High Prevalence of Atrial Fibrillation in a Lithuanian Stroke Patient Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Baseline Characteristics
2.3. Follow-Up and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Antithrombotic Treatment
3.3. Screening for AF
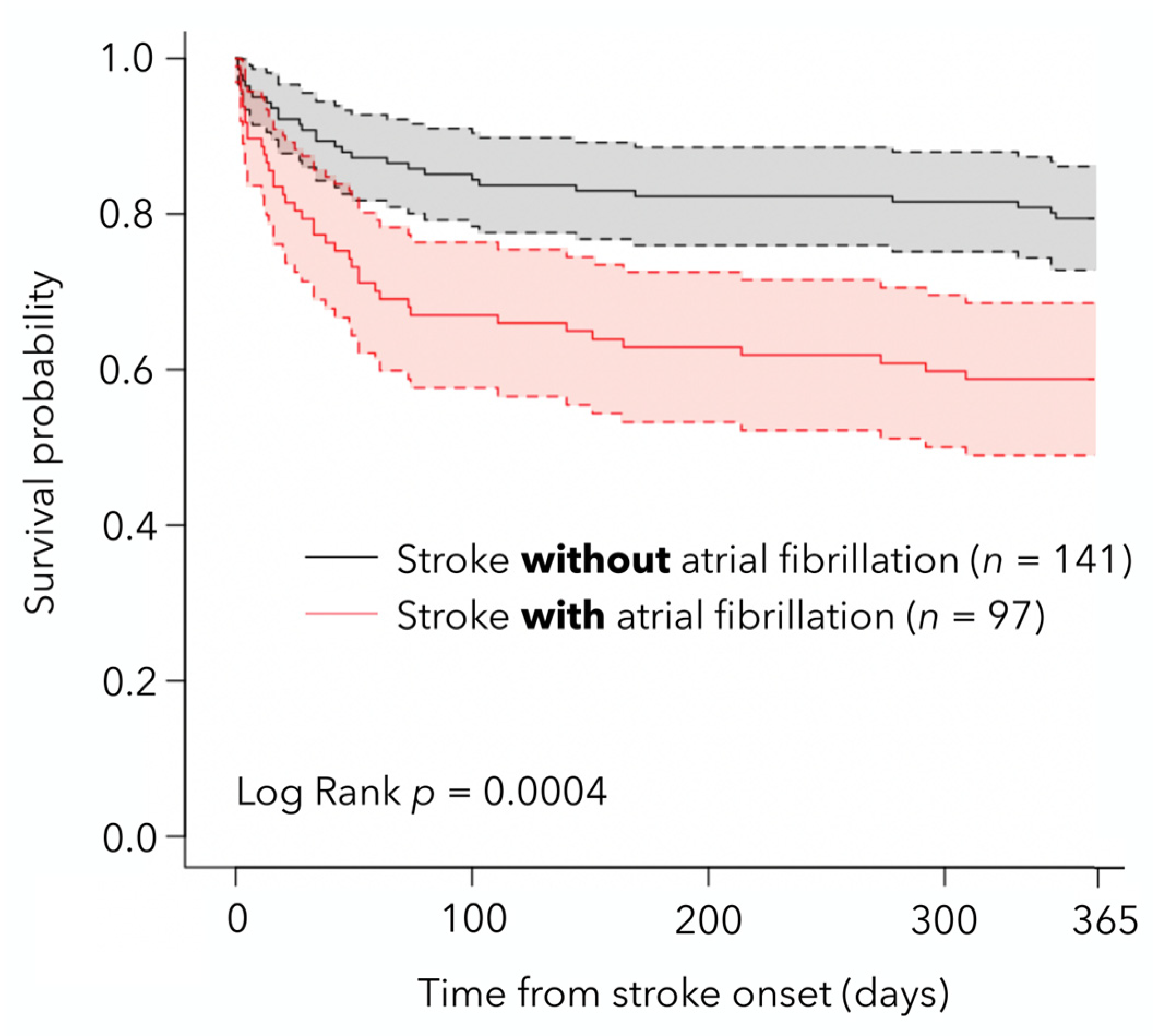
3.4. Patient Survival, Functional Outcome, and HRQoL
3.5. Stroke Patients with AFDAS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AFDAS | Atrial fibrillation detected after stroke |
| AIS | Acute ischemic stroke |
| CI | Confidence intervals |
| CTA | Computed tomography angiography |
| EQ-5D-3L | EuroQoL five-dimensional three-level descriptive system |
| EQ-VAS | EQ-5D-3L with a self-rated visual analog scale |
| HR | Hazard ratio |
| HRQoL | Health-related quality of life |
| INR | International Normalized Ratio |
| IQR | Interquartile range |
| LVO | Large vessel occlusion |
| mRS | modified Rankin Scale |
| NIHSS | National Institutes of Health Stroke Scale |
| NOACs | non-vitamin K antagonist oral anticoagulants |
| OAC | Oral anticoagulation |
| OR | Odds ratio |
| SD | Standard deviation |
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide Epidemiology of Atrial Fibrillation. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundel, B.J.J.M.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial Fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.T.H.; Campbell, B.C.V.; Christensen, S.; Desmond, P.M.; Silva, D.A.D.; Parsons, M.W.; Churilov, L.; Lansberg, M.G.; Mlynash, M.; Olivot, J.; et al. Worse Stroke Outcome in Atrial Fibrillation Is Explained by More Severe Hypoperfusion, Infarct Growth, and Hemorrhagic Transformation. Int. J. Stroke 2012, 10, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial Fibrillation as an Independent Risk Factor for Stroke: The Framingham Study. Stroke 2018, 22, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef]
- Kolominsky-Rabas, P.L.; Weber, M.; Gefeller, O.; Neundoerfer, B.; Heuschmann, P.U. Epidemiology of Ischemic Stroke Subtypes According to TOAST Criteria. Stroke 2001, 32, 2735–2740. [Google Scholar] [CrossRef] [Green Version]
- Seiffge, D.J.; Marchis, G.M.D.; Koga, M.; Paciaroni, M.; Wilson, D.; Cappellari, M.; Macha, K.; Tsivgoulis, G.; Ambler, G.; Arihiro, S.; et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann. Neurol. 2020, 87, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Schnabel, R.B.; Haeusler, K.G.; Healey, J.S.; Freedman, B.; Boriani, G.; Brachmann, J.; Brandes, A.; Bustamante, A.; Casadei, B.; Crijns, H.J.G.M.; et al. Searching for Atrial Fibrillation Poststroke. Circulation 2019, 140, 1834–1850. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Grory, B.M.; Köhrmann, M.; Ricci, B.A.; Tsioufis, K.; Cutting, S.; Krogias, C.; Schellinger, P.D.; Campello, A.R.; et al. Prolonged Cardiac Rhythm Monitoring and Secondary Stroke Prevention in Patients with Cryptogenic Cerebral Ischemia. Stroke 2019, 50, 2175–2180. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Freedman, B.; Caterina, R.D.; Potpara, T.S. Stroke Prevention in Atrial Fibrillation: Past, Present and Future. Thromb. Haemostasis. 2017, 117, 1230–1239. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Anjum, I.; Kashif, T.; Ahmed, M.M.; Sohail, W.; Sarwar, M.; Khokhar, I. Dual or Mono Antiplatelet Therapy for the Prevention of Ischemic Stroke: A Literature Review. Cureus 2018, 10, e2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, B.; Potpara, T.S.; Lip, G.Y.H. Stroke Prevention in Atrial Fibrillation. Lancet 2016, 388, 806–817. [Google Scholar] [CrossRef]
- Bo, M.; Grisoglio, E.; Brunetti, E.; Falcone, Y.; Marchionni, N. Oral Anticoagulant Therapy for Older Patients with Atrial Fibrillation: A Review of Current Evidence. Eur. J. Intern. Med. 2017, 41, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.N.; Brown, J.D. Real-World Adherence for Direct Oral Anticoagulants in a Newly Diagnosed Atrial Fibrillation Cohort: Does the Dosing Interval Matter? BMC Cardiovasc. Disor. 2019, 19, 64. [Google Scholar] [CrossRef] [Green Version]
- Salmasi, S.; Loewen, P.S.; Tandun, R.; Andrade, J.G.; Vera, M.A.D. Adherence to Oral Anticoagulants among Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis of Observational Studies. BMJ Open 2020, 10, e034778. [Google Scholar] [CrossRef] [Green Version]
- Wachter, R.; Weber-Krüger, M.; Hamann, G.F.; Kermer, P.; Liman, J.; Mende, M.; Seegers, J.; Wasser, K.; Gröschel, S.; Uphaus, T.; et al. Long-Term Follow-up of Enhanced Holter-Electrocardiography Monitoring in Acute Ischemic Stroke. J. Stroke 2022, 24, 98–107. [Google Scholar] [CrossRef]
- Collaborators, G.S.; Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Masiliūnas, R.; Vilionskis, A.; Bornstein, N.M.; Rastenytė, D.; Jatužis, D. The Impact of a Comprehensive National Policy on Improving Acute Stroke Patient Care in Lithuania. Eur. Stroke J. 2022, 7, 134–142. [Google Scholar] [CrossRef]
- Laucevičius, A.; Rinkūnienė, E.; Petrulionienė, Ž.; Ryliškytė, L.; Jucevičienė, A.; Puronaitė, R.; Badarienė, J.; Navickas, R.; Mikolaitytė, J.; Gargalskaitė, U.; et al. Trends in Cardiovascular Risk Factor Prevalence among Lithuanian Middle-Aged Adults between 2009 and 2018. Atherosclerosis 2020, 299, 9–14. [Google Scholar] [CrossRef]
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Karelis, G.; Micule, M.; Klavina, E.; Haritoncenko, I.; Kikule, I.; Tilgale, B.; Polaka, I. The Riga East University Hospital Stroke Registry—An Analysis of 4915 Consecutive Patients with Acute Stroke. Medicina 2021, 57, 632. [Google Scholar] [CrossRef] [PubMed]
- Vibo, R.; Schneider, S.; Kõrv, L.; Mallene, S.; Torop, L.-A.; Kõrv, J. Estonian Young Stroke Registry: High Burden of Risk Factors and High Prevalence of Cardiomebolic and Large-Artery Stroke. Eur. Stroke J. 2021, 6, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Jurjans, K.; Vikmane, B.; Vetra, J.; Miglane, E.; Kalejs, O.; Priede, Z.; Millers, A. Is Anticoagulation Necessary for Severely Disabled Cardioembolic Stroke Survivors? Medicina 2019, 55, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sposato, L.A.; Chaturvedi, S.; Hsieh, C.-Y.; Morillo, C.A.; Kamel, H. Atrial Fibrillation Detected after Stroke and Transient Ischemic Attack: A Novel Clinical Concept Challenging Current Views. Stroke 2022, 53, e94–e103. [Google Scholar] [CrossRef]
- Rabin, R.; Charro, F. de EQ-SD: A Measure of Health Status from the EuroQol Group. Ann. Med. 2009, 33, 337–343. [Google Scholar] [CrossRef]
- EuroQol Group. EQ-5D-3L. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-3l-about (accessed on 8 May 2022).
- Greiner, W.; Weijnen, T.; Nieuwenhuizen, M.; Oppe, S.; Badia, X.; Busschbach, J.; Buxton, M.; Dolan, P.; Kind, P.; Krabbe, P.; et al. A Single European Currency for EQ-5D Health States. Eur. J. Health Econ. Former. Hepac. 2003, 4, 222–231. [Google Scholar] [CrossRef]
- Ministry of Health of The Republic of Lithuania. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/82898401b32d11e79583aefa27fdb44b (accessed on 8 May 2022).
- Beumer, D.; Mulder, M.J.H.L.; Saiedie, G.; Fonville, S.; van Oostenbrugge, R.J.; van Zwam, W.H.; Homburg, P.J.; van der Lugt, A.; Dippel, D.W.J. Occurrence of Intracranial Large Vessel Occlusion in Consecutive, Non-Referred Patients with Acute Ischemic Stroke. Neurovasc. Imaging 2016, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Vemmos, K.N.; Bots, M.L.; Tsibouris, P.K.; Zis, V.P.; Takis, C.E.; Grobbee, D.E.; Stamatelopoulos, S. Prognosis of Stroke in the South of Greece: 1 Year Mortality, Functional Outcome and Its Determinants: The Arcadia Stroke Registry. J. Neurol. Neurosurg. Psychiatry 2000, 69, 595. [Google Scholar] [CrossRef]
- Stevens, E.; Emmett, E.; Wang, Y.; McKevitt, C.; Wolfe, C.D. The Burden of Stroke in Europe; Stroke Alliance for Europe: London, UK, 2017. [Google Scholar]
- Táborský, M.; Dušek, L.; Kautzner, J.; Vícha, M.; Aiglová, R.; Gloger, V.; Fedorco, M.; Duba, J.; Dušek, L.; Jarkovský, J.; et al. SETAP: Epidemiology and Prevention of Stroke and Transient Ischaemic Attack in Czech Patients with Atrial Fibrillation. EP Eur. 2020, 23, 539–547. [Google Scholar] [CrossRef]
- Inoue, M.; Noda, R.; Yamaguchi, S.; Tamai, Y.; Miyahara, M.; Yanagisawa, S.; Okamoto, K.; Hara, T.; Takeuchi, S.; Miki, K.; et al. Specific Factors to Predict Large-Vessel Occlusion in Acute Stroke Patients. J. Stroke Cerebrovasc. Dis. 2018, 27, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Gornbein, J.; Saver, J.L. Ischemic Strokes Due to Large-Vessel Occlusions Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Front. Neurol. 2017, 8, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, R.G.; Benavente, O.; McBride, R.; Pearce, L.A. Antithrombotic Therapy To Prevent Stroke in Patients with Atrial Fibrillation: A Meta-Analysis. Ann. Intern. Med. 1999, 131, 492. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 42, ehaa612. [Google Scholar] [CrossRef]
- Rubiera, M.; Aires, A.; Antonenko, K.; Lémeret, S.; Nolte, C.H.; Putaala, J.; Schnabel, R.B.; Tuladhar, A.M.; Werring, D.J.; Zeraatkar, D.; et al. European Stroke Organisation (ESO) Guideline on Screening for Subclinical Atrial Fibrillation after Stroke or Transient Ischaemic Attack of Undetermined Origin. Eur. Stroke J. 2022. [Google Scholar] [CrossRef]
- Jatužis, D.; Rastenytė, D.; Vilionskis, A.; Matijošaitis, V.; Ryliškienė, K. Galvos Smegenų Insulto Diagnostikos, Gydymo Ir Profilaktikos Metodika; Vilniaus universiteto leidykla: Vilnius, Lithuania, 2021; ISBN 978-609-07-0584-1. [Google Scholar]
- Svennberg, E.; Tjong, F.; Goette, A.; Akoum, N.; Biaise, L.D.; Bordachar, P.; Boriani, G.; Burri, H.; Conte, G.; Deharo, J.-C.; et al. How to Use Digital Devices to Detect and Manage Arrhythmias: An EHRA Practical Guide. EP Eur. 2022. [Google Scholar] [CrossRef]
- Guo, Y.; Lane, D.A.; Wang, L.; Zhang, H.; Wang, H.; Zhang, W.; Wen, J.; Xing, Y.; Wu, F.; Xia, Y.; et al. Mobile Health Technology to Improve Care for Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 1523–1534. [Google Scholar] [CrossRef]
- McGrath, E.R.; Kapral, M.K.; Fang, J.; Eikelboom, J.W.; O’Conghaile, A.; Canavan, M.; O’Donnell, M.J. Association of Atrial Fibrillation with Mortality and Disability after Ischemic Stroke. Neurology 2013, 81, 825–832. [Google Scholar] [CrossRef]
- Tsalta-Mladenov, M.; Andonova, S. Health-Related Quality of Life after Ischemic Stroke: Impact of Sociodemographic and Clinical Factors. Neurol. Res. 2021, 43, 553–561. [Google Scholar] [CrossRef]
- Sadlonova, M.; Wasser, K.; Nagel, J.; Weber-Krüger, M.; Gröschel, S.; Uphaus, T.; Liman, J.; Hamann, G.F.; Kermer, P.; Gröschel, K.; et al. Health-Related Quality of Life, Anxiety and Depression up to 12 Months Post-Stroke: Influence of Sex, Age, Stroke Severity and Atrial Fibrillation—A Longitudinal Subanalysis of the Find-AFRANDOMISED Trial. J. Psychosom. Res. 2021, 142, 110353. [Google Scholar] [CrossRef] [PubMed]
- Golicki, D.; Niewada, M.; Buczek, J.; Karlińska, A.; Kobayashi, A.; Janssen, M.F.; Pickard, A.S. Validity of EQ-5D-5L in Stroke. Qual. Life Res. 2014, 24, 845–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
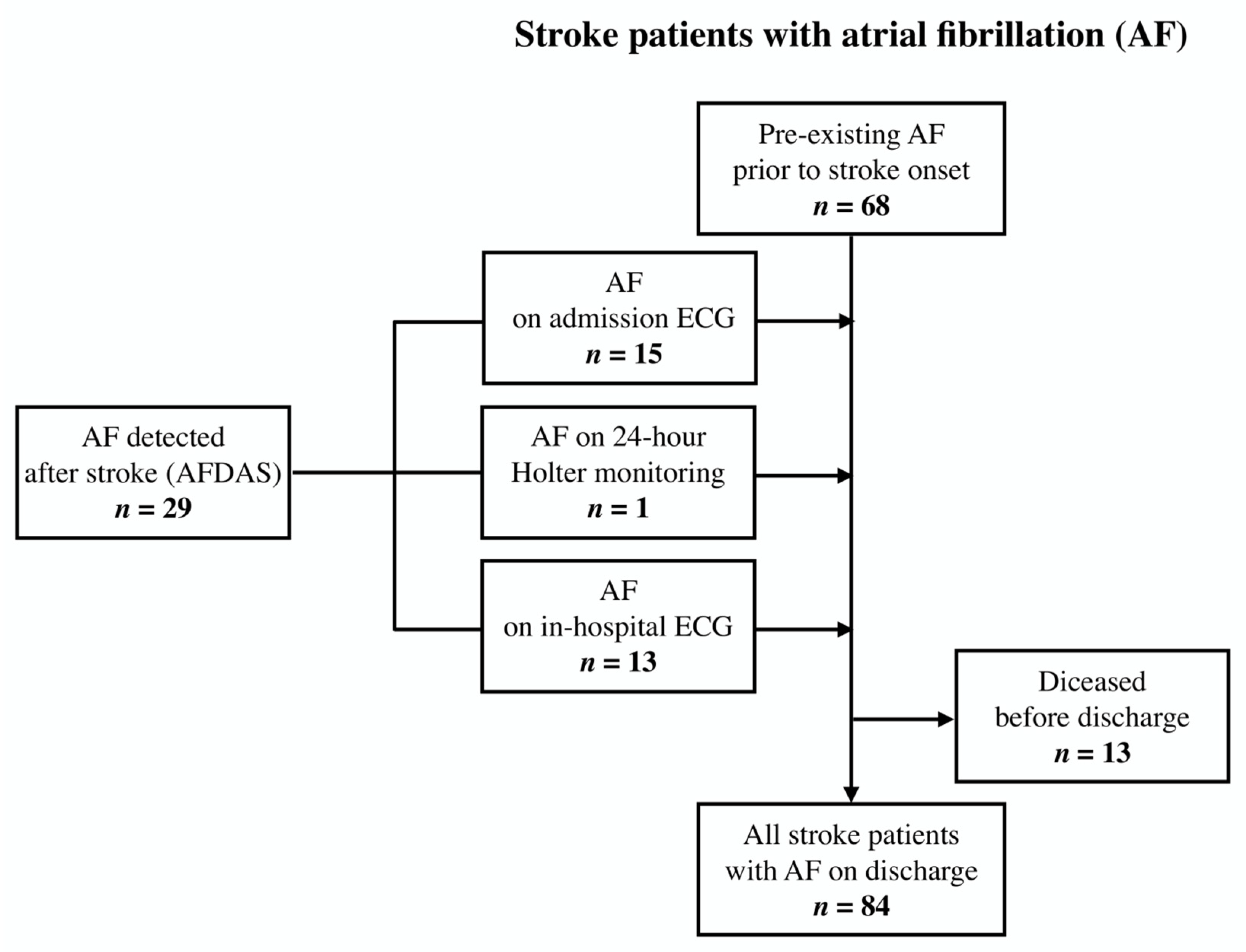
| All Stroke Patients (n = 238) | Stroke Patients with AF (n = 97) | Stroke Patients without AF (n = 141) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Female, n (%) | 107 | (45.0) | 57 | (58.8) | 50 | (35.5) | <0.001 |
| Mean age, years (SD) | 71.4 | (11.9) | 75.7 | (11.0) | 68.4 | (11.5) | <0.001 |
| Baseline median mRS ≤ 2, n (%) | 213 | (89.5) | 88 | (90.7) | 125 | (88.7) | 0.609 |
| Baseline NIHSS, median (IQR) | 6 | (4–12) | 9 | (6–16) | 6 | (3–9) | <0.001 |
| Risk factors, n (%) | |||||||
| Hypertension | 217 | (91.2) | 93 | (95.9) | 124 | (87.9) | 0.034 |
| Diabetes mellitus | 50 | (21.0) | 26 | (26.8) | 24 | (17.0) | 0.069 |
| Dyslipidemia | 185 | (77.7) | 65 | (67.0) | 120 | (84.1) | <0.001 |
| History of stroke/TIA | 51 | (21.4) | 25 | (25.8) | 26 | (18.4) | 0.175 |
| Congestive heart failure | 91 | (38.2) | 58 | (59.8) | 33 | (23.4) | <0.001 |
| Coronary artery disease | 82 | (34.5) | 51 | (52.6) | 31 | (22.0) | <0.001 |
| Peripheral artery disease | 12 | (5.0) | 5 | (5.2) | 7 | (5.0) | 0.947 |
| Underlying malignancy | 15 | (6.3) | 4 | (4.1) | 11 | (7.8) | 0.251 |
| CTA performed, n (%) | 160 | (67.2) | 67 | (69.1) | 93 | (66.0) | 0.615 |
| Reperfusion, n (%) | 91 | (38.2) | 38 | (39.2) | 53 | (37.6) | |
| Not eligible | 147 | (61.8) | 59 | (60.8) | 88 | (62.4) | 0.805 |
| IVT | 41 | (17.2) | 13 | (13.4) | 28 | (19.9) | 0.195 |
| EVT | 41 | (17.2) | 20 | (20.6) | 21 | (14.9) | 0.250 |
| Combined treatment | 9 | (3.8) | 5 | (5.2) | 4 | (2.8) | 0.357 |
| Large vessel occlusion, n (%) † | 82 | (51.3) | 44 | (65.7) | 38 | (40.9) | 0.002 |
| Anterior | 61 | (38.1) | 37 | (55.2) | 24 | (25.8) | <0.001 |
| Posterior | 17 | (10.6) | 5 | (7.5) | 12 | (12.9) | 0.271 |
| Both | 4 | (2.5) | 2 | (3.2) | 2 | (2.0) | 0.739 |
| None | 78 | (48.8) | 23 | (34.3) | 55 | (59.1) | 0.002 |
| 24-h Holter monitoring, n (%) ‡ | |||||||
| Performed | 5 | (3.5) | |||||
| Not performed | 136 | (96.5) | |||||
| Pre-Existing AF before Stroke Onset (n = 68) | All AF on Discharge ¶ (n = 84) | |||
|---|---|---|---|---|
| Antithrombotic treatment status, n (%) | ||||
| No treatment | 23 | (33.8) | 0 | (0) |
| Antiplatelets | 8 | (11.8) | 10 | (11.9) |
| LMWH † | 0 | (0) | 10 | (11.9) |
| Warfarin † | 26 | (36.8) | 23 | (27.4) |
| NOACs † | 11 | (16.2) | 41 | (48.8) |
| INR, median (IQR) ‡ | 1.28 | (1.20–1.79) | ||
| INR within therapeutic range, n (%) ‡ | 5 | (19.2) | ||
| Insufficient anticoagulation, n (%) § | 55 | (80.9) | ||
| All Stroke Patients (n = 238) | Stroke Patients with AF (n = 97) | Stroke Patients without AF (n = 141) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Case fatality, n (%) | |||||||
| In-hospital case fatality | 19 | (8.0) | 13 | (13.4) | 6 | 4.3) | 0.011 |
| 90-day case fatality | 52 | (21.8) | 31 | (32.0) | 21 | (14.9) | 0.002 |
| 1-year case fatality | 69 | (29.0) | 40 | (41.2) | 29 | (20.6) | <0.001 |
| Median mRS ≤ 2 at 90 days, n (%) † | 78 | (58.6) | 27 | (56.3) | 51 | (60.0) | 0.673 |
| Missing mRS, n (%) | 53 | (28.5) | 18 | (27.3) | 35 | (29.2) | 0.784 |
| EQ-5D domain, n (%) | |||||||
| Decreased mobility | 48 | (36.6) | 17 | (36.2) | 31 | (36.9) | 0.933 |
| Difficulty with self-care | 54 | (41.2) | 26 | (48.9) | 31 | (36.9) | 0.041 |
| Problems performing usual activities | 88 | (67.7) | 31 | (67.4) | 55 | (65.5) | 0.825 |
| Pain or discomfort | 56 | (43.8) | 21 | (45.7) | 35 | (42.7) | 0.745 |
| Anxious or depressed | 51 | (40.5) | 18 | (39.1) | 33 | (40.7) | 0.935 |
| EQ-5D score index, mean (SD) | 0.61 | (0.32) | 0.62 | (0.32) | 0.58 | (0.33) | 0.549 |
| Missing EQ-5D, n (%) | 60 | (32.3) | 21 | (31.8) | 39 | (32.5) | 0.924 |
| EQ-VAS, median (IQR) | 50 | (40–70) | 50 | (30–70) | 60 | (40–76.25) | 0.022 |
| Missing EQ-VAS, n (%) | 75 | (40.3) | 27 | (40.9) | 48 | (40.0) | 0.904 |
| Covariates | Univariate | Multivariable † | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||||
| Age | 1.05 | (1.03–1.07) | <0.001 | 1.03 | (1.01–1.06) | 0.007 | |
| Gender | Female | 1.00 | (reference) | ||||
| Male | 0.69 | (0.43–1.11) | 0.125 | ||||
| Atrial fibrillation | No | 1.00 | (reference) | 1.00 | (reference) | ||
| Yes | 2.33 | (1.44–3.75) | <0.001 | 1.27 | (0.74–2.19) | 0.385 | |
| Reperfusion treatment | No | 1.00 | (reference) | ||||
| Yes | 1.18 | (0.73–1.90) | 0.507 | ||||
| Baseline mRS ≤ 2 | No | 1.00 | (reference) | 1.00 | (reference) | ||
| Yes | 0.33 | (0.19–0.59) | <0.001 | 0.56 | (0.30–1.05) | 0.072 | |
| Baseline NIHSS | 1.13 | (1.10–1.17) | <0.001 | 1.11 | (1.07–1.14) | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masiliūnas, R.; Dapkutė, A.; Grigaitė, J.; Lapė, J.; Valančius, D.; Bacevičius, J.; Katkus, R.; Vilionskis, A.; Klimašauskienė, A.; Ekkert, A.; et al. High Prevalence of Atrial Fibrillation in a Lithuanian Stroke Patient Cohort. Medicina 2022, 58, 800. https://doi.org/10.3390/medicina58060800
Masiliūnas R, Dapkutė A, Grigaitė J, Lapė J, Valančius D, Bacevičius J, Katkus R, Vilionskis A, Klimašauskienė A, Ekkert A, et al. High Prevalence of Atrial Fibrillation in a Lithuanian Stroke Patient Cohort. Medicina. 2022; 58(6):800. https://doi.org/10.3390/medicina58060800
Chicago/Turabian StyleMasiliūnas, Rytis, Austėja Dapkutė, Julija Grigaitė, Jokūbas Lapė, Domantas Valančius, Justinas Bacevičius, Rimgaudas Katkus, Aleksandras Vilionskis, Aušra Klimašauskienė, Aleksandra Ekkert, and et al. 2022. "High Prevalence of Atrial Fibrillation in a Lithuanian Stroke Patient Cohort" Medicina 58, no. 6: 800. https://doi.org/10.3390/medicina58060800
APA StyleMasiliūnas, R., Dapkutė, A., Grigaitė, J., Lapė, J., Valančius, D., Bacevičius, J., Katkus, R., Vilionskis, A., Klimašauskienė, A., Ekkert, A., & Jatužis, D. (2022). High Prevalence of Atrial Fibrillation in a Lithuanian Stroke Patient Cohort. Medicina, 58(6), 800. https://doi.org/10.3390/medicina58060800









