New Perspectives in Therapeutic Vaccines for HPV: A Critical Review
Abstract
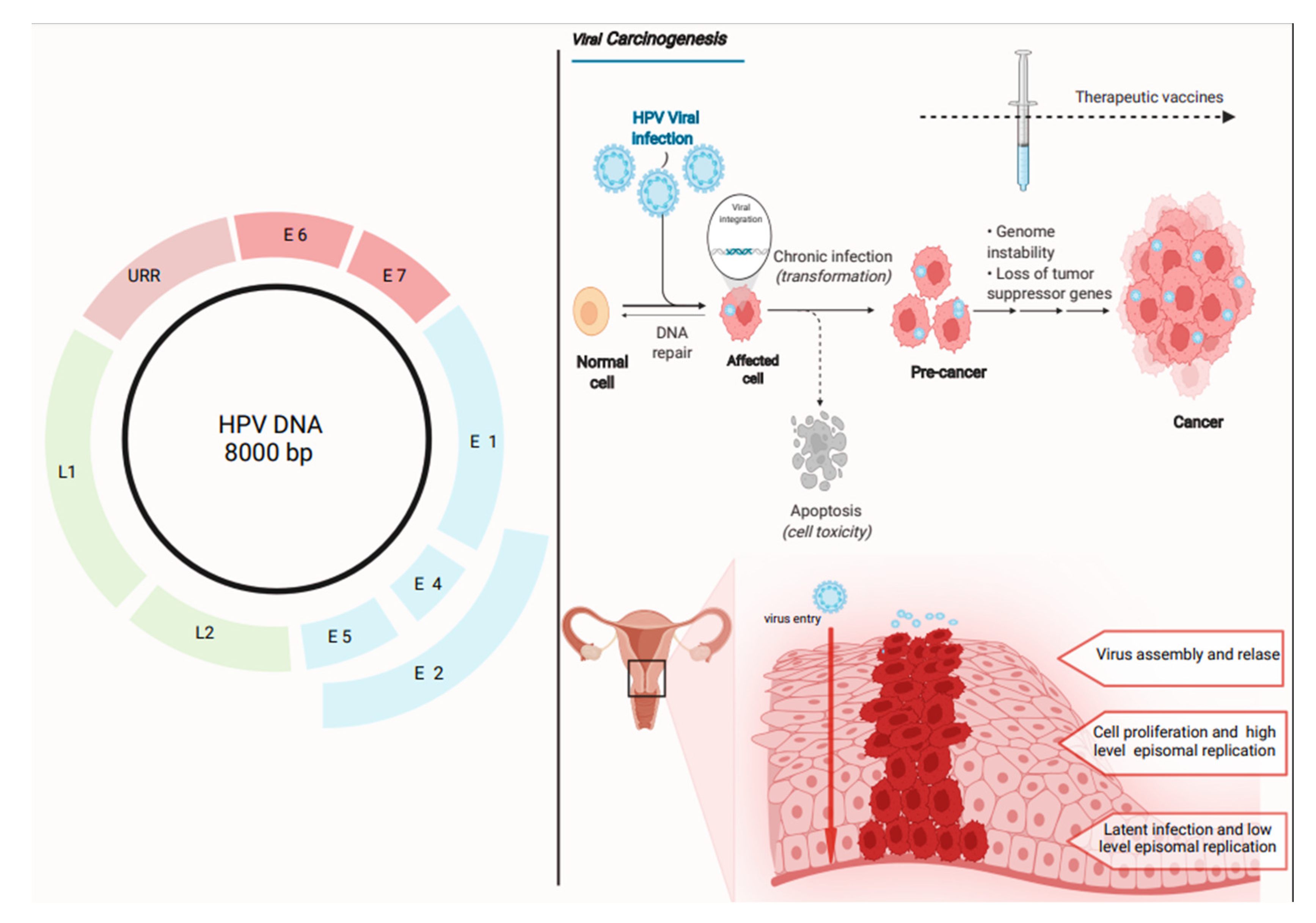
1. Introduction
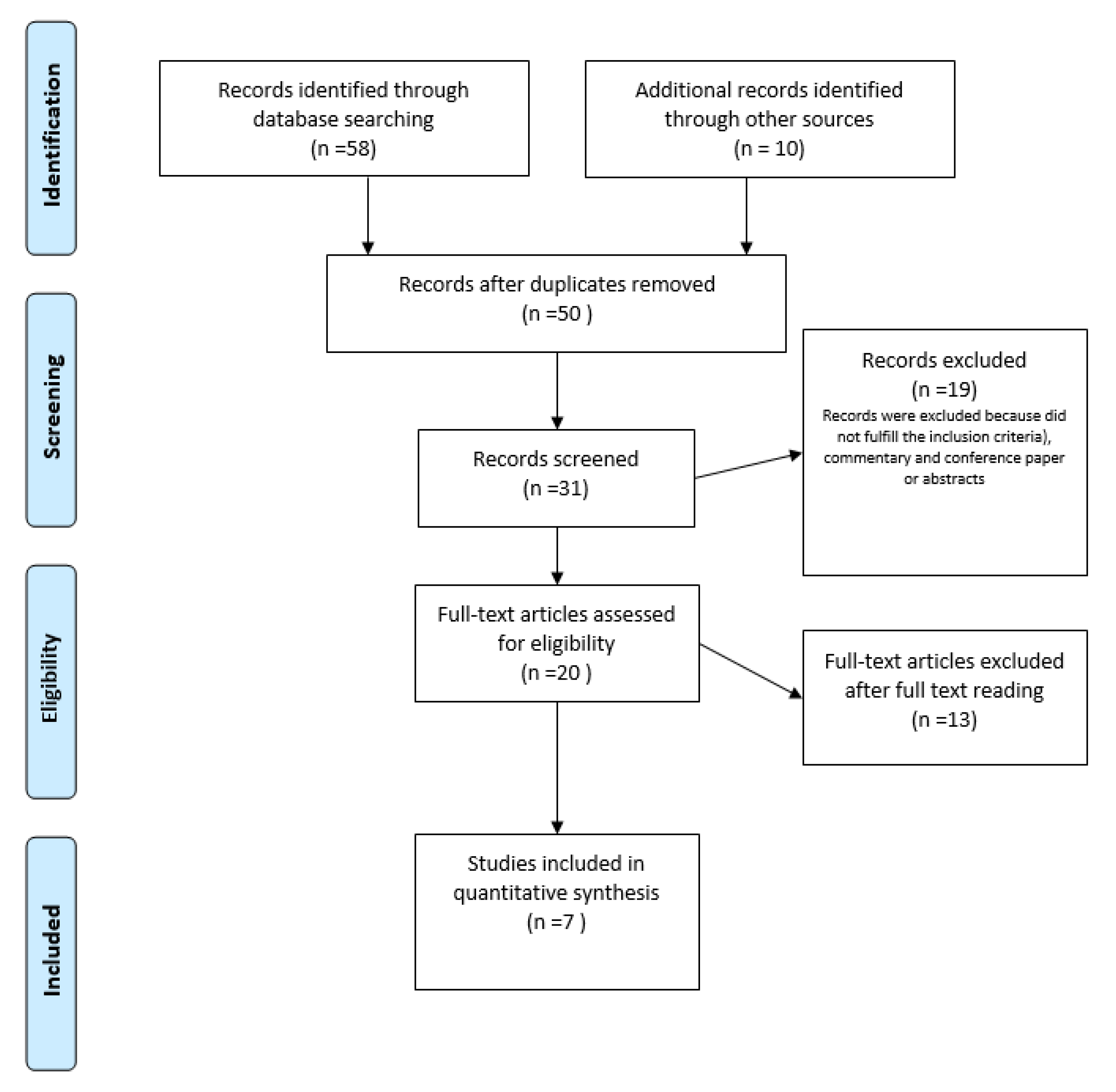
2. Materials and Methods
3. Results
4. Discussion
4.1. HPV Vaccines Based on L2 Protein: Rationale
4.2. HPV Immunological Response and L2 Therapeutic Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Rylander, E.; Larsson, B.; Strand, A.; Silfversvärd, C.; Wilander, E. The role of Human Papillomavirus in cervical adenocarcinoma carcinogenesis. Eur. J. Cancer 2001, 3, 246–250. [Google Scholar] [CrossRef]
- Graham, S.V. Keratinocyte Differentiation-Dependent Human Papillomavirus Gene Regulation. Viruses 2017, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Kayyal, M.; Bolhassani, A.; Noormohammadi, Z.; Sadeghizadeh, M. In Silico Design and Immunological Studies of Two Novel Multiepitope DNA-Based Vaccine Candidates Against High-Risk Human Papillomaviruses. Mol. Biotechnol. 2021, 63, 1192–1222. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Human Papillomavirus Vaccines. WHO position paper. Wkly. Epidemiol. Rec. 2009, 84, 118–131. [Google Scholar]
- Ghelardi, A.; Marrai, R.; Bogani, G.; Sopracordevole, F.; Bay, P.; Tonetti, A.; Lombardi, S.; Bertacca, G.; Joura, E.A. Surgical Treatment of Vulvar HSIL: Adjuvant HPV Vaccine Reduces Recurrent Disease. Vaccines 2021, 9, 83. [Google Scholar] [CrossRef]
- Hagensee, M.E.; Yaegashi, N.; Galloway, D.A. Self-assembly of Human Papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 1993, 67, 315–322. [Google Scholar] [CrossRef]
- Harper, D.M.; De Mars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef]
- Olczak, P.; Roden, R.B.S. Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases. Vaccines 2020, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Rafael, T.S.; Rotman, J.; Brouwer, O.R.; van der Poel, H.G.; Mom, C.H.; Kenter, G.G.; de Gruijl, T.D.; Jordanova, E.S. Immunotherapeutic Approaches for the Treatment of HPV-Associated (Pre-)Cancer of the Cervix, Vulva and Penis. J. Clin. Med. 2022, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Bywaters, S.M.; Brendle, S.A.; Ashley, R.E.; Makhov, A.M.; Conway, J.F.; Christensen, N.D.; Hafenstein, S. Cryoelectron Microscopy Maps of Human Papillomavirus 16 Reveal L2 Densities and Heparin Binding Site. Structure 2017, 25, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.J.; Boswell, C.M.; Sehr, P.; Pawlita, M.; Tomlinson, A.E.; McVey, R.J.; Dobson, J.; Roberts, J.S.; Hickling, J.; Kitchener, H.C.; et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding Human Papillomavirus 16/18 oncoproteins. Cancer Res. 2003, 63, 6032–6041. [Google Scholar]
- Moody, C.A.; Laimins, L.A. Human Papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Graham, S.V. Human Papillomavirus: Gene expression, regulation and prospects for novel diagnostic methods and antiviral therapies. Future Microbiol. 2010, 5, 1493–1506. [Google Scholar] [CrossRef]
- Karimi, H.; Soleimanjahi, H.; Abdoli, A.; Banijamali, R.S. Combination therapy using human papilloma-virus L1/E6/E7 genes and archaeosome: A nanovaccine confer immuneadjuvanting effects to fight cervical cancer. Sci. Rep. 2020, 10, 5787. [Google Scholar] [CrossRef]
- Wang, J.W.; Roden, R.B.S. L2, the minor capsid protein of papillomavirus. Virology 2013, 445, 175–186. [Google Scholar] [CrossRef]
- Keiffer, T.R.; Soorya, S.; Sapp, M.J. Recent Advances in Our Understanding of the Infectious Entry Pathway of Human Papillomavirus Type 16. Microorganisms 2021, 9, 2076. [Google Scholar] [CrossRef]
- Goetschius, D.J.; Hartmann, S.R.; Subramanian, S.; Bator, C.M.; Christensen, N.D.; Hafenstein, S.L. High resolution cryo EM analysis of HPV16 identifies minor structural protein L2 and describes capsid flexibility. Sci. Rep. 2021, 11, 3498. [Google Scholar] [CrossRef]
- Broniarczyk, J.; Massimi, P.; Pim, D.; Bergant Marušič, M.; Myers, M.P.; Garcea, R.L.; Banks, L. Phosphorylation of Human Papillomavirus Type 16 L2 Contributes to Efficient Virus Infectious Entry. J. Virol. 2019, 93, e00128-19. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for sys-tematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Boutron, I.; Page, M.; Higgins, J.; Altman, D.; Lundh, A.; Hróbjartsson, A.; Cochrane Bias Methods Group. Chapter 7: Considering bias and conflicts of interest among the included studies. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2, Updated February 2021; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: www.training.cochrane.org/handbook (accessed on 13 May 2022).
- Schünemann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H.; Cochrane GRADEing Methods Group; Cochrane Statistical Methods Group. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidencele. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2, Updated February 2021; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: www.training.cochrane.org/handbook (accessed on 13 May 2022).
- Higgins, J.P.T.; Churchill, R.; Chandler, J.; Cumpston, M.S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.2.0, Updated June 2017, Chapter 8; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: www.training.cochrane.org/handbook (accessed on 13 May 2022).
- De Jong, A.; O’Neill, T.; Khan, A.Y.; Kwappenberg, K.M.; Chisholm, S.E.; Whittle, N.R.; Dobson, J.A.; Jack, L.C.; St Clair Roberts, J.A.; Offringa, R.; et al. Enhancement of human papillo-mavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 2002, 20, 3456–3464. [Google Scholar] [CrossRef]
- Smyth, L.J.; Van Poelgeest, M.I.; Davidson, E.J.; Kwappenberg, K.M.; Burt, D.; Sehr, P.; Pawlita, M.; Man, S.; Hickling, J.K.; Fiander, A.N.; et al. Immunological responses in women with Human Papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin. Cancer Res. 2004, 10, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.J.; Faulkner, R.L.; Sehr, P.; Pawlita, M.; Smyth, L.J.; Burt, D.J.; Tomlinson, A.E.; Hickling, J.; Kitchener, H.C.; Stern, P.L. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7). Vaccine 2004, 22, 2722–2729. [Google Scholar] [CrossRef]
- Fiander, A.N.; Tristram, A.J.; Davidson, E.J.; Tomlinson, A.E.; Man, S.; Baldwin, P.J.; Sterling, J.C.; Kitchener, H.C. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: Clinical results from a multicenter phase II trial. Int. J. Gynecol. Cancer 2006, 16, 1075–1081. [Google Scholar] [CrossRef]
- Daayana, S.; Elkord, E.; Winters, U.; Pawlita, M.; Roden, R.; Stern, P.L.; Kitchener, H.C. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Cancer 2010, 102, 1129–1136. [Google Scholar] [CrossRef]
- Safety and Feasibility of TA-CIN Vaccine in HPV16 Associated Cervical Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02405221 (accessed on 13 May 2022).
- Buck, C.B.; Trus, B.L. The papillomavirus virion: A machine built to hide molecular Achilles’ heels. Adv. Exp. Med. Biol. 2012, 726, 403–422. [Google Scholar]
- Rubio, I.; Bolchi, A.; Moretto, N.; Canali, E.; Gissmann, L.; Tommasino, M.; Muller, M.; Ottonello, S. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine 2009, 27, 1949–1956. [Google Scholar] [CrossRef]
- Seitz, H.; Canali, E.; Ribeiro-Muller, L.; Palfi, A.; Bolchi, A.; Tommasino, M.; Ottonello, S.; Muller, M. A three component mix of thioredoxin-L2 antigens elicits broadly neutralizing responses against on-cogenic Human Papillomaviruses. Vaccine 2014, 32, 2610–2617. [Google Scholar] [CrossRef]
- Kalnin, K.; Chivukula, S.; Tibbitts, T.; Yan, Y.; Stegalkina, S.; Shen, L.; Cieszynski, J.; Costa, V.; Sabharwal, R.; Anderson, S.F.; et al. Incorporation of RG1 epitope concatemers into a self-adjuvanting Flagellin-L2 vaccine broaden durable protection against cutaneous challenge with diverse human papil-lomavirus genotypes. Vaccine 2017, 35, 4942–4951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, X.; Liu, H.; Bao, Q.; Wang, Z.; Liao, G.; Xu, X. A rationally designed flagellin-L2 fusion protein induced serum and mucosal neutralizing antibodies against multiple HPV types. Vaccine 2019, 37, 4022–4030. [Google Scholar] [CrossRef] [PubMed]
- Jagu, S.; Karanam, B.; Gambhira, R.; Chivukula, S.V.; Chaganti, R.J.; Lowy, D.R.; Schiller, J.T.; Roden, R.B. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillo-mavirus vaccines. J. Natl. Cancer Inst. 2009, 101, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Jagu, S.; Kwak, K.; Garcea, R.L.; Roden, R.B. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine 2010, 28, 4478–4486. [Google Scholar] [CrossRef]
- Pouyanfard, S.; Spagnoli, G.; Bulli, L.; Balz, K.; Yang, F.; Odenwald, C.; Seitz, H.; Mariz, F.C.; Bolchi, A.; Ottonello, S.; et al. Minor Capsid Protein L2 Polytope Induces Broad Protection against Oncogenic and Mucosal Human Papillomaviruses. J. Virol. 2018, 92, e01930-17. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Ribeiro-Muller, L.; Canali, E.; Bolchi, A.; Tommasino, M.; Ottonello, S.; Muller, M. Robust In Vitro and In Vivo Neutralization against Multiple High-Risk HPV Types Induced by a Thermostable Thioredoxin-L2 Vaccine. Cancer Prev. Res. 2015, 8, 932–941. [Google Scholar] [CrossRef][Green Version]
- Spagnoli, G.; Pouyanfard, S.; Cavazzini, D.; Canali, E.; Maggi, S.; Tommasino, M.; Bolchi, A.; Muller, M.; Ottonello, S. Broadly neutralizing antiviral responses induced by a single-molecule HPV vac-cine based on thermostable thioredoxin-L2 multiepitope nanoparticles. Sci. Rep. 2017, 7, 18000. [Google Scholar] [CrossRef]
- Pett, M.; Coleman, N. Integration of high-risk Human Papillomavirus: A key event in cervical carcino-genesis? J. Pathol. 2007, 212, 356–367. [Google Scholar] [CrossRef]
- van Poelgeest, M.I.; Nijhuis, E.R.; Kwappenberg, K.M.; Hamming, I.E.; Wouter Drijfhout, J. Distinct regu-lation and impact of type 1 T-cell immunity against HPV16 L1, E2 and E6 antigens during HPV16-induced cervical infection and neoplasia. Int. J. Cancer 2006, 118, 675–683. [Google Scholar] [CrossRef]
- van Poelgeest, M.I.; van Seters, M.; van Beurden, M.; Kwappenberg, K.M.; Heijmans-Antonissen, C.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G.; Helmerhorst, T.J.M.; Offringa, R.; et al. Detection of Human Papillomavirus (HPV) 16-specific CD4+ T-cell immunity in pa-tients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin. Cancer Res. 2005, 11, 5273–5280. [Google Scholar] [CrossRef]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Van der Burg, S.H.; Palefsky, J.M. Human Immunodeficiency Virus and Human Papilloma Virus—Why HPV-induced lesions do not spontaneously resolve and why therapeutic vaccination can be successful. J. Transl. Med. 2009, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Greenfield, W.W.; Cannon, M.J.; Coleman, H.N.; Spencer, H.J.; Nakagawa, M. CD4+ T-cell re-sponse against Human Papillomavirus type 16 E6 protein is associated with a favorable clinical trend. Cancer Immunol. Immunother. 2012, 61, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Capobianchi, M.R.; Del Porto, P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Beachler, D.C.; Jenkins, G.; Safaeian, M.; Kreimer, A.R.; Wentzensen, N. Natural acquired immunity against subsequent genital Human Papillomavirus infection: A systematic review and meta-analysis. J. Infect. Dis. 2016, 213, 1444–1454. [Google Scholar] [CrossRef]
- Vermaelen, K. Vaccine strategies to improve anti-cancer cellular immune responses. Front. Immunol. 2019, 10, 8. [Google Scholar] [CrossRef]
- Gambhira, R.; Gravitt, P.E.; Bossis, I.; Stern, P.L.; Viscidi, R.P.; Roden, R.B. Vaccination of healthy volun-teers with Human Papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 2006, 66, 11120–11124. [Google Scholar] [CrossRef]
- Welters, M.J.; van der Logt, P.; van den Eeden, S.J.; Kwappenberg, K.M.; Drijfhout, J.W.; Fleuren, G.J.; Kenter, G.G.G.; Melief, C.J.M.; van der Burg, S.H.; Offringa, R. Detection of Human Papillomavirus type 18 E6 and E7-specific CD4+ T-helper 1 immunity in relation to health versus disease. Int. J. Cancer 2006, 118, 950–956. [Google Scholar] [CrossRef]
- Coleman, H.N.; Moscicki, A.B.; Farhat, S.N.; Gupta, S.K.; Wang, X.; Nakagawa, M. CD8 T-cell responses in incident and prevalent Human Papillomavirus types 16 and 18 infections. ISRN Obstet. Gynecol. 2012, 2012, 854237. [Google Scholar] [CrossRef]
- Piersma, S.J.; Jordanova, E.S.; Van Poelgeest, M.I.; Kwappenberg, K.M.; Van Der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.; Kenter, G.G.; Fleuren, G.J.; Offringa, R. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the ab-sence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef]
- Carter, J.J.; Koutsky, L.A.; Wipf, G.C.; Christensen, N.D.; Lee, S.K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. The natural history of Human Papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 1996, 174, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ermel, A.; Tu, W.; Shew, M.; Brown, D.R. Association of HPV types 6, 11, 16, and 18 DNA detection and serological response in unvaccinated adolescent women. J. Med. Virol. 2013, 85, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Beachler, D.C.; Viscidi, R.; Sugar, E.A.; Minkoff, H.; Strickler, H.D.; Cranston, R.D.; Wiley, D.J.; Jacobson, L.P.; Weber, K.M.; Margolick, J.B.; et al. A longitudinal study of Human Papillomavirus 16 L1, e6, and e7 seropositivity and oral Human Papillomavirus 16 infection. Sex. Transm. Dis. 2015, 42, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, M.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Wacholder, S.; Gonzalez, P.; Quint, W.; van Doorn, L.J.; Sherman, M.E.; Xhenseval, V.; et al. Epidemiologi-cal study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and −18 infections. J. Natl. Cancer Inst. 2010, 102, 1653–1662. [Google Scholar] [CrossRef]
- Franceschi, S.; Baussano, I. Naturally acquired immunity against Human Papillomavirus (HPV): Why it matters in the HPV vaccine era. J. Infect. Dis. 2014, 210, 507–509. [Google Scholar] [CrossRef]
- Castellsagué, X.; Naud, P.; Chow, S.N.; Wheeler, C.M.; Germar, M.J.V.; Lehtinen, M.; Paavonen, J.; Jaisamrarn, U.; Garland, S.M.; Salmerón, J.; et al. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired Human Papillomavirus type 16/18 antibodies: Analysis of the control arm of PATRICIA. J. Infect. Dis. 2014, 210, 517–534. [Google Scholar] [CrossRef]
- Karanam, B.; Gambhira, R.; Peng, S.; Jagu, S.; Kim, D.J.; Ketner, G.W.; Stern, P.L.; Adams, R.J.; Roden, R.B. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine 2009, 27, 1040–1049. [Google Scholar] [CrossRef]
- Huber, B.; Wang, J.W.; Roden, R.B.S.; Kirnbauer, R. RG1-VLP and Other L2-Based, Broad-Spectrum HPV Vaccine Candidates. J. Clin. Med. 2021, 10, 1044. [Google Scholar] [CrossRef]
- Hassett, K.J.; Meinerz, N.M.; Semmelmann, F.; Cousins, M.C.; Garcea, R.L.; Randolph, T.W. Development of a highly thermostable, adjuvanted Human Papillomavirus vaccine. Eur. J. Pharm. Biopharm. 2015, 94, 220–228. [Google Scholar] [CrossRef]
- Zacharia, A.; Harberts, E.; Valencia, S.M.; Myers, B.; Sanders, C.; Jain, A.; Larson, N.R.; Middaugh, C.R.; Picking, W.D.; Difilippantonio, S.; et al. Optimization of RG1-VLP vaccine performance in mice with novel TLR4 agonists. Vaccine 2021, 39, 292–302. [Google Scholar] [CrossRef]
- Hu, J.; Balogh, K.; Matsui, K.; Tan, H.; Olczak, P.; Buchman, G.; Howard, B.; White, J.; Kennedy, M.; Sei, S.; et al. Abstract LB-200: A cGMP-grade chimeric papillomavirus candidate vaccine (HPV16 RG1-VLP) confers long term cross-protection compared to a nonavalent hpv vaccine in a pre-clinical papillomavirus animal model. Immunology 2019, 79, LB-200. [Google Scholar] [CrossRef]
- Peng, S.; Song, L.; Knoff, J.; Wang, J.W.; Chang, Y.N.; Hannaman, D.; Wu, T.C.; Alvarez, R.D.; Roden, R.B.; Hung, C.F. Control of HPV-associated tumors by innovative therapeutic HPV DNA vaccine in the ab-sence of CD4+ T cells. Cell Biosci. 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Tomaić, V. Functional Roles of E6 and E7 Oncoproteins in HPV-Induced Malignancies at Diverse Anatomical Sites. Cancers 2016, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Tumban, E.; Peabody, J.; Tyler, M.; Peabody, D.S.; Chackerian, B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against Human Papillomavirus. PLoS ONE 2012, 7, e49751. [Google Scholar]
- Yadav, R.; Zhai, L.; Tumban, E. Virus-like Particle-Based L2 Vaccines against HPVs: Where Are We Today? Viruses 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
| Study Author and Years | Composition | Main Advantages Reported | Develop Phase |
|---|---|---|---|
| De Jong et al., 2002 [27] | TA-CIN vaccine (recombinant HPV-16 L2 E6 E7) | Immunological profile: revealed a significant anti HPV-16 E6 and E7 T cell response in 8/11 patients treated with TA-CIN vaccine. Immunological response with IgG production was reported. No reported serious or severe adverse events. | Phase I |
| Davidson et al., 2003 [15] | Therapeutic vaccine based on modified HPV-16 and 18 E6–E7 proteins | Evaluation of vaccines in vulvar intraepithelial neoplasia. Ten patients demonstrated an increase in HPV-16 specific T-CELL response. In one patient, there was a complete regression of vulvar lesions and viral clearance by PCR evaluation. In eight patients, a reduction in vulvar lesions of at least 50% of diameter was reported, and in 6 of these women, a reduction in viral load. Four patients showed a decrease in symptoms. All patients reported an increase in both serological and cell-mediated immunity. | Phase II |
| Smith et al., 2004 [28] | TA-CIN vaccine (recombinant HPV-16 L2E6E7) | Evaluation of vaccine application in high-grade ano-genital intraepithelial neoplasm. Regarding the proliferative responses against the oncoproteins E6 and E7, there was only a significant increase in the response for E6. HPV-16 E6-specific T cell responses in 9 patients, induced by vaccination. In two women, HPV-16 E7-specific T cells were enhanced after all vaccinations. Three patients reported enhanced HPV-18 E6-specific T-cell activity after vaccination. No significant changes were reported in serological responses to HPV-18 E6 or E7 after vaccination. Among 19 patients with stable disease, 8 reported an immunogenic response, and in the 4 patients with disease progression, two did not show a T-cell response and two revealed an absence of both a T-cell and a humoral response | Phase II |
| Davidson et al., 2004 [29] | TA-CIN vaccine (recombinant HPV-16 L2 E6 E7) and TA-HPV (vaccine based on virus encoding HPV16/18 and E7) | Ten women affected by HPV-16 related VIN were enrolled. Three patients reported a clinical response, while 6 patients maintained stable disease. Two patients reported a partial or complete clinical response, which was confirmed by the reduction in lesion diameter of ≥50%. Six patients maintained stable disease, one patient reported significant symptomatic relief following vaccination, while one patient reported disease progression (increase in lesion diameter of ≥25% post-vaccination). IgG levels did not demonstrate a significant change. Regarding HPV-specific proliferative responses, all patients demonstrated a proliferative response. | Phase II |
| Fainder et al., 2006 [30] | TA-CIN vaccine (recombinant HPV-16 L2 E6 E7) and TA-HPV (vaccine based on virus encoding HPV16/18 and E7) | Patients with ano-genital intraepithelial neoplasia were vaccinated with 3 doses of TA-CIN followed by one dose of a recombinant vaccine with virus encoding HPV-16 and 18 E6/E7 (TA-HPV). Two with VAIN 3, 27 with VIN3 and 2 patients with VIN3 also had anal intraepithelial neoplasia. A complete response was seen in 1 patient, partial response in 5, stable disease in 18, and progression in 5 of them. None of the patients developed invasive lesions. | Phase II |
| Dayana et al. 2010 [31] | TA-CIN vaccine (recombinant HPV-16 L2 E6 E7) and Imiquimod | Women with VIN2 and VIN3 were enrolled. A complete regression of VIN in 12/19 at week 52 of treatment (63%). In responder patients, there was a significant increase in a CD4+ and a CD8+ response. The authors concluded that the modulation of local and systemic immune response plays a role in the therapeutic vaccine effect. | Phase II |
| Safety and Feasibility of TA-CIN Vaccine in HPV16 Associated Cervical Cancer [31] | TA-CIN vaccine (recombinant HPV-16 L2 E6 E7) | (ongoing study) The first aim of this trial is to provide evidence for the safety and feasibility of the vaccine and the different immune responses when the dose is administered at different locations such as the thigh or the arm in order to determine the site that can elicit the most potent immunological effect. Secondary aims were the evaluation of the level of circulating antibodies to HPV-16 E6, E7, and L2, the level of circulating T-Cells and the mononucleocyte response in the peripheral blood before and after vaccination | Phase I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardella, B.; Gritti, A.; Soleymaninejadian, E.; Pasquali, M.F.; Riemma, G.; La Verde, M.; Schettino, M.T.; Fortunato, N.; Torella, M.; Dominoni, M. New Perspectives in Therapeutic Vaccines for HPV: A Critical Review. Medicina 2022, 58, 860. https://doi.org/10.3390/medicina58070860
Gardella B, Gritti A, Soleymaninejadian E, Pasquali MF, Riemma G, La Verde M, Schettino MT, Fortunato N, Torella M, Dominoni M. New Perspectives in Therapeutic Vaccines for HPV: A Critical Review. Medicina. 2022; 58(7):860. https://doi.org/10.3390/medicina58070860
Chicago/Turabian StyleGardella, Barbara, Andrea Gritti, Ehsan Soleymaninejadian, Marianna Francesca Pasquali, Gaetano Riemma, Marco La Verde, Maria Teresa Schettino, Nicola Fortunato, Marco Torella, and Mattia Dominoni. 2022. "New Perspectives in Therapeutic Vaccines for HPV: A Critical Review" Medicina 58, no. 7: 860. https://doi.org/10.3390/medicina58070860
APA StyleGardella, B., Gritti, A., Soleymaninejadian, E., Pasquali, M. F., Riemma, G., La Verde, M., Schettino, M. T., Fortunato, N., Torella, M., & Dominoni, M. (2022). New Perspectives in Therapeutic Vaccines for HPV: A Critical Review. Medicina, 58(7), 860. https://doi.org/10.3390/medicina58070860






