Nutritional Interventions to Improve Cachexia Outcomes in Cancer—A Systematic Review
Abstract
1. Introduction
1.1. Pathophysiology
1.2. Assessment
1.3. Diagnosis Criteria and Staging
- To screen all of the patients with cancer for nutritional risk as soon as possible, regardless of body mass index and weight history;
- To include multiple nutrition-related assessment practices: measures of anorexia, BWC, inflammatory biomarkers, resting energy expenditure (REE), and physical function;
- To implement multimodal nutritional interventions with individualized plans [31]
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process and Data Items
2.6. Study Risk of Bias Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishikawa, H.; Goto, M.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Cancer Cachexia: Its Mechanism and Clinical Significance. Int. J. Mol. Sci. 2021, 22, 8491. [Google Scholar] [CrossRef] [PubMed]
- Esper, D.H.; Harb, W.A. The cancer cachexia syndrome: A review of metabolic and clinical manifestations. Nutr Clin. Pract. 2005, 20, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.C.; Garcia, J.M.; Sansgiry, S.; Walder, A.; Berger, D.H.; Anaya, D.A. Preoperative cancer cachexia and short-term outcomes following surgery. J. Surg. Res. 2016, 205, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Bennani-Baiti, N.; Walsh, D. What is cancer anorexia-cachexia syndrome? A historical perspective. J. R. Coll Physicians Edinb. 2009, 39, 257–262. [Google Scholar]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Peterson, S.J.; Mozer, M. Differentiating Sarcopenia and Cachexia among Patients with Cancer. Nutr. Clin. Pract. 2017, 32, 30–39. [Google Scholar] [CrossRef]
- Berardi, E.; Madaro, L.; Lozanoska-Ochser, B.; Adamo, S.; Thorrez, L.; Bouche, M.; Coletti, D. A Pound of Flesh: What Cachexia Is and What It Is Not. Diagnostics 2021, 11, 116. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Masoni, M.C.; Fini, M.; Pagani, S.; Giampietro, O.; Carpi, A. Malnutrition, anorexia and cachexia in cancer patients: A mini-review on pathogenesis and treatment. Biomed. Pharm. 2013, 67, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Tijerina, A.J. The biochemical basis of metabolism in cancer cachexia. Dimens Crit. Care Nurs. 2004, 23, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, R.N.; Hardee, J.P.; VanderVeen, B.N.; Carson, J.A. Resistance Exercise’s Ability to Reverse Cancer-Induced Anabolic Resistance. Exerc. Sport Sci. Rev. 2018, 46, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Dreicer, R.; Walsh, D.; Lagman, R.; LeGrand, S.B. Appetite and cancer-associated anorexia: A review. J. Clin. Oncol. 2004, 22, 1510–1517. [Google Scholar] [CrossRef]
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer cachexia: A nutritional or a systemic inflammatory syndrome? Br. J. Cancer 2022, 1–4, Advance online publication. [Google Scholar] [CrossRef]
- Dev, R. Measuring cachexia-diagnostic criteria. Ann. Palliat. Med. 2019, 8, 24–32. [Google Scholar] [CrossRef]
- Talbert, E.E.; Guttridge, D.C. Emerging signaling mediators in the anorexia-cachexia syndrome of cancer. Trends Cancer 2022, 8, 397–403. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Reber, E.; Schönenberger, K.A.; Vasiloglou, M.F.; Stanga, Z. Nutritional Risk Screening in Cancer Patients: The First Step Toward Better Clinical Outcome. Front. Nutr. 2021, 8, 603936. [Google Scholar] [CrossRef]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef]
- Read, J.A.; Crockett, N.; Volker, D.H.; MacLennan, P.; Choy, S.T.B.; Beale, P.; Clarke, S.J. Nutritional assessment in cancer: Comparing the Mini-Nutritional Assessment (MNA) with the scored Patient-Generated Subjective Global Assessment (PGSGA). Nutr. Cancer 2005, 53, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Boléo-Tomé, C.; Monteiro-Grillo, I.; Camilo, M.; Ravasco, P. Validation of the Malnutrition Universal Screening Tool (MUST) in cancer. Br. J. Nutr. 2012, 108, 343–348. [Google Scholar] [CrossRef]
- Sanson, G.; Sadiraj, M.; Barbin, I.; Confezione, C.; De Matteis, D.; Boscutti, G.; Zaccari, M.; Zanetti, M. Prediction of early- and long-term mortality in adult patients acutely admitted to internal medicine: NRS-2002 and beyond. Clin. Nutr. 2020, 39, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Arribas, L.; Hurtós, L.; Sendrós, M.J.; Peiró, I.; Salleras, N.; Fort, E.; Sánchez-Migallón, J.M. NUTRISCORE: A new nutritional screening tool for oncological outpatients. Nutrition 2017, 33, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Vagnildhaug, O.M.; Blum, D.; Wilcock, A.; Fayers, P.; Strasser, F.; Baracos, V.E.; Hjermstad, M.J.; Kaasa, S.; Laird, B.; Solheim, T.S. The applicability of a weight loss grading system in cancer cachexia: A longitudinal analysis. J. Cachexia Sarcopenia Muscle 2017, 8, 789–797. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Purcell, S.A.; Elliott, S.A.; Baracos, V.E.; Chu, Q.S.; Prado, C.M. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur. J. Clin. Nutr. 2016, 70, 1230–1238. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Toledo, M.; Betancourt, A.; Serpe, R.; Busquets, S. The cachexia score (CASCO): A new tool for staging cachectic cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 87–93. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, B.; Liu, H.; Yang, K.; Thapa, S.; Zhang, H.; Li, L.; Yu, S. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 306–314. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakamura, S.; Narimatsu, H. Nutritional Approach to Cancer Cachexia: A Proposal for Dietitians. Nutrients 2022, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Review Manager Web (RevMan Web). Version (5.3). The Cochrane Collaboration. 2020. Available online: revman.cochrane.org (accessed on 17 May 2022).
- Higgins, J.P.T.; Sterne, J.A.C. (Eds.) The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. Available online: www.cochrane-handbook.org (accessed on 17 May 2022).
- Akitaae, H.; Takahashia, H.; Asukaia, K.; Tomokunia, A.; Wadaa, H.; Marukawab, S.; Yamasakib, T.; Yanagimotoa, Y.; Takahashia, Y.; Sugimura, K.; et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin. Nutr. ESPEN. 2019, 33, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.D.; Ross, J.A.; Voss, A.C.; Tisdale, M.J.; Fearon, K.C. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br. J. Cancer 1999, 81, 80–86. [Google Scholar] [CrossRef]
- Bayram, I.; Erbey, F.; Celik, N.; Nelson, J.L.; Tanyeli, A. The use of a protein and energy dense eicosapentaenoic acid containing supplement for malignancy-related weight loss in children. Pediat. Blood Cancer 2009, 52, 571–574. [Google Scholar] [CrossRef]
- Berk, L.; James, J.; Schwartz, A.; Hug, E.; Mahadevan, A.; Samuels, M.; Kachnic, L. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer 2008, 16, 1179–1188. [Google Scholar] [CrossRef]
- Bruera, E.; Strasser, F.; Palmer, J.L.; Willey, J.; Calder, K.; Amyotte, G.; Baracos, V. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: A double-blind, placebo-controlled study. J. Clin. Oncol. 2003, 21, 129–134. [Google Scholar] [CrossRef]
- Burden, S.T.; Gibson, D.J.; Lal, S.; Hill, J.; Pilling, M.; Soop, M.; Ramesh, A.; Todd, C. Pre-operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight-losing patients with colorectal cancer: Single-blind randomized controlled trial. J. Cachex Sarcopenia Muscle 2017, 8, 437–446. [Google Scholar] [CrossRef]
- Gomez-Candela, C.; Sanz, M.; Horrisberger, A.; Kohen, V.; Bermejo, L.; Auñón, P. Efficacy evaluation of an oral powder supplement enriched with eicosapentaenoic acid in cancer patients. Nutr. Hosp. Organo Of. Soc. Española Nutr. Parenter. Enter. 2011, 26, 1385–1593. [Google Scholar]
- Engelen, M.P.K.J.; Safar, A.M.; Bartter, T.; Koeman, F.; Deutz, N.E.P. High anabolic potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann. Oncol. 2015, 26, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Hanai, N.; Terada, H.; Hirakawa, H.; Suzuki, H.; Nishikawa, D.; Beppu, S.; Hasegawa, Y. Prospective randomized investigation implementing immunonutritional therapy using a nutritional supplement with a high blend ratio of ω-3 fatty acids during the perioperative period for head and neck carcinomas. Jpn J. Clin. Oncol. 2018, 48, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Rowland, K.; Loprinzi, C.L.; Sloan, J.A.; Dakhil, S.R.; Macdonald, N.; Gagnon, B.; Novotny, P.J.; Mailliard, J.A.; Bushey, T.I.; et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: A North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J. Clin. Oncol. 2004, 22, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simone, G.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Holzhauer, P.; et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—A randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Wang, H.M.; Chang, J.W.; Huang, J.S.; Lai, C.H.; Lan, Y.J.; Wu, T.H.; Chang, P.H.; Wang, H.; Wu, C.J.; et al. Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 41–48. [Google Scholar] [CrossRef]
- Solís-Martínez, O.; Plasa-Carvalho, V.; Phillips-Sixtos, G.; Trujillo-Cabrera, Y.; Hernández-Cuellar, A.; Queipo-García, G.E.; Meaney-Mendiolea, E.; Ceballos, G.; Fuchs-Tarlovsky, V. Effect of Eicosapentaenoic Acid on Body Composition and Inflammation Markers in Patients with Head and Neck Squamous Cell Cancer from a Public Hospital in Mexico. Nutr. Cancer 2018, 70, 663–670. [Google Scholar] [CrossRef]
- May, P.E.; Barber, A.; D’Olimpio, J.T.; Hourihane, A.; Abumrad, N.N. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef]
- Palma, C.G.L.; Lera, A.T.; Lerner, T.; De Oliveira, M.M.; De Borta, T.M.; Barbosa, R.P.; Brito, G.M.; Guazzelli, C.A.; Cruz, F.J.M.; del Giglio, A. Guarana (Paullinia cupana) Improves Anorexia in Patients with Advanced Cancer. J. Diet. Suppl. 2015, 13, 221–231. [Google Scholar] [CrossRef]
- Persson, C.; Glimelius, B.; Rönnelid, J.; Nygren, P. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: A randomized pilot study. Nutrition 2005, 21, 170–178. [Google Scholar] [CrossRef]
- Schmidt, N.; Møller, G.; Bæksgaard, L.; Østerlind, K.; Stark, K.D.; Lauritzen, L.; Andersen, J.R. Fish oil supplementation in cancer patients. Capsules or nutritional drink supplements? A controlled study of compliance. Clin. Nutr. ESPEN 2020, 35, 63–68. [Google Scholar] [CrossRef]
- Storck, L.J.; Ruehlin, M.; Gaeumann, S.; Gisi, D.; Schmocker, M.; Meffert, P.J.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effect of a leucine-rich supplement in combination with nutrition and physical exercise in advanced cancer patients: A randomized controlled intervention trial. Clin. Nutr. 2020, 39, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Strasser, F.; Lüftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.-D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of Orally Administered Cannabis Extract and Delta-9-Tetrahydrocannabinol in Treating Patients With Cancer-Related Anorexia-Cachexia Syndrome: A Multicenter, Phase III, Randomized, Double-Blind, Placebo-Controlled Clinical Trial From the Cannabis-InCachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar] [PubMed]
- Szefel, J.; Kruszewski, W.J.; Ciesielski, M.; Szajewski, M.; Kawecki, K.; Aleksandrowicz-Wrona, E.; Jankun, J.; Łysiak-Szydłowska, W. L-carnitine and cancer cachexia. I. L-carnitine distribution and metabolic disorders in cancer cachexia. Oncol. Rep. 2012, 28, 319–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tayek, J.A.; Bistrian, B.R.; Hehir, D.J.; Martin, R.; Moldawer, L.L.; Blackburn, G.L. Improved protein kinetics and albumin synthesis by branched chain amino acid-enriched total parenteral nutrition in cancer cachexia. A prospective randomized crossover trial. Cancer 1986, 58, 147–157. [Google Scholar] [CrossRef]
- Taylor, L.A.; Pletschen, L.; Arends, J.; Unger, C.; Massing, U. Marine phospholipids—A promising new dietary approach to tumor-associated weight loss. Support Care Cancer 2010, 18, 159–170. [Google Scholar] [CrossRef]
- Turcott, J.G.; del Rocío Guillen Núñez, M.; Flores-Estrada, D.; Oñate-Ocaña, L.F.; Zatarain-Barrón, Z.L.; Barrón, F.; Arrieta, O. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Support Care Cancer 2018, 26, 3029–3038. [Google Scholar] [CrossRef]
- Werner, K.; De Gaudry, D.K.; Taylor, L.A.; Keck, T.; Unger, C.; Hopt, U.T.; Massing, U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: Marine phospholipids versus fish oil—A randomized controlled double-blind trial. Lipids Health Dis. 2017, 16, 104. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Barber, M.D.; Ross, J.A.; Tisdale, M.J.; Fearon, K.C.H. Effect of Oral Eicosapentaenoic Acid on Weight Loss in Patients With Pancreatic Cancer. Nutrition and cancer. Nutr. Cancer 2000, 36, 177–184. [Google Scholar] [CrossRef]
- Zuijdgeest-Van Leeuwen, S.D.; Dagnelie, P.C.; Wattimena, J.L.; Van den Berg, J.W.; van der Gaast, A.; Swart, G.R.; Wilson, J.F. Eicosapentaenoic acid ethyl ester supplementation in cachectic cancer patients and healthy subjects: Effects on lipolysis and lipid oxidation. Clin. Nutr. 2000, 19, 417–423. [Google Scholar] [CrossRef]
- Barber, M.D.; Fearon, K.C.; Tisdale, M.J.; McMillan, D.C.; Ross, J.A. Effect of a fish oil-enriched nutritional supplement on metabolic mediators in patients with pancreatic cancer cachexia. Nutr. Cancer 2001, 40, 118–124. [Google Scholar] [CrossRef]
- Hussey, H.J.; Tisdale, M.J. Effect of a cachectic factor on carbohydrate metabolism and attenuation by eicosapentaenoic acid. Br. J. Cancer 1999, 80, 1231–1235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorite, M.J.; Cariuk, P.; Tisdale, M.J. Induction of muscle protein degradation by a tumour factor. Br. J. Cancer 1997, 76, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J.; Beck, S.A. Inhibition of tumour-induced lipolysis in vitro and cachexia and tumour growth in vivo by eicosapentaenoic acid. Biochem. Pharm. 1991, 41, 103–107. [Google Scholar] [CrossRef]
- Lorite, M.J.; Thompson, M.G.; Drake, J.L.; Carling, G.; Tisdale, M.J. Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br. J. Cancer 1998, 78, 850–856. [Google Scholar] [CrossRef]
- Whitehouse, A.S.; Tisdale, M.J. Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-kappaB. Br. J. Cancer 2003, 89, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.; Pearson, S.; Allen, J. The omega-3 fatty acid, eicosapentaenoic acid (EPA), prevents the damaging effects of tumour necrosis factor (TNF)-alpha during murine skeletal muscle cell differentiation. Lipids Health Dis. 2008, 7, 24. [Google Scholar] [CrossRef]
- Nicastro, H.; Da Luz, C.R.; Chaves, D.F.; Bechara, L.R.; Voltarelli, V.A.; Rogero, M.M.; Lancha, A.H. Does Branched-Chain Amino Acids Supplementation Modulate Skeletal Muscle Remodeling through Inflammation Modulation? Possible Mechanisms of Action. J. Nutr. Metab. 2012, 2012, 136937. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem. J. 2007, 407, 113–120. [Google Scholar] [CrossRef]

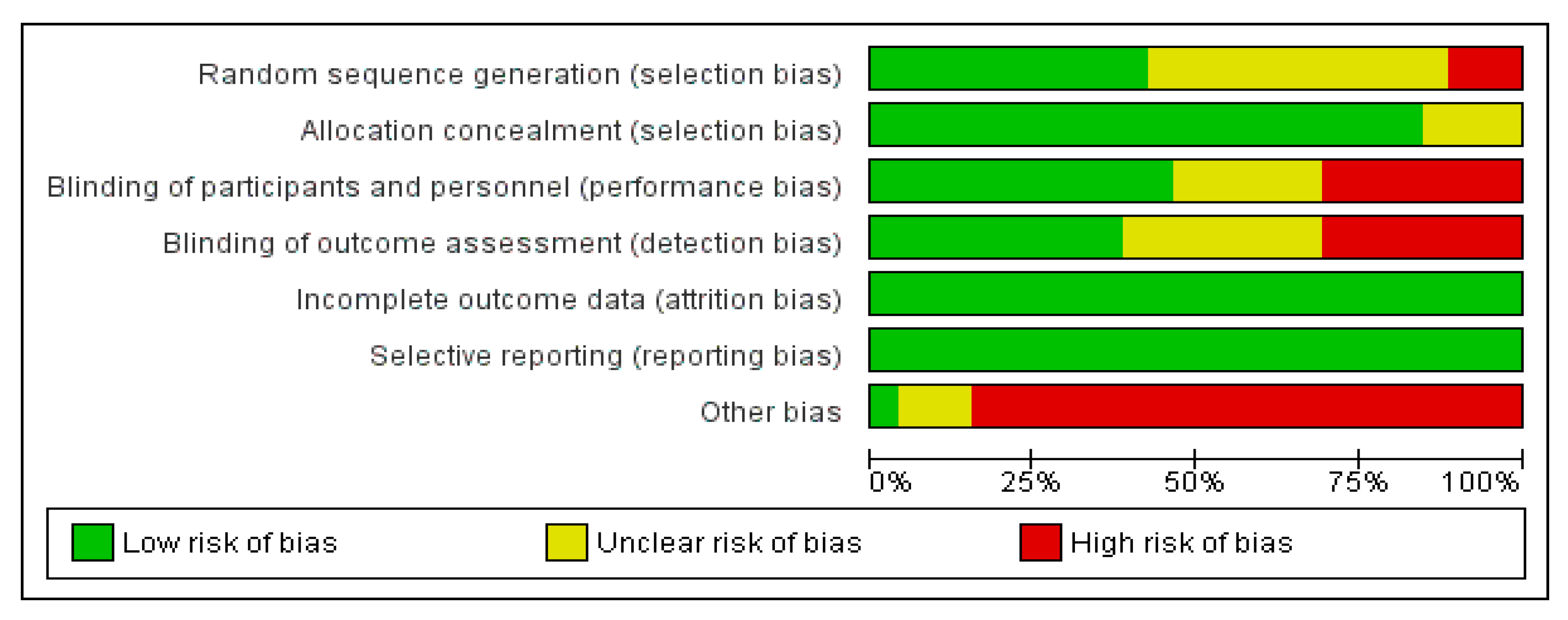
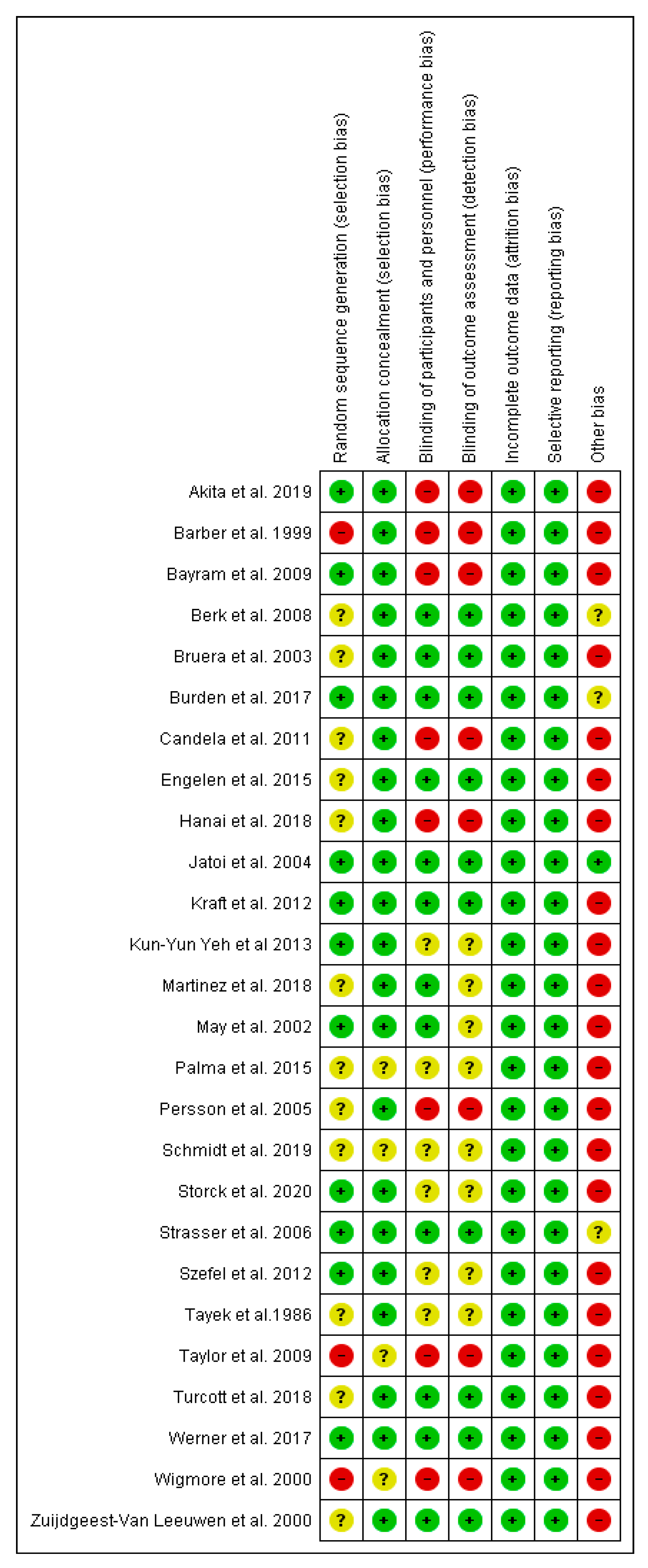
| Study ID | Design | Type of Cancer | n * | Intervention | Duration | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Akita et al., 2019 [36] | RCT | Pancreatic | 31 | EPA | 5 weeks | Improvement of SMM and PMA before and after CRT | The post/pre ratio of SMM in the nutritional intervention (NI) group increased with increases in supplement intake (p = 0.02). The post/pre ratio of PMA in the NI group was significantly higher than that of the normal diet (ND) group |
| Barber et al., 1999 [37] | CT | Pancreatic | 20 | EPA | 7 weeks | Weight gain (BWC, dietary intake, REE and performance status) | Weight-gain at both 3 (median 1 kg, p = 0.02) and 7 weeks (median 2 kg, p = 0.03). Dietary intake increased significantly by almost 400 kcal per day (−1) (p = 0.002). REE per kg BW and lean body mass (LBM) fell significantly. Performance status and appetite were significantly improved at 3 weeks. |
| Bayram et al., 2009 [38] | RCT | Pediatric | 33 | EPA | 24 weeks | BW, BMI, and weight percentile | Decrease in % of patients with weight loss in BW (6.1% vs. 47.4%; p = 0.001), BMI (12.1% vs. 52.6%; p = 0.002), and a negative deviation in weight percentile (6.1% vs.31.6%; p = 0.021) |
| Berk et al., 2008 [39] | RCT | Various | 235 | 3 g of HMB, 14 g arginine, and 14 g of glutamine | 8 weeks | LBM, body plethysmography, weight, the Schwartz Fatigue Scale, and the Spitzer QoL Scale | No statistically significant difference |
| Bruera et al., 2003 [40] | RCT | Various, advanced | 46 | FO | 12 days | Appetite, tiredness, nausea, well-being, caloric intake, nutritional status, and function were prospectively assessed on days 1 and 14 | No significant influence on appetite, tiredness, nausea, well-being, caloric intake, nutritional status, or function after two weeks |
| Burden et al., 2017 [41] | RCT | Colorectal | 55 | 250 mL/day ONS and dietary advice | 1 week | % weight loss, total complications, and BWC measurements | Less weight loss following surgery for colorectal cancer |
| Candela et al., 2011 [42] | RCT | Various | 16 | EPA | 4 weeks | Anthropometric and biological parameters, QoL (SF-36 questionnaire) | No significant changes in anthropometric and biological parameters except significantly decreased interferon gamma (INF-γ) values (0.99 ± 0.95 vs. 0.65 ± 0.92 pg/mL, p < 0.05 |
| Engelen et al., 2015 [43] | RCT | Advanced non-small-cell lung cancer | 13 | EAA, high leucine mixture | - | BW, height, fat, fat-free mass (FFM), respiratory muscle function, handgrip strength, and endurance. Protein anabolism | High leucine levels in the EAA/Leucine mixture were of no anabolic benefit. A highly significant linear relationship between net protein anabolism and the amount of EAA available in the systemic circulation (R (2): 0.85, p < 0.001) was found in both groups. |
| Hanai et al., 2018 [44] | RCT | Head and neck squamous cell carcinoma | 14 | ω-3 fatty acids | 28 days perioperative | Weight, lean body mass, albumin, prealbumin, CRP, IL6, white blood cell count, body temperature, postoperative complications | Not effective for maintaining the nutritional status |
| Jatoi et al., 2004 [45] | CT | Various | 421 in 3 arms | EPA versus MA, or both | 4 weeks | A 10% weight gain above baseline | No improvement in weight or appetite |
| Kraft et al., 2012 [46] | RCT | Pancreatic | 38 | L-carnitine | 12 weeks | Adverse effects, QoL, fatigue, BMI, BWC, survival time, L-carnitine level, CRP, CA 19-9, albumin, leucocytes | Body-mass-index increased by 3.4 ± 1.4%; nutritional status (body cell mass, body fat) and QoL parameters improved |
| Kun-Yun Yeh et al., 2013 [47] | RCT | Head and neck cancer | 31 | EE regimen enriched with ω-3 fatty acids, micronutrients, and probiotics | 12 weeks | BW changes, serum albumin and prealbumin levels | Significantly increased BW and maintained higher serum albumin and prealbumin levels |
| Martinez et al., 2018 [48] | RCT | Head and neck squamous cell cancer | 32 | EPA | 6 weeks | BWC, IL-1β, IL-6, TNF-α and IFN-γ, CRP, serum proteins, and blood count | A decrease in serum levels of IL-1β, IL-6, TNF-α, and IFN-γ, and regulation of BW (−0.3 ± 5.9 vs. −2.1 ± 3.7), LBM (−0.2 ± 3.8 versus −1.3 ± 3.6), BFM (0.2 ± 3.5 vs. −1.2 ± 3.8), and QoL (10 ± 33 vs. 5 ± 34). |
| May et al., 2002 [49] | RCT | Solid tumors | 18 | β-HMB, arginine, and glutamine | 24 weeks | Change in body mass and fat-free mass (FFM) | Weight gain of 0.95 +/− 0.66 kg in 4 weeks, significant FFM increase of 1.12 +/− 0.68 kg |
| Palma et al., 2015 [50] | CT | Various, advanced | 18 | Guarana (Paullinia cupana) | 4 weeks | A positive response in the first phase to be at least 5% weight gain or a three-point improvement in the appetite scale in at least three of the first 18 evaluable patients | Only two patients had weight gain above 5% from their baseline, whereas six patients had at least a three-point improvement in the visual appetite scale; a significant decrease in the lack of appetite and in somnolence |
| Persson et al., 2005 [51] | RCT | Advanced GIC | 13 | FO and melatonin | 4 weeks | Tumor necrosis factor-alpha, IL-1β, soluble IL-2 receptor, IL-6, IL-8, and EPA, DHA, arachidonic acid, and linoleic acid. | No major changes in biochemical variables and cytokines were observed with any intervention. In the FO group, 5 of 13 patients (38%) showed weight stabilization or gain compared with 3 of 11 patients (27%) in the MLT group. |
| Schmidt et al., 2019 [52] | CT | GIC | 13 | FO | 4 weeks | Acceptability and compliance, nutritional status and side effects, leukocyte, platelet counts, and markers of dose-limiting toxicities of chemotherapy. | FO in capsules appeared to result in better compliance than a nutritional drink with an equivalent dose of ω-3 LC PUFAs. There were no differences between the groups concerning changes in whole blood ω-3 LC PUFAs, weight, nutritional status, acceptability, or side effects; in the capsule group, the whole blood ω-3 LC PUFAs correlated negatively with the increase in nausea. No changes in median thrombocyte or leukocyte blood counts were observed. |
| Storck et al., 2020 [53] | RCT | Various, advanced | 27 | Leucine-rich supplement (whey protein) | 12 weeks | Physical function, physical performance tests, nutritional status, dietary intake, fatigue, QoL, and clinical course | The secondary endpoint handgrip strength improved significantly. No significant differences between the other outcomes |
| Strasser et al., 2006 [54] | RCT | Various, advanced | 164 | CE and THC | 6 weeks | Appetite, mood, and nausea, QoL CE-related toxicity was assessed every 2 weeks. | Increased appetite was reported by 73%, 58%, and 69% of patients receiving CE, THC, or PL, respectively. |
| Szefel et al., 2012 [55] | RCT | Various | 25 | TPN with LCTs, or LCTs plus MCTs as 50/50. | 10 days | L-Carnitine distribution and the effects of parenteral lipid emulsions on plasma L-Carnitine levels and urinary excretion | A diet of MCTs/LCTs reduces L-carnitine release from muscle to plasma and urine more effectively than LCTs. |
| Tayek et al.,1986 [56] | RCT | Intra-abdominal metastatic adenocarcinoma | 10 | Conventional TPN (19% BCAA) and a BCAA-enriched TPN formula (50% BCAA) | - | Changes in the whole-body leucine kinetics and fractional rates of albumin synthesis | BCAA-enriched formulas improve whole body leucine kinetics, fractional rates of albumin synthesis, and leucine balance, and thus may favorably influence protein metabolism in cancer cachexia. |
| Taylor et al., 2009 [57] | CT | Various | 17 | MPL | 6 weeks | Compliance, changes in BW, appetite, and QoL, fatty acid profile in plasma and blood cells | Significantly reducing the ω-6 to ω-3 fatty acid ratio, median weight change of +0.6% after 6 weeks), while appetite and QoL improved. |
| Turcott et al., 2018 [58] | RCT | Advanced non-small cell lung cancer | 9 | Drugs derived from cannabinoids (Nabilone) | 6 weeks | Appetite, nutritional status, and QoL | Increased caloric intake (342 kcal), and significantly higher intake of carbohydrates (64 g) compared to patients receiving PL (p = 0.040). QoL also showed significant improvements |
| Werner et al., 2017 [59] | RCT | Pancreatic | 31 | FO, MPL | 6 weeks | Routine blood parameters, lipid profiles, BW, and appetite | 50% of the FO group gained BW during the 6-week intervention. In the MPL group, 47% gained weight; no significant change in fat mass, muscle mass, and body water; no significant statistical difference in BMI in both groups |
| Wigmore et al., 2000 [60] | CT | Advanced pancreatic cancer | 26 | High-purity EPA | 12 weeks | Overall survival, changes in BWC, hematologic and clinical chemistry variables, acute-phase protein response, and performance status | A median weight gain of 0.5 kg persisted over the 12-week study period. TBW as a % of BW remained stable, as did the % of patients with an acute-phase protein response, nutritional intake, and performance status. Overall median survival from diagnosis was 203 days. |
| Zuijdgeest-Van Leeuwen et al., 2000 [61] | RCT | Various | 17 | EPA-EE (6 g/d) or PL OA-EE; 6 g/d) | 7 days | Whole-body lipolysis and palmitic acid release were measured in the overnight fasting state, changes in weight, plasma free fatty acids (FFA), triacylglycerols (TAG), CRP, albumin, and prealbumin | No significant effects of EPA-EE on whole-body lipolysis, palmitic acid release, or palmitate oxidation were detected in cancer patients or healthy subjects compared to OA-EE. EPA-EE reduced plasma-FFA and TAG concentrations significantly in healthy subjects but not in cancer patients. |
| Study ID | Intervention | n * | Age (Years) | Baseline BMI (kg/m2) | Effect on BW | p ** |
|---|---|---|---|---|---|---|
| Akita et al., 2019 [36] | EPA | 31 | 67.8 ± 10.7 | 22.3 ± 2.39 | Decrease | 0.01 |
| Barber et al., 1999 [37] | EPA | 20 | 62 (51–75) | 19.8 (17.8–21.8) | Increase | 0.03 |
| Bayram et al., 2009 [38] | EPA | 33 | 7.7 ± 2.7 | - | Decrease | NS |
| Berk et al., 2008 [39] | 3 g of βHMB, 14 g arginine, and 14 g of glutamine | 235 | 67 (23–91) | - | Increase | NS |
| Bruera et al., 2003 [40] | FO | 46 | 63.0 ± 9.1 | - | Neutral | NS |
| Burden et al., 2017 [41] | 250 mL/day ONS and dietary advice | 55 | 70.5 ± 11.66 | - | Neutral | NS |
| Candela et al., 2011 [42] | EPA | 16 | 61.31 ± 12.07 | 20.94 ± 3.72 | Neutral | NS |
| Engelen et al., 2015 [43] | EAA, high leucine mixture | 13 | 68.5 ± 2.1 | 26.5 ± 1.1 | N/A | N/A |
| Hanai et al., 2018 [44] | ω-3 fatty acids | 14 | 61.5 (45–77) | - | Decrease | N/A |
| Jatoi et al., 2004 [45] | EPA or MA versus, or both | 421 in 3 arms | 65 ± 11 | - | Neutral | NS |
| Kraft et al., 2012 [46] | L-carnitine | 38 | 64.4 ± 1.67 | 24.7 ± 0.65 | Increase | 0.01 |
| Kun-Yun Yeh et al., 2013 [47] | EE regimen enriched with ω-3 fatty acids, micronutrients, and probiotics | 31 | 54.1± 9.3 | 20.0 ± 3.1 | Increase | <0.05 |
| Martinez et al., 2018 [48] | EPA | 32 | 60± 14 | - | Neutral | NS |
| May et al., 2002 [49] | β-HMB, arginine, and glutamine | 18 | 65.9 ±2.0 | - | Increase | <0.05 |
| Palma et al., 2015 [50] | Guarana | 18 | 65 (49–81) | - | Neutral | NS |
| Persson et al., 2005 [51] | FO and melatonin | 13 | 66 ±9 | 21.6± 4.1 | Increase | NS |
| Schmidt et al., 2019 [52] | FO capsules | 13 | 68 (59–69) | 27.0 (24.1–28.5) | Neutral | NS |
| FO nutritional drink group | 61 (57–66.8) | 25.8 (23.8–27.8) | ||||
| Storck et al., 2020 [53] | Leucine-rich supplement (whey protein) | 27 | 62.0 ±11.4 | 25.0 ±4.6 | Increase | NS |
| Strasser et al., 2006 [54] | CE and THC | 164 | 61 ± 12 | - | Decrease | NS |
| Szefel et al., 2012 [55] | TPN, LCTs, or LCTs plus MCTs as 50/50. | 25 | 66 ± 11 | 21 ± 5 | Decrease | NS |
| Tayek et al., 1986 [56] | TPN formula (19% BCAA) and a TPN formula (50% BCAA) | 10 | 59.6 ± 4.6 | - | Increase | N/A |
| Taylor et al., 2009 [57] | MPL | 17 | 62.2 ± 8.9 | 20.2 ± 3.7 | Increase | 0.006 |
| Turcott et al., 2018 [58] | Nabilone | 9 | 61.1 ± 10.6 | 20.9 ± 3.5 | Decrease | NS |
| Werner et al., 2017 [59] | FO supplementation, MPL | 31 | 70.3 ± 8.24 | 21.3 ± 1.73 | Neutral | N/A |
| Wigmore et al., 2000 [60] | High-purity EPA | 26 | 56 (39–75) | 23.2 (21.1–27.4) | Increase | <0.05 |
| Zuijdgeest-Van Leeuwen et al., 2000 [61] | EPA-EE or OA-EE | 17 | 64 ± 10 | 22 ± 3 | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braha, A.; Albai, A.; Timar, B.; Negru, Ș.; Sorin, S.; Roman, D.; Popovici, D. Nutritional Interventions to Improve Cachexia Outcomes in Cancer—A Systematic Review. Medicina 2022, 58, 966. https://doi.org/10.3390/medicina58070966
Braha A, Albai A, Timar B, Negru Ș, Sorin S, Roman D, Popovici D. Nutritional Interventions to Improve Cachexia Outcomes in Cancer—A Systematic Review. Medicina. 2022; 58(7):966. https://doi.org/10.3390/medicina58070966
Chicago/Turabian StyleBraha, Adina, Alin Albai, Bogdan Timar, Șerban Negru, Săftescu Sorin, Deiana Roman, and Dorel Popovici. 2022. "Nutritional Interventions to Improve Cachexia Outcomes in Cancer—A Systematic Review" Medicina 58, no. 7: 966. https://doi.org/10.3390/medicina58070966
APA StyleBraha, A., Albai, A., Timar, B., Negru, Ș., Sorin, S., Roman, D., & Popovici, D. (2022). Nutritional Interventions to Improve Cachexia Outcomes in Cancer—A Systematic Review. Medicina, 58(7), 966. https://doi.org/10.3390/medicina58070966







