Eosinophilic Angiocentric Fibrosis of the Nasal Cavities: A Report of an Uncommon Lesion with Emphasis on the Etiology and Differential Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
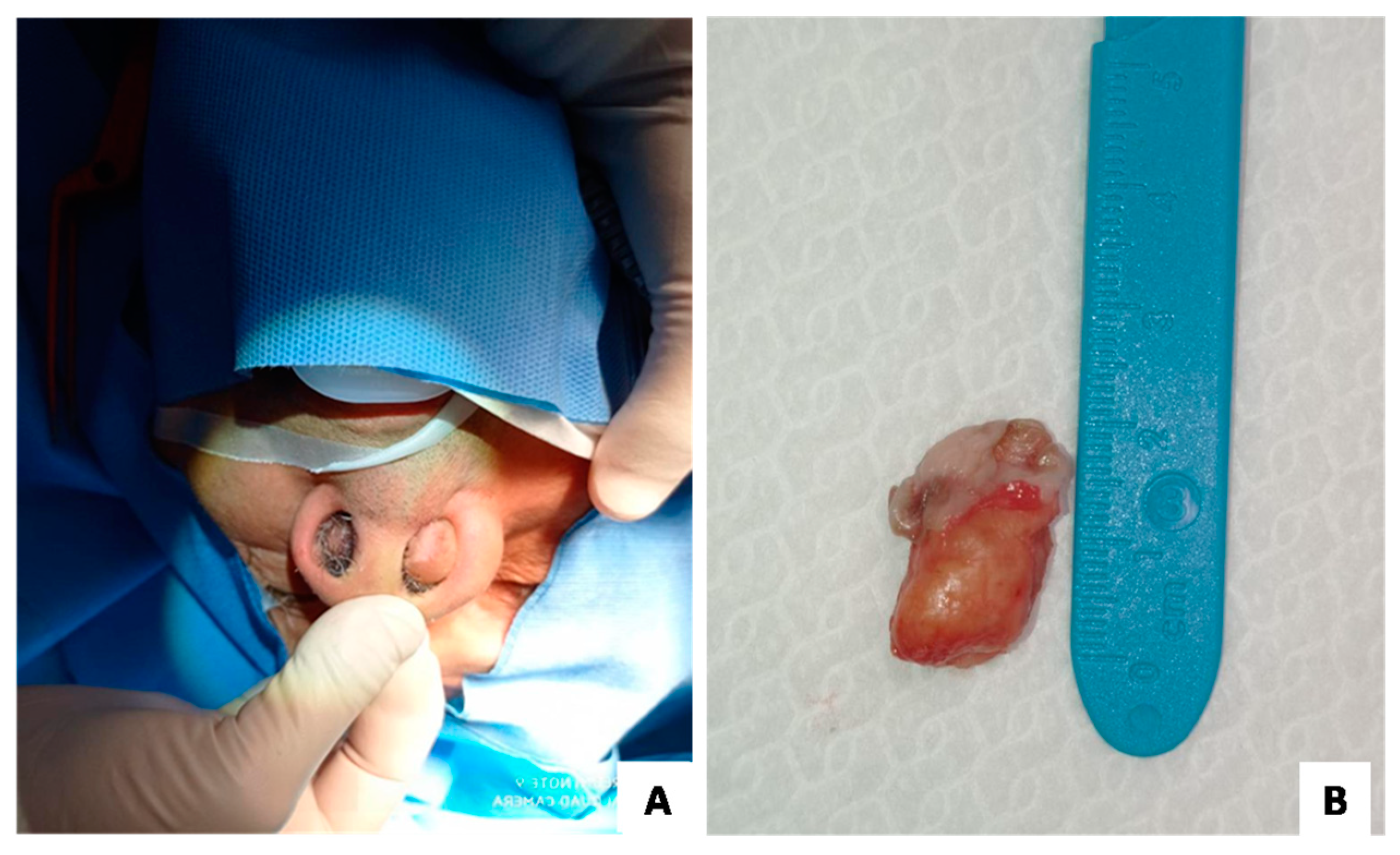
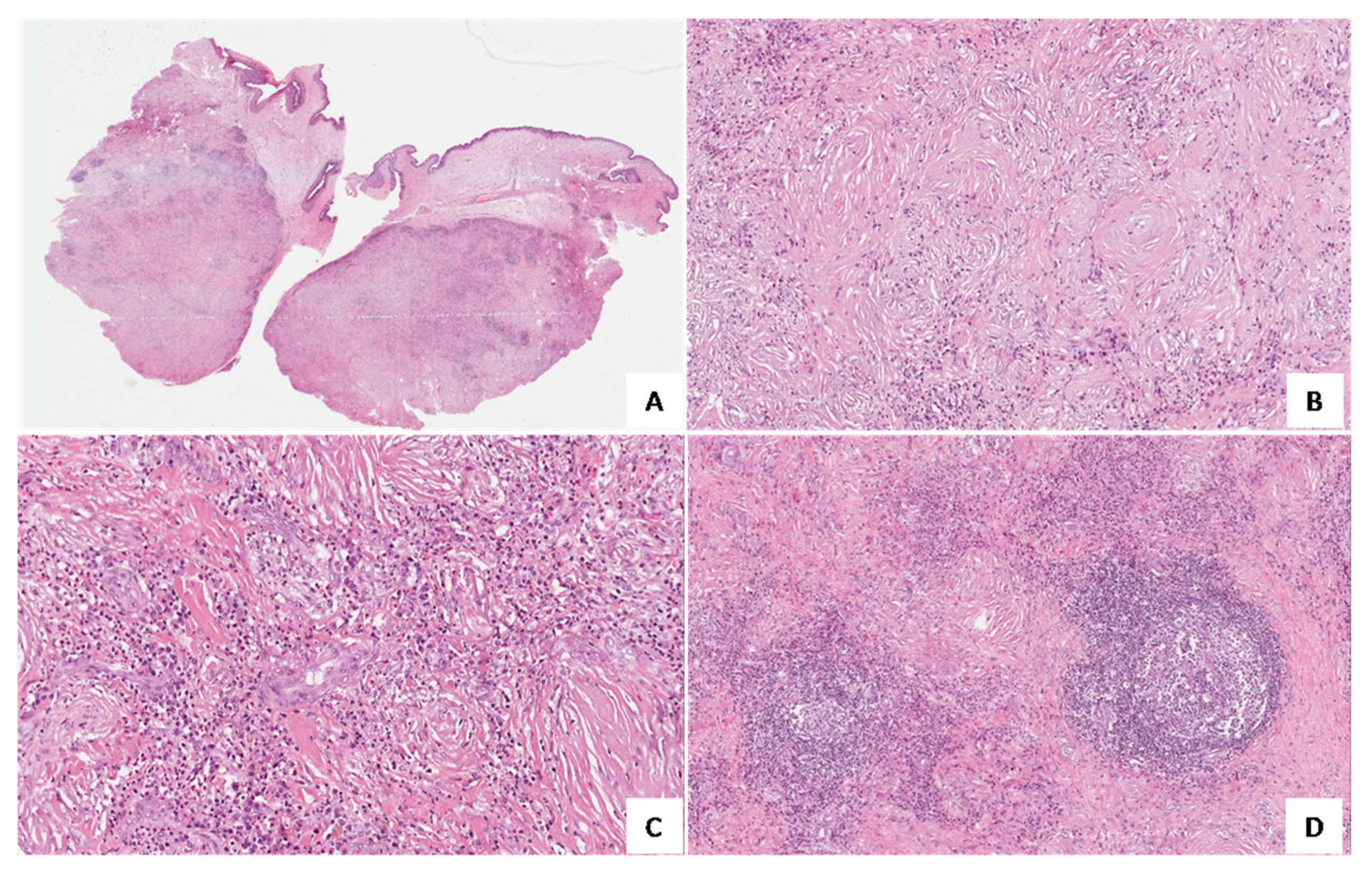
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, J.; Flanagan, M. Eosinophilic Angiocentric Fibrosis: A Review and Update of Its Association With Immunoglobulin G4-Related Disease. Arch. Pathol. Lab. Med. 2018, 142, 1560–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, P.F.; McCann, B.G. Eosinophilic Angiocentric Fibrosis of the Upper Respiratory Tract: A Mucosal Variant of Granuloma Faciale? A Report of Three Cases. Histopathology 1985, 9, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R. Algorithmic Approach to Fibroinflammatory Sinonasal Tract Lesions. Head Neck Pathol. 2021, 15, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Heedari, H.M.; Ciotoracu, A.C.; Mitulescu, T.C.; Dimancescu, M.G.; Enache, S.; Predețeanu, D. A Clinical Case of Orbital Inflammatory Pseudotumor as the Primary Expression of Eosinophilic Angiocentric Fibrosis. Rom. J. Ophthalmol. 2021, 65, 411. [Google Scholar] [CrossRef]
- Suárez, A.R.; Capote, A.C.; Barrientos, Y.F.; Soto, M.L.; Moreno, S.G.; Martín, F.G. Ocular Involvement in Idiopathic Hypertrophic Pachymeningitis Associated with Eosinophilic Angiocentric Fibrosis: A Case Report. Arq. Bras. Oftalmol. 2018, 81, 250–253. [Google Scholar] [CrossRef]
- Legare, N.; Frosh, S.; Vasquez, J.B.; Ho, S.T. Eosinophilic Angiocentric Fibrosis: A Sino-Orbital Masquerader. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef]
- Jurkov, M.; Olze, H.; Klauschen, F.; Bertelmann, E.; Schneider, U.; Arens, P. IgG4-Related Orbitopathy as an Important Differential Diagnosis of Advanced Silent Sinus Syndrome. HNO 2020, 68, 65–68. [Google Scholar] [CrossRef]
- Okuyama, S.; Yazu, H.; Ito, Y.; Minato, H.; Fujishima, H. Eosinophilic Angiocentric Fibrosis in Bilateral Upper Eyelid Conjunctivas: A First Case Report. Am. J. Case Rep. 2020, 21, e924042-1–e924042-6. [Google Scholar] [CrossRef]
- Heft Neal, M.E.; Rowan, N.R.; Willson, T.J.; Wang, E.W.; Lee, S.E. A Case Report and Systematic Review of Eosinophilic Angiocentric Fibrosis of the Paranasal Sinuses. Ann. Otol. Rhinol. Laryngol. 2017, 126, 415–423. [Google Scholar] [CrossRef]
- Han, S.C.; Park, J.H.; Hong, S.N. Eosinophilic Angiocentric Fibrosis Invading the Nasal Septum: A Case Report and Review of Literature. Ear Nose Throat J. 2021, 100, 557–561. [Google Scholar] [CrossRef]
- Nutalapati, S.; O’Neal, R.; O’Connor, W.; Comer, B.T.; Hildebrandt, G.C. Challenges in Medicine: The Odyssey of a Patient with Isolated IgG4-Related Eosinophilic Angiocentric Fibrosis Presenting as a Locally Destructive Sinonasal Mass. Case Rep. Rheumatol. 2021, 2021, 6668184. [Google Scholar] [CrossRef] [PubMed]
- Keogh, I.; O’Connell, R.; Hynes, S.; Lang, J. Eosinophilic Angiocentric Fibrosis as a Stenosing Lesion in the Subglottis. Case Rep. Otolaryngol. 2017, 2017, 2381786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidghamat, E.; Groves, R.; Robson, A. Eosinophilic Angiocentric Fibrosis Presenting as Asymptomatic Cutaneous Nodules. Clin. Exp. Dermatol. 2015, 40, 85–86. [Google Scholar] [CrossRef]
- Deshpande, V.; Khosroshahi, A.; Nielsen, G.P.; Hamilos, D.L.; Stone, J.H. Eosinophilic Angiocentric Fibrosis Is a Form of IgG4-Related Systemic Disease. Am. J. Surg. Pathol. 2011, 35, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.J.C.; Lee, M.-H.H.; Chung, H.W.; Tang, P.Y. Eosinophilic Angiocentric Fibrosis and IgG4-Related Disease Revisited. Histopathology 2022. [Google Scholar] [CrossRef]
- Yung, A.; Wachsmuth, R.; Ramnath, R.; Merchant, W.; Myatt, A.E.; Sheehan-Dare, R. Eosinophilic Angiocentric Fibrosis—A Rare Mucosal Variant of Granuloma Faciale Which May Present to the Dermatologist. Br. J. Dermatol. 2005, 152, 574–576. [Google Scholar] [CrossRef]
- Nigar, E.; Dhillon, R.; Carr, E.; Matin, R.N. Eosinophilic Angiocentric Fibrosis and Extrafacial Granuloma Faciale. Histopathology 2007, 51, 729–731. [Google Scholar] [CrossRef]
- Holme, S.A.; Laidler, P.; Holt, P.J.A. Concurrent Granuloma Faciale and Eosinophilic Angiocentric Fibrosis. Br. J. Dermatol. 2005, 153, 851–853. [Google Scholar] [CrossRef]
- Depaepe, L.; Chouvet, B.; Claudy, A.; Thomas, L.; Berger, F.; Balme, B. Eosinophilic Angiocentric Fibrosis and Granuloma Facial with Extra Facial Presentation, the Same Pathology? Ann. Pathol. 2011, 31, 138–141. [Google Scholar] [CrossRef]
- Burns, B.V.; Roberts, P.F.; De Carpentier, J.; Zarod, A.P. Eosinophilic Angiocentric Fibrosis Affecting the Nasal Cavity. A Mucosal Variant of the Skin Lesion Granuloma Faciale. J. Laryngol. Otol. 2001, 115, 223–226. [Google Scholar] [CrossRef]
- Loane, J.; Jaramillo, M.; Young, H.A.; Kerr, K.M. Eosinophilic Angiocentric Fibrosis and Wegener’s Granulomatosis: A Case Report and Literature Review. J. Clin. Pathol. 2001, 54, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Benlemlih, A.; Szableski, V.; Bendahou, M.; Rivière, S.; Villain, M.; Costes, V. Eosinophilic Angiocentric Fibrosis: A Form of IgG4-Related Systemic Disease? Ann. Pathol. 2012, 32, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Zen, Y.; Chan, J.K.C.; Yi, E.E.; Sato, Y.; Yoshino, T.; Klöppel, G.; Godfrey Heathcote, J.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus Statement on the Pathology of IgG4-Related Disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorostis, S.; Bacha, M.; Gravier, S.; Raguin, T. Right Ethmoid Eosinophilic Angiocentric Fibrosis with Orbital Extension. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 351–354. [Google Scholar] [CrossRef] [PubMed]
| Disease | Usual Sites | Laboratory Tests | Histopathological Features |
|---|---|---|---|
| Eosinophilic angiocentric fibrosis | Upper respiratory tract and orbit | IgG4 serum levels (not specific) | “Onion skin-like” perivascular fibrosis, eosinophil-rich inflammatory infiltrate |
| Granuloma faciale | Skin (face) | None | Fibrosis and inflammatory infiltrate |
| Wegener’s granulomatosis | Upper respiratory tract, lungs, and kidneys | c-ANCA | Foreign-body giant cells, geographic necrosis, and granuloma |
| Churg–Strauss syndrome | Upper and lower respiratory tract, kidney, heart, and gastrointestinal tract | p-ANCA and blood eosinophilia | Fibrinoid necrosis and extravascular granulomas with eosinophil-rich inflammatory infiltrate |
| Kimura’s disease | Skin (head and neck) | Blood eosinophilia and raised IgE serum levels | Fibrosis and lymphoid aggregates |
| Site | Nasal Region (n = 6) [2,9,10,11,14] | Orbital Region, Including Meninges and Ocular Adnexa (n = 7) [5,6,8,14,24] | Subglottis (n = 1) [12] | Upper Arms and Chest (n = 1) [13] |
|---|---|---|---|---|
| IgG4 serum levels (normal, 8 to 140 mg/dL) | Low (n = 1) [6] | Normal (n = 3) [5,10,11] | Elevated (n = 3) [8,14,24] | Not available (n = 8) [2,9,12,13,14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, J.; Broggi, G.; Federico, C.; Zanelli, M.; Palicelli, A.; Caltabiano, R. Eosinophilic Angiocentric Fibrosis of the Nasal Cavities: A Report of an Uncommon Lesion with Emphasis on the Etiology and Differential Diagnosis. Medicina 2022, 58, 865. https://doi.org/10.3390/medicina58070865
Farina J, Broggi G, Federico C, Zanelli M, Palicelli A, Caltabiano R. Eosinophilic Angiocentric Fibrosis of the Nasal Cavities: A Report of an Uncommon Lesion with Emphasis on the Etiology and Differential Diagnosis. Medicina. 2022; 58(7):865. https://doi.org/10.3390/medicina58070865
Chicago/Turabian StyleFarina, Jessica, Giuseppe Broggi, Carmelo Federico, Magda Zanelli, Andrea Palicelli, and Rosario Caltabiano. 2022. "Eosinophilic Angiocentric Fibrosis of the Nasal Cavities: A Report of an Uncommon Lesion with Emphasis on the Etiology and Differential Diagnosis" Medicina 58, no. 7: 865. https://doi.org/10.3390/medicina58070865
APA StyleFarina, J., Broggi, G., Federico, C., Zanelli, M., Palicelli, A., & Caltabiano, R. (2022). Eosinophilic Angiocentric Fibrosis of the Nasal Cavities: A Report of an Uncommon Lesion with Emphasis on the Etiology and Differential Diagnosis. Medicina, 58(7), 865. https://doi.org/10.3390/medicina58070865









