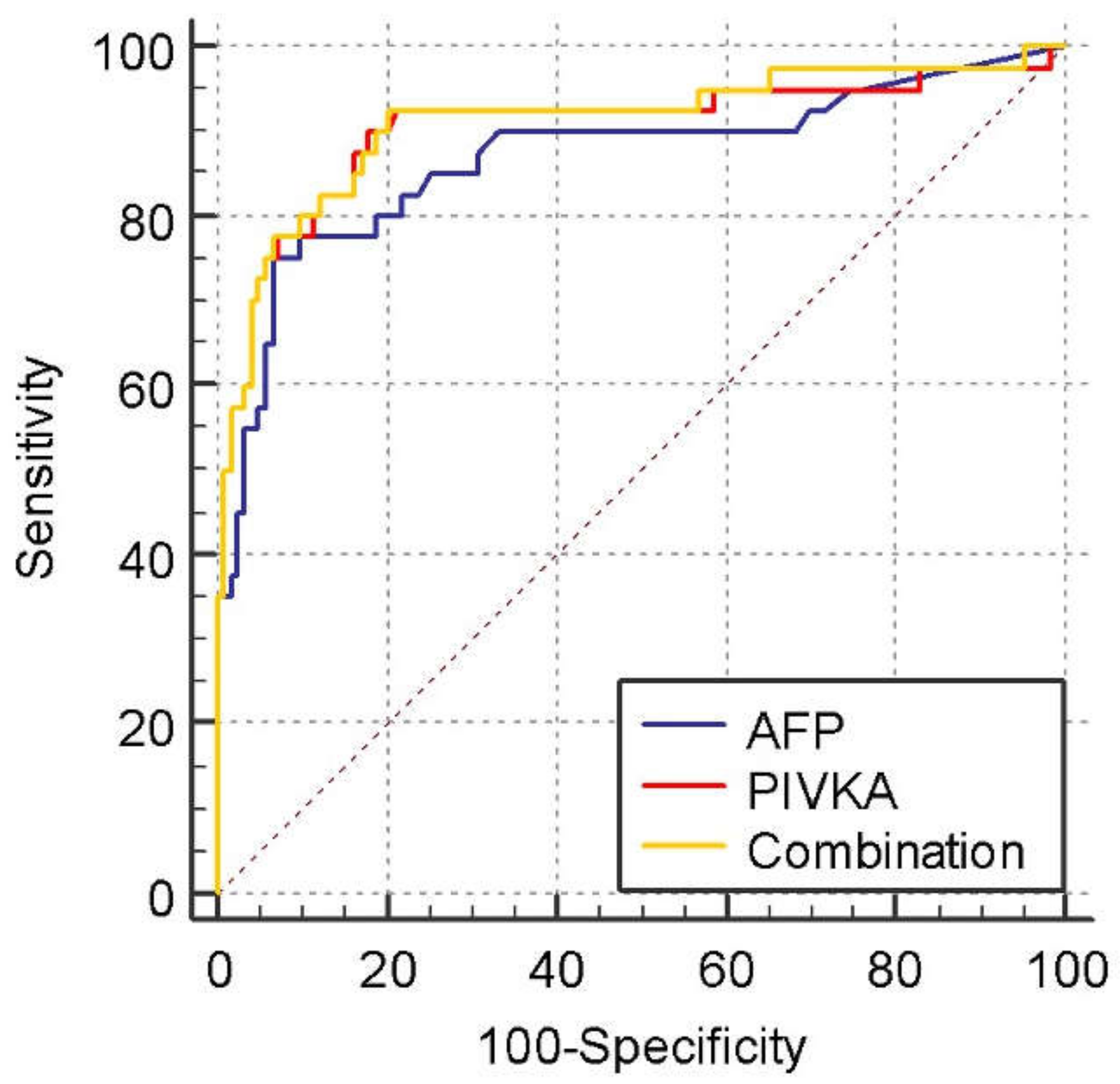
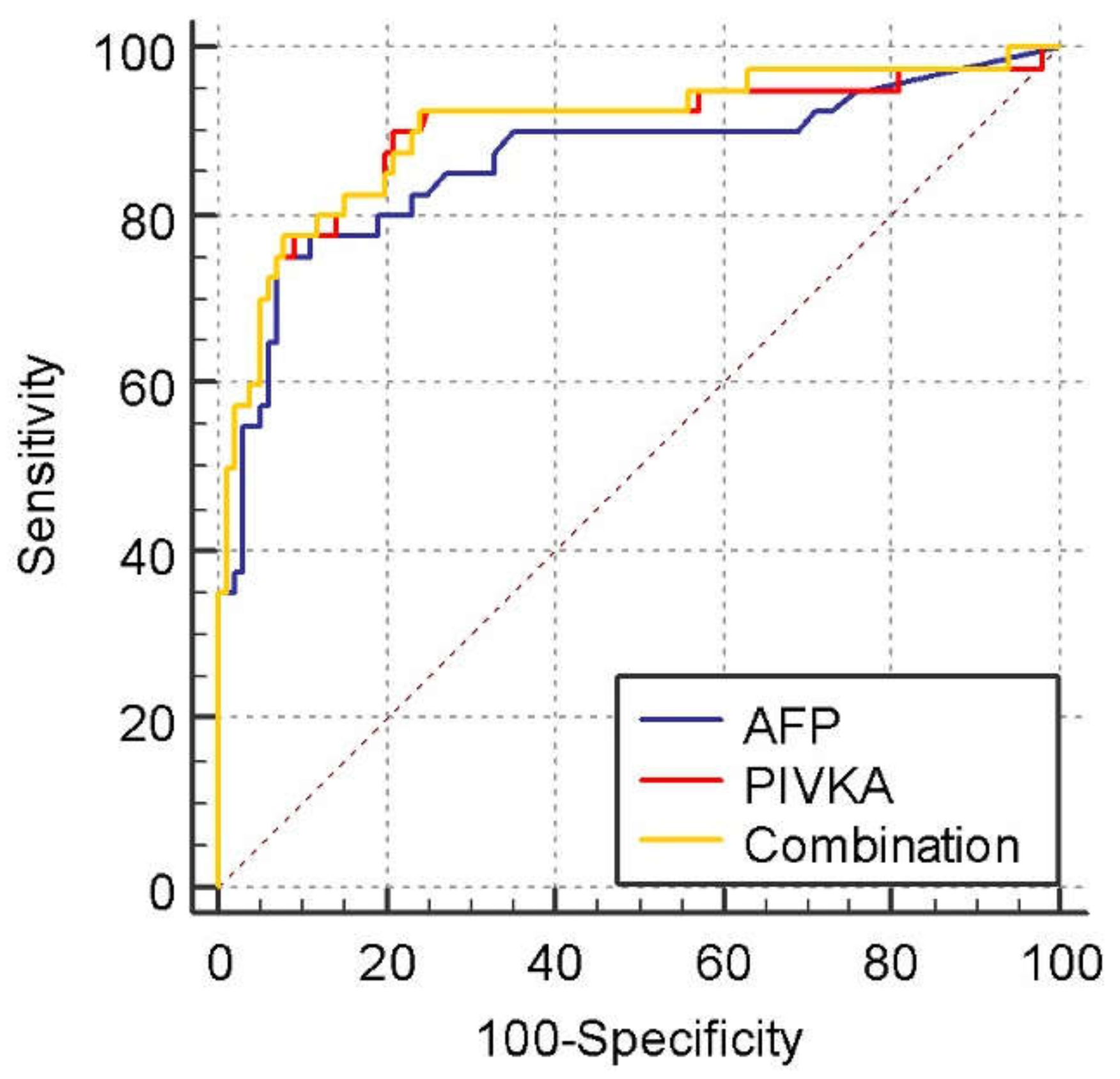
3.2. Diagnostic Values for PIVKA-II and AFP in the Differentiation of HCC from NMHR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Qu, C.; Zhang, S.; Zeng, H.; Sun, K.; Gu, X.; Xia, C.; Yang, Z.; Li, H.; Wei, W.; et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res. 2018, 30, 571–579. Available online: http://www.cjcrcn.org/article/html_9835.html (accessed on 3 April 2022). [CrossRef] [PubMed]
- Ahmed Mohammed, H.F.; Roberts, L.R. Should AFP (or Any Biomarkers) Be Used for HCC Surveillance? Curr. Hepatol. Rep. 2017, 16, 137–145. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef] [Green Version]
- Song, P.-P.; Xia, J.-F.; Inagaki, Y.; Hasegawa, K.; Sakamoto, Y.; Kokudo, N.; Tang, W. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 262–274. [Google Scholar] [CrossRef]
- Liebman, H.A.; Furie, B.C.; Tong, M.J.; Blanchard, R.A.; Lo, K.J.; Lee, S.D.; Coleman, M.S.; Furie, B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 1984, 310, 1427–1431. [Google Scholar] [CrossRef]
- Zinkin, N.T.; Grall, F.; Bhaskar, K.; Otu, H.H.; Spentzos, D.; Kalmowitz, B.; Wells, M.; Guerrero, M.; Asara, J.M.; Libermann, T.A.; et al. Serum proteomics and biomarkers in hepatocellular carcinoma and chronic liver disease. Clin. Cancer Res. 2008, 14, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Ikeda, K.; Kawamura, Y.; Yatsuji, H.; Hosaka, T.; Sezaki, H.; Akuta, N.; Suzuki, F.; Suzuki, Y.; Saitoh, S.; et al. High serum des-gamma-carboxy prothrombin level predicts poor prognosis after radiofrequency ablation of hepatocellular carcinoma. Cancer 2009, 115, 571–580. [Google Scholar] [CrossRef]
- Ferrante, N.D.; Pillai, A.; Singal, A.G. Update on the Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterol. Hepatol. 2020, 16, 506. [Google Scholar]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Seo, S.I.; Kim, H.S.; Kim, W.J.; Shin, W.G.; Kim, D.J.; Kim, K.H.; Jang, M.K.; Lee, J.H.; Kim, J.S.; Kim, H.Y.; et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis b virus-associated hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 3928–3935. [Google Scholar] [CrossRef]
- Marrero, J.A.; Su, G.L.; Wei, W.; Emick, D.; Conjeevaram, H.S.; Fontana, R.J.; Lok, A.S. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology 2003, 37, 1114–1121. [Google Scholar] [CrossRef]
- Yang, J.D.; Heimbach, J.K. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 2020, 371, m3544. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.D.; Browning, T.; Singal, A.G. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Loglio, A.; Iavarone, M.; Facchetti, F.; Di Paolo, D.; Perbellini, R.; Lunghi, G.; Ceriotti, F.; Galli, C.; Sandri, M.T.; Viganò, M.; et al. The combination of PIVKA-II and AFP improves the detection accuracy for HCC in HBV caucasian cirrhotics on long-term oral therapy. Liver Int. 2020, 40, 1987–1996. [Google Scholar] [CrossRef]
- Si, Y.Q.; Wang, X.Q.; Fan, G.; Wang, C.Y.; Zheng, Y.W.; Song, X.; Pan, C.-C.; Chu, F.-L.; Liu, Z.-F.; Lu, B.-R.; et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect. Agents Cancer 2020, 15, 70. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; He, W.; Song, D.; Ji, X.; Shao, J. The diagnostic value of serum PIVKA-II alone or in combination with AFP in Chinese hepatocellular carcinoma patients. Dis. Markers 2021, 2021, 8868370. [Google Scholar] [CrossRef]
- Feng, H.; Li, B.; Li, Z.; Wei, Q.; Ren, L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer 2021, 21, 401. [Google Scholar] [CrossRef]
- Lim, T.S.; Kim, D.Y.; Han, K.-H.; Kim, H.-S.; Shin, S.H.; Jung, K.S.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand. J. Gastroenterol. 2016, 51, 344–353. [Google Scholar] [CrossRef]
- Basit, A.; Prasad, B.; Estergreen, J.K.; Sabath, D.E.; Alade, N.; Veenstra, D.L.; Rettie, A.E.; Thummel, K.E. A Novel LC-MS/MS Assay for Quantification of Des-carboxy Prothrombin and Characterization of Warfarin-Induced Changes. Clin. Transl. Sci. 2020, 13, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Anuj, P.; Parul, S.; Kumar, A.A. Assessment of Pivka II as Tumour Marker in Hepatocellular Carcinoma. Int. J. Cancer Clin. Res. 2021, 8, 151. [Google Scholar] [CrossRef]
- Su, T.H.; Peng, C.Y.; Chang, S.H.; Tseng, T.C.; Liu, C.J.; Chen, C.L.; Liu, C.H.; Yang, H.C.; Chen, P.J.; Kao, J.H. Serum PIVKA-II and alpha-fetoprotein at virological remission predicts hepatocellular carcinoma in chronic hepatitis B related cirrhosis. J. Formos. Med. Assoc. 2022, 121, 703–711. [Google Scholar] [CrossRef]
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. α-Fetoprotein, Des-γ Carboxyprothrombin, and Lectin-Bound α-Fetoprotein in Early Hepatocellular Carcinoma. Gastroenterology 2009, 137, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Jasirwan, C.O.M.; Fahira, A.; Siregar, L.; Loho, I. The alpha-fetoprotein serum is still reliable as a biomarker for the surveillance of hepatocellular carcinoma in Indonesia. BMC Gastroenterol. 2020, 20, 215. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Zhang, P.; Zhang, J.; Li, X.; Gan, D.; Cao, X.; Han, M.; Du, H.; Ye, Y. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0228857. [Google Scholar] [CrossRef]
- Li, M.; Zhu, M.; Li, W.; Lu, Y.; Xie, X.; Wu, Y.; Zheng, S. Alpha-fetoprotein receptor as an early indicator of HBx-driven hepatocarcinogenesis and its applications in tracing cancer cell metastasis. Cancer Lett. 2013, 330, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zhao, J.; Lu, F. Alpha-fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection-related hepatocellular carcinoma. Oncotarget 2016, 7, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Liu, H.; Li, H.; Jiang, W.; Hou, W.; McNutt, M.A.; Lu, F.; Li, G. Alpha fetoprotein mediates HBx induced carcinogenesis in the hepatocyte cytoplasm. Int. J. Cancer 2015, 137, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Au, N.; Xing, J.; Yang, H. Virus infection—Results from a clinic-based longitudinal cohort. Eur J Cancer 2013, 48, 2319–2327. [Google Scholar]
- Liu, C.; Xiao, G.Q.; Yan, L.N.; Li, B.; Jiang, L.; Wen, T.F.; Wang, W.-T.; Xu, M.-Q.; Yang, J.-Y. Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Murugavel, K.G.; Mathews, S.; Jayanthi, V.; Shankar, E.M.; Hari, R.; Surendran, R.; Vengatesan, A.; Raghuram, K.; Rajasambandam, P.; Murali, A.; et al. Alpha-fetoprotein as a tumor marker in hepatocellular carcinoma: Investigations in south Indian subjects with hepatotropic virus and aflatoxin etiologies. Int. J. Infect. Dis. 2008, 12, e71–e76. [Google Scholar] [CrossRef] [Green Version]
- Kobeisy, M.A.; Morsy, K.H.; Galal, M.; Sayed, S.K.; Ashmawy, M.M.; Mohammad, F.M. Clinical significance of elevated alpha-foetoprotein (AFP) in patients with chronic hepatitis C without hepatocellular carcinoma in upper EGYPT. Arab J. Gastroenterol. 2012, 13, 49–53. [Google Scholar] [CrossRef]
- Fattovich, G.; Giustina, G.; Degos, F.; Tremolada, F.; Diodati, G.; Almasio, P.; Nevens, F.; Solinas, A.; Mura, D.; Brouwer, J.T.; et al. Morbidity and mortality in compensated cirrhosis type C: A retrospective follow-up study of 384 patients. Gastroenterology 1997, 112, 463–472. [Google Scholar] [CrossRef]
- Best, J.; Bechmann, L.P.; Sowa, J.P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735.e4. [Google Scholar] [CrossRef] [Green Version]
| Non-Malignant High-Risk Group | HCC | p-Value | ||
|---|---|---|---|---|
| Non-Cirrhotic High-Risk Group | Liver Cirrhosis | |||
| Number of patients (n) | 23 | 100 | 40 | |
| Age, median (IQR) | 50 (17) | 63 (14) | 64.5 (12) | 0.001 b |
| Gender | ||||
| Male | 17 (73.9%) | 70 (70%) | 34 (85%) | |
| Female | 6 (26.1%) | 30 (30%) | 6 (15%) | |
| Child-Pugh score | <0.0001 a | |||
| A | 23 (100%) | 76 (76%) | 11 (27.5%) | |
| B | - | 15 (15%) | 16 (40%) | |
| C | - | 9 (9%) | 13 (32.5%) | |
| BCLC stage | NA | NA | ||
| A | 7 (17.5%) | |||
| B | 10 (25%) | |||
| C | 17 (42.5%) | |||
| D | 6 (15%) | |||
| Albumin, median (IQR) | 40 (4) | 35(9) | 27 (10) | <0.0001 b |
| TB | 13 (6) | 20 (24) | 32.5 (50.3) | <0.0001 b |
| ALT | 23 (25) | 30 (26) | 46 (60) | 0.002 b |
| INR | 1.0 (0) | 1.2 (0.2) | 1.3 (0.3) | <0.0001 b |
| Parameters | LC | HCC | Statistical Value | p-Value |
| AFP, ng/ml | 2.8 (3.7) | 65.5 (1986.7) | 541.5 a | p < 0.001 a |
| PIVKA, mAU/ml | 26.5 (13.9) | 1170.1 (29863) | 408.5 a | p < 0.001 a |
| Parameters | NCHR | HCC | Statistical value | p-Value |
| AFP, ng/ml | 3.1 (2.2) | 65.5 (1986.7) | 105 a | p < 0.001 a |
| PIVKA, mAU/ml | 26.4 (6.8) | 1170.1 (29863) | 61 a | p < 0.001 a |
| Parameters | AFP Value | PIVKA Value | ||
|---|---|---|---|---|
| Correlation Coefficient (r) | p | Correlation Coefficient (r) | p | |
| Child-Pugh score | 0.32 | <0.0001 a | 0.45 | <0.0001 a |
| BCLC staging | 0.46 | 0.003 a | 0.46 | 0.003 a |
| Albumin | −0.31 | <0.0001 a | −0.38 | <0.0001 a |
| TB | 0.29 | <0.0001 a | 0.29 | <0.0001 a |
| ALT | 0.39 | <0.0001 a | 0.29 | <0.0001 a |
| INR | 0.25 | 0.001 a | 0.26 | 0.001 a |
| Cut-Off Value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| AFP (ng/mL) | ||||
| HCC vs. NMHR: >14 | 75 | 93.5 | 26.3 | 99.2 |
| HCC vs. LC: >14 | 75 | 93 | 48.2 | 97.7 |
| HCC vs. NMHR: >19 | 62.5 | 94.3 | 25.4 | 98.8 |
| HCC vs. LC: >19 | 62.5 | 94 | 47.5 | 96.6 |
| HCC vs. NMHR: >196 | 37.5 | 98.4 | 41.6 | 98.1 |
| HCC vs. LC: >196 | 37.5 | 98 | 62 | 94.7 |
| PIVKA (mAU/mL) | ||||
| HCC vs. NMHR: >37 | 90 | 82.1 | 13.5 | 99.6 |
| HCC vs. LC: >37 | 90 | 79 | 27.1 | 98.9 |
| HCC vs. NMHR: >100 | 77.5 | 92.7 | 24.7 | 99.3 |
| HCC vs. LC: >100 | 77.5 | 91 | 42.8 | 97.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, H.; Wan Shuaib, W.M.A.; Raja Ali, R.A.; Othman, H. Utility of PIVKA-II and AFP in Differentiating Hepatocellular Carcinoma from Non-Malignant High-Risk Patients. Medicina 2022, 58, 1015. https://doi.org/10.3390/medicina58081015
Hadi H, Wan Shuaib WMA, Raja Ali RA, Othman H. Utility of PIVKA-II and AFP in Differentiating Hepatocellular Carcinoma from Non-Malignant High-Risk Patients. Medicina. 2022; 58(8):1015. https://doi.org/10.3390/medicina58081015
Chicago/Turabian StyleHadi, Hana, Wan Muhammad Azfar Wan Shuaib, Raja Affendi Raja Ali, and Hanita Othman. 2022. "Utility of PIVKA-II and AFP in Differentiating Hepatocellular Carcinoma from Non-Malignant High-Risk Patients" Medicina 58, no. 8: 1015. https://doi.org/10.3390/medicina58081015
APA StyleHadi, H., Wan Shuaib, W. M. A., Raja Ali, R. A., & Othman, H. (2022). Utility of PIVKA-II and AFP in Differentiating Hepatocellular Carcinoma from Non-Malignant High-Risk Patients. Medicina, 58(8), 1015. https://doi.org/10.3390/medicina58081015






