Clinical Manifestations and Laboratory Findings of Kawasaki Disease: Beyond the Classic Diagnostic Features
Abstract
1. Introduction
2. The Global Epidemiology of KD
3. Diagnostic Clinical Features of KD
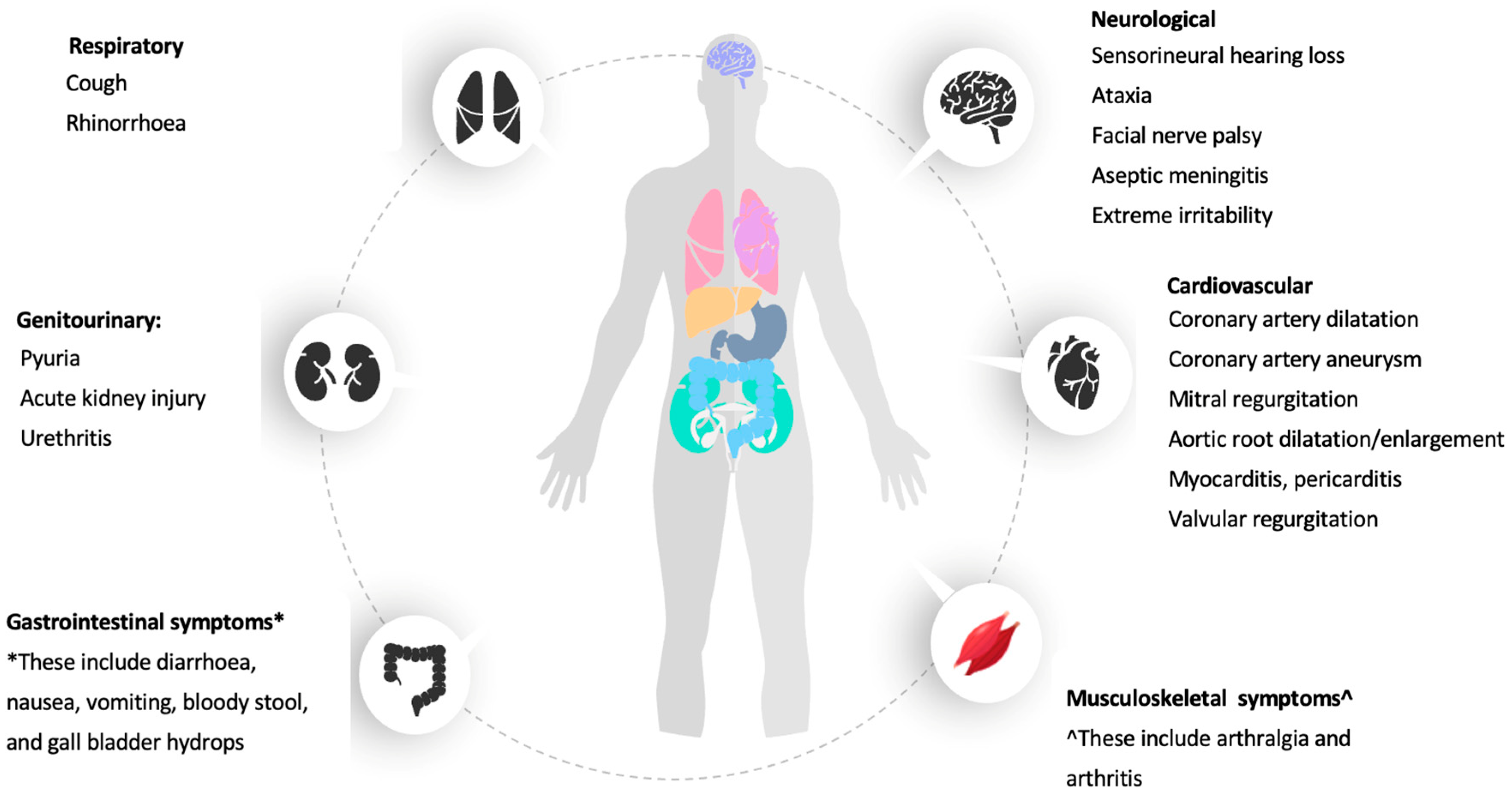
4. Other Clinical Manifestations of KD
4.1. Cardiac Manifestation of KD
4.2. Gastrointestinal System
4.3. Genitourinary System
4.4. Musculoskeletal System
4.5. Respiratory System
4.6. Neurological System
5. Laboratory Characteristics of KD
5.1. Full Blood Count and Inflammatory Markers (CRP, ESR, PCT, Serum Ferritin, D-Dimer/Fibrinogen Level)
5.2. Liver Function Test and Lipid Profile
5.3. Renal Function and Urinary Sample Assessment
5.4. Autoimmune and Cardiovascular Biomarkers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967, 16, 178–222. [Google Scholar] [PubMed]
- Onouchi, Y. The genetics of Kawasaki disease. Int. J. Rheum. Dis. 2017, 21, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-Y.; Lu, C.-Y.; Shao, P.-L.; Lee, P.-I.; Lin, M.-T.; Fan, T.-Y.; Cheng, A.-L.; Lee, W.-L.; Hu, J.-J.; Yeh, S.-J.; et al. Viral infections associated with Kawasaki disease. J. Formos. Med. Assoc. 2014, 113, 148–154. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsukahara, H.; Doi, H. Early childhood exposure to maternal smoking and Kawasaki Disease: A longitudinal survey in Japan. Sci. Total Environ. 2018, 655, 141–146. [Google Scholar] [CrossRef]
- Uehara, R.; Belay, E.D. Epidemiology of Kawasaki Disease in Asia, Europe, and the United States. J. Epidemiol. 2012, 22, 79–85. [Google Scholar] [CrossRef]
- Makino, N.; Nakamura, Y.; Yashiro, M.; Ae, R.; Tsuboi, S.; Aoyama, Y.; Kojo, T.; Uehara, R.; Kotani, K.; Yanagawa, H. Descriptive Epidemiology of Kawasaki Disease in Japan, 2011–2012: From the Results of the 22nd Nationwide Survey. J. Epidemiol. 2015, 25, 239–245. [Google Scholar] [CrossRef]
- Sotimehin, S.A.; Ogunlesi, T.A.; Adekanmbi, A.F.; Fetuga, M.B.; Odumuyiwa, E.A.; Olowu, O.A. Kawasaki Disease in A Nigerian Child- a case report. Niger. Med. Pract. 2010, 57, 4. [Google Scholar] [CrossRef]
- Agha, H.M.; Hamza, H.S. Incomplete Kawasaki disease in Egypt. Glob. Cardiol. Sci. Pract. 2017, 3, e201724. [Google Scholar] [CrossRef][Green Version]
- Zhu, F.H.; Ang, J.Y. The Clinical Diagnosis and Management of Kawasaki Disease: A Review and Update. Curr. Infect. Dis. Rep. 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Mori, M.; Miyamae, T.; Imagawa, T.; Katakura, S.; Kimura, K.; Yokota, S. Meta-analysis of the results of intravenous gamma globulin treatment of coronary artery lesions in Kawasaki disease. Mod. Rheumatol. 2004, 14, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Oates-Whitehead, R.M.; Baumer, J.H.; Haines, L.; Love, S.; Maconochie, I.K.; Gupta, A.; Roman, K.; Dua, J.S.; Flynn, I. Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 2003, 4. [Google Scholar] [CrossRef]
- Baumer, J.H.; Love, S.J.; Gupta, A.; Haines, L.C.; Maconochie, I.; Dua, J.S. Salicylate for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 2006, 4. [Google Scholar] [CrossRef] [PubMed]
- Ae, R.; Makino, N.; Kosami, K.; Kuwabara, M.; Matsubara, Y.; Nakamura, Y. Epidemiology, Treatments, and Cardiac Complications in Patients with Kawasaki Disease: The Nationwide Survey in Japan, 2017–2018. J. Pediatr. 2020, 225, 23–29.e2. [Google Scholar] [CrossRef] [PubMed]
- Makino, N.; Nakamura, Y.; Yashiro, M.; Sano, T.; Ae, R.; Kosami, K.; Kojo, T.; Aoyama, Y.; Kotani, K.; Yanagawa, H. Epidemiological observations of Kawasaki disease in Japan, 2013–2014. Pediatr. Int. 2018, 60, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.S. Kawasaki disease in Malaysia. Ann. Trop. Paediatr. 1996, 16, 213–220. [Google Scholar] [CrossRef]
- Bah, M.N.M.; Alias, E.Y.; Razak, H.; Sapian, M.H.; Foo, F.H.; Abdullah, N. Epidemiology, clinical characteristics, and immediate outcome of Kawasaki disease: A population-based study from a tropical country. Eur. J. Pediatr. 2021, 180, 2599–2606. [Google Scholar] [CrossRef]
- Lee, B.W.; Tay, J.S.H.; Yip, W.C.L.; Yap, H.K.; Chan, K.Y.; Low, P.S. Kawasaki syndrome in Chinese children. Ann. Trop. Paediatr. 1989, 9, 147–151. [Google Scholar] [CrossRef]
- Yung, C.F.; Nadua, K.D.; Oh, B.K.; Thoon, K.C. Epidemiologic trends in Kawasaki disease during coronavirus disease-19 in Singapore. J. Pediatr. 2020, 226, 314–315. [Google Scholar] [CrossRef]
- Durongpisitkul, K.; Sangtawesin, C.; Khongphatthanayopthin, A.; Panamonta, M.; Sopontammarak, S.; Sittiwangkul, R.; Pongpanich, B. Epidemiologic study of Kawasaki disease and cases resistant to IVIG therapy in Thailand. Asian Pac. J. Allergy Immunol. 2006, 24, 27. [Google Scholar]
- Chen, J.-J.; Ma, X.-J.; Liu, F.; Yan, W.-L.; Huang, M.-R.; Huang, M.; Huang, G.-Y. Epidemiologic Features of Kawasaki Disease in Shanghai from 2008 through 2012. Pediatr. Infect. Dis. J. 2016, 35, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Li, X.J.; Li, H.; Xu, M.; Zhou, M. Epidemiological Survey of Kawasaki Disease in Sichuan Province of China. J. Trop. Pediatr. 2007, 54, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-D.; Zhao, D.; Du, J.; Zhang, Y.-L.; Lin, Y.; Liu, C.; Zhang, T. Epidemiologic study on kawasaki disease in beijing from 2000 through 2004. Pediatr. Infect. Dis. J. 2007, 26, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Wu, M.-H. The global epidemiology of Kawasaki disease: Review and future perspectives. Glob. Cardiol. Sci. Pract. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Rizk, S.R.; El Said, G.; Daniels, L.B.; Burns, J.C.; El Said, H.; Sorour, K.A.; Gharib, S.; Gordon, J.B. Acute Myocardial Ischemia in Adults Secondary to Missed Kawasaki Disease in Childhood. Am. J. Cardiol. 2015, 115, 423–427. [Google Scholar] [CrossRef]
- Manlhiot, C.; O’Shea, S.; Bernknopf, B.; LaBelle, M.; Chahal, N.; Dillenburg, R.F.; Lai, L.S.; Bock, D.; Lew, B.; Masood, S.; et al. Epidemiology of Kawasaki Disease in Canada 2004 to 2014: Comparison of Surveillance Using Administrative Data vs Periodic Medical Record Review. Can. J. Cardiol. 2017, 34, 303–309. [Google Scholar] [CrossRef]
- Harnden, A.; Mayon-White, R.; Perera, R.; Yeates, D.; Goldacre, M.; Burgner, D. Kawasaki Disease in England. Pediatr. Infect. Dis. J. 2009, 28, 21–24. [Google Scholar] [CrossRef]
- Saundankar, J.; Yim, D.; Itotoh, B.; Payne, R.; Maslin, K.; Jape, G.; Ramsay, J.; Kothari, D.; Cheng, A.; Burgner, D. The Epidemiology and Clinical Features of Kawasaki Disease in Australia. Pediatrics 2014, 133, e1009–e1014. [Google Scholar] [CrossRef]
- Kim, G.B. Reality of Kawasaki disease epidemiology. Korean J. Pediatr. 2019, 62, 292–296. [Google Scholar] [CrossRef]
- Singh, S.; Vignesh, P.; Burgner, D. The epidemiology of Kawasaki disease: A global update. Arch. Dis. Child. 2015, 100, 1084–1088. [Google Scholar] [CrossRef]
- Holman, R.C.; Belay, E.D.; Christensen, K.Y.; Folkema, A.M.; Steiner, C.A.; Schonberger, L.B. Hospitalizations for Kawasaki Syndrome Among Children in the United States, 1997–2007. Pediatr. Infect. Dis. J. 2010, 29, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Hinze, C.H.; Graham, T.B.; Sutherell, J.S. Kawasaki disease without fever. Pediatr. Infect. Dis. J. 2009, 28, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, A. Kawasaki disease for dermatologists. Indian Dermatol. Online J. 2016, 7, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Gámez-González, L.B.; Yamazaki-Nakashimada, M.A. Erythema Multiforme. JCR J. Clin. Rheumatol. 2020, 26, e181–e182. [Google Scholar] [CrossRef] [PubMed]
- Shwe, S.; Kraus, C.N.; Linden, K.G.; Rojek, N.W. Erythema multiforme in a child with Kawasaki disease. JAAD Case Rep. 2019, 5, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Pilania, R.K.; Palla, S.; Vignesh, P.; Nada, R.; Singh, S. Palmoplantar psoriasis in a child with Kawasaki disease. Rheumatology 2020, 59, 2187–2188. [Google Scholar] [CrossRef]
- Haddock, E.S.; Calame, A.; Shimizu, C.; Tremoulet, A.H.; Burns, J.C.; Tom, W.L. Psoriasiform eruptions during Kawasaki disease (KD): A distinct phenotype. J. Am. Acad. Dermatol. 2016, 75, 69–76.e2. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Huang, Y.-H.; Guo, M.M.-H.; Chang, L.-S.; Kuo, H.-C. Kawasaki Disease and Allergic Diseases. Front. Pediatr. 2021, 8, 905. [Google Scholar] [CrossRef]
- Woon, P.Y.; Chang, W.C.; Liang, C.-C.; Hsu, C.H.; Klahan, S.; Huang, Y.-H.; Chang, W.-P.; Kuo, H.-C. Increased Risk of Atopic Dermatitis in Preschool Children with Kawasaki Disease: A Population-Based Study in Taiwan. Evid.-Based Complement. Altern. Med. 2013, 2013, 605123. [Google Scholar] [CrossRef]
- Jun, W.Y.; Ann, Y.K.; Kim, J.Y.; Son, J.S.; Kim, S.-J.; Yang, H.S.; Bae, S.H.; Chung, S.; Kim, K.S. Kawasaki Disease with Fever and Cervical Lymphadenopathy as the Sole Initial Presentation. Korean Circ. J. 2017, 47, 107–114. [Google Scholar] [CrossRef]
- Park, B.S.; Bang, M.H.; Kim, S.H. Imaging and Clinical Data Distinguish Lymphadenopathy-First-Presenting Kawasaki Disease from Bacterial Cervical Lymphadenitis. J. Cardiovasc. Imaging 2018, 26, 238. [Google Scholar] [CrossRef] [PubMed]
- Kanegaye, J.T.; Van Cott, E.; Tremoulet, A.H.; Salgado, A.; Shimizu, C.; Kruk, P.; Hauschildt, J.; Sun, X.; Jain, S.; Burns, J.C. Lymph-Node-First Presentation of Kawasaki Disease Compared with Bacterial Cervical Adenitis and Typical Kawasaki Disease. J. Pediatr. 2013, 162, 1259–1263.e2. [Google Scholar] [CrossRef] [PubMed]
- April, M.M.; Burns, J.C.; Newburger, J.W.; Healy, G.B. Kawasaki Disease and Cervical Adenopathy. Arch. Otolaryngol.—Head Neck Surg. 1989, 115, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-T.; Lee, Y.-S.; Lin, J.-J.; Chung, H.-T.; Huang, Y.-C.; Su, K.-W. Kawasaki Shock Syndrome with Initial Presentation as Neck lymphadenitis: A Case Report. Children 2022, 9, 56. [Google Scholar] [CrossRef]
- Çakan, M.; Ayaz, N.A.; Keskindemirci, G.; Onan, S.H.; Saydam, F.A. A Case of Kawasaki Disease with Severe Lip and Oral Mucosa Involvement Complicated with Microstomia and Corrected With Surgery. Arch. Rheumatol. 2018, 33, 238–240. [Google Scholar] [CrossRef]
- Grouteau, E.; Debuisson, C.; Brochard, K.; Paranon, S.; Beaudon, C.L.; Pajot, C.; Claudet, I. Severe Global Inflammatory Involvement of Ocular Segments and Optic Disc Swelling in a 12-Year-Old Girl with Kawasaki Disease. Eur. J. Ophthalmol. 2011, 21, 112–114. [Google Scholar] [CrossRef]
- Kang, J.I.; Lee, Y.S.; Lee, S.W.; Sohn, S.; Hong, Y.M. Kawasaki Disease with Optic Disc Swelling and Uveitis. Ewha Med. J. 2016, 39, 133–136. [Google Scholar] [CrossRef]
- Choi, H.S.; Lee, S.B.; Kwon, J.H.; Kim, H.S.; Sohn, S.; Hong, Y.M. Uveitis as an important ocular sign to help early diagnosis in Kawasaki disease. Korean J. Pediatr. 2015, 58, 374–379. [Google Scholar] [CrossRef][Green Version]
- Kadyan, A.; Choi, J.; Headon, M.P. Disciform keratitis and optic disc swelling in Kawasaki disease: An unusual presentation. Eye 2006, 20, 976–977. [Google Scholar] [CrossRef][Green Version]
- Ohno, S.; Miyajima, T.; Higuchi, M.; Yoshida, A.; Matsuda, H.; Saheki, Y.; Nagamatsu, I.; Togashi, T.; Matsumoto, S. Ocular Manifestations of Kawasaki’s Disease (Mucocutaneous Lymph Node Syndrome). Am. J. Ophthalmol. 1982, 93, 713–717. [Google Scholar] [CrossRef]
- Sadeghi, P.; Izadi, A.; Mojtahedi, S.Y.; Khedmat, L.; Jafari, M.; Afshin, A.; Yarahmadi, P.; Beigi, E.H. A 10-year cross-sectional retrospective study on Kawasaki disease in Iranian children: Incidence, clinical manifestations, complications, and treatment patterns. BMC Infect. Dis. 2021, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.R.D.M.; de Magalhães, C.M.R.; Almeida, R.D.F.R.; dos Santos, R.C.R.; Gandolfi, L.; Pratesi, R. Prospective study of Kawasaki disease complications: Review of 115 cases. Rev. Assoc. Médica Bras. 2011, 57, 295–300. [Google Scholar] [CrossRef]
- Watanabe, T. Pyuria in patients with Kawasaki disease. World J. Clin. Pediatr. 2015, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, X.; Duan, Z.; Deng, Y.; Cai, S.; Wang, Z.; Xu, K.; Kang, H.; Jiang, M.; Li, L.; et al. Prevalence and characteristics of arthritis in Kawasaki disease: A Chinese cohort study. Clin. Exp. Med. 2019, 19, 167–172. [Google Scholar] [CrossRef]
- Yun, S.H.; Yang, N.R.; Park, S.A. Associated Symptoms of Kawasaki Disease. Korean Circ. J. 2011, 41, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, A.; Jindal, A.K.; Gupta, A.; Suri, D.; Rawat, A.; Vaidya, P.C.; Singh, M. Pulmonary Presentation of Kawasaki Disease-A Diagnostic Challenge. Pediatric Pulmonol. 2018, 53, 103–107. [Google Scholar] [CrossRef]
- Printz, B.F.; Sleeper, L.A.; Newburger, J.W.; Minich, L.L.; Bradley, T.; Cohen, M.S.; Frank, D.; Li, J.S.; Margossian, R.; Shirali, G.; et al. Noncoronary Cardiac Abnormalities Are Associated with Coronary Artery Dilation and With Laboratory Inflammatory Markers in Acute Kawasaki Disease. J. Am. Coll. Cardiol. 2011, 57, 86–92. [Google Scholar] [CrossRef]
- Sánchez-Manubens, J.; Anton, J.; Prada, F.; Bou, R.; Iglesias, E.; Calzada-Hernandez, J.; Torrente-Segarra, V.; Hernandez, S.; Ricart, S.; Tobeña, M.; et al. Incidence and clinical features of kawasaki disease in Catalonia (Spain). Pediatr. Rheumatol. 2014, 12, P123. [Google Scholar] [CrossRef]
- Ae, R.; Maddox, R.A.; Abrams, J.Y.; Schonberger, L.B.; Nakamura, Y.; Kuwabara, M.; Makino, N.; Kosami, K.; Matsubara, Y.; Matsubara, D.; et al. Kawasaki Disease with Coronary Artery Lesions Detected at Initial Echocardiography. J. Am. Hear. Assoc. 2021, 10, 7. [Google Scholar] [CrossRef]
- Pinto, F.F.; Laranjo, S.; Carmo, M.M.; Brito, M.J.; Ferreira, R.C. Twelve Years of Kawasaki Disease in Portugal. Pediatr. Infect. Dis. J. 2017, 36, 364–368. [Google Scholar] [CrossRef]
- Kim, G.B.; Park, S.; Eun, L.Y.; Han, J.W.; Lee, S.Y.; Yoon, K.L.; Yu, J.J.; Choi, J.-W.; Lee, K.-Y. Epidemiology and Clinical Features of Kawasaki Disease in South Korea, 2012–2014. Pediatr. Infect. Dis. J. 2017, 36, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-P.; Yan, W.-L.; Huang, M.; Huang, M.-R.; Chen, S.; Huang, G.-Y.; Liu, F. Epidemiologic Features of Kawasaki Disease in Shanghai from 2013 through 2017. J. Epidemiol. 2020, 30, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Son, N.H.; Hao, T.K.; Anh, N.T.H. A Retrospective Cohort Study of Kawasaki Disease in Hue Central Hospital for 10 Years (2010–2019). Open Access Maced. J. Med. Sci. 2020, 8, 99–103. [Google Scholar] [CrossRef]
- Kim, J.-J.; Hong, Y.M.; Yun, S.W.; Han, M.K.; Lee, K.-Y.; Song, M.S.; Lee, H.-D.; Kim, D.S.; Sohn, S.; Ha, K.-S.; et al. Assessment of Risk Factors for Korean Children with Kawasaki Disease. Pediatr. Cardiol. 2012, 33, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S. Kawasaki Disease. Yonsei Med. J. 2006, 47, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Cimaz, R.; Fanti, E.; Mauro, A.; Voller, F.; Rusconi, F. Epidemiology of Kawasaki disease in Italy: Sur.veillance from national hospitalization records. Eur. J. Pediatr. 2017, 176, 1061–1065. [Google Scholar] [CrossRef]
- Singh, R.; Ward, C.; Walton, M.; Persad, R. Atypical Kawasaki disease and gastrointestinal manifestations. Paediatr. Child Health 2007, 12, 235–237. [Google Scholar] [CrossRef]
- Zulian, F.; Falcini, F.; Zancan, L.; Martini, G.; Secchieri, S.; Luzzatto, C.; Zacchello, F. Acute surgical abdomen as presenting manifestation of Kawasaki disease. J. Pediatr. 2003, 142, 731–735. [Google Scholar] [CrossRef]
- Fabi, M.; Corinaldesi, E.; Pierantoni, L.; Mazzoni, E.; Landini, C.; Bigucci, B.; Ancora, G.; Malaigia, L.; Bodnar, T.; Di Fazzio, G.; et al. Gastrointestinal presentation of Kawasaki disease: A red flag for severe disease? PLoS ONE 2018, 13, e0202658. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, X.; Duan, Z.; Cai, S.; Duan, J.; Zhou, Y. Age-related differences in clinical characteristics of Kawasaki disease. Braz. J. Med. Biol. Res. 2021, 54, e10281. [Google Scholar] [CrossRef]
- Franken, E.A.; Kleiman, M.B.; Norins, A.L.; Smith, J.A.; Smith, W.L. Intestinal Pseudo-Obstruction in Mucocutaneous Lymph-Node Syndrome. Radiology 1979, 130, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Kidney and Urinary Tract Involvement in Kawasaki Disease. Int. J. Pediatr. 2013, 2013, 831834. [Google Scholar] [CrossRef] [PubMed]
- Ulinski, T.; Sellier-Leclerc, A.-L.; Tudorache, E.; Bensman, A.; Aoun, B. Acute tubulointerstitial nephritis. Pediatr. Nephrol. 2011, 27, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Nardi, P.M.; Haller, J.O.; Friedman, A.P.; Slovis, T.L.; Schaffer, R.M. Renal manifestations of Kawasaki’s disease. Pediatr. Radiol. 1985, 15, 116–118. [Google Scholar] [CrossRef]
- Dong, S.; Bout-Tabaku, S.; Texter, K.; Jaggi, P. Diagnosis of Systemic-Onset Juvenile Idiopathic Arthritis after Treatment for Presumed Kawasaki Disease. J. Pediatr. 2015, 166, 1283–1288. [Google Scholar] [CrossRef]
- Go, E.; van Veenendaal, M.; Manlhiot, C.; Schneider, R.; McCrindle, B.W.; Yeung, R.S.M. Kawasaki Disease and Systemic Juvenile Idiopathic Arthritis—Two Ends of the Same Spectrum. Front. Pediatr. 2021, 9, 333. [Google Scholar] [CrossRef]
- Kanemasa, H.; Nanishi, E.; Takada, H.; Ishimura, M.; Nishio, H.; Honjo, S.; Masuda, H.; Nagai, N.; Nishihara, T.; Ishii, T.; et al. Overlapping Features in Kawasaki Disease-Related Arthritis and Systemic-Onset Juvenile Idiopathic Arthritis: A Nationwide Study in Japan. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef]
- Koutras, A. Myositis with Kawasaki’s Disease. Arch. Pediatr. Adolesc. Med. 1982, 136, 78–79. [Google Scholar] [CrossRef]
- Gama, C.; Breeden, K.; Miller, R. Myositis in Kawasaki disease. Pediatr. Neurol. 1990, 6, 135–136. [Google Scholar] [CrossRef]
- Agarwal, S.; Gupta, A.; Suri, D.; Rawat, A.; Singh, S. Proximal Muscle Weakness in a Child with Kawasaki Disease. Indian J. Pediatr. 2015, 82, 866. [Google Scholar] [CrossRef]
- He, T.; Yang, Z.; Wang, X.; Yang, J. Kawasaki disease associated pulmonary involvement in infants. Pediatr. Pulmonol. 2021, 56, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Saji, T.; Matsuo, N.; Odagiri, K. Chest x-ray findings in the acute phase of Kawasaki disease. Pediatr. Radiol. 1989, 20, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Na Lee, M.; Cha, J.H.; Ahn, H.M.; Yoo, J.H.; Kim, H.S.; Sohn, S.; Hong, Y.M. Mycoplasma pneumoniaeinfection in patients with Kawasaki disease. Korean J. Pediatr. 2011, 54, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sittiwangkul, R.; Pongprot, Y. Large pleural effusion: An unusual manifestation of Kawasaki disease. Clin. Pediatr. 2004, 43, 389–391. [Google Scholar] [CrossRef]
- Sengler, C.; Gaedicke, G.; Wahn, U.; Keitzer, R. Pulmonary symptoms in kawasaki disease. Pediatr. Infect. Dis. J. 2004, 23, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Filjak, I.S.; Batinica, M.; Rešić, A.; Gagro, A. 450 Kawasaki Disease with Pulmonary Manifestations—A case Report of Three Patients.In Proceedings of the Abstarcts. BMJ Publ. Group Ltd. R. Coll. Paediatr. Child Health 2021, A188–A189. [Google Scholar] [CrossRef]
- Leahy, T.R.; Cohen, E.; Allen, U.D. Incomplete Kawasaki Disease Associated with Complicated Streptococcus pyogenes Pneumonia: A Case Report. Can. J. Infect. Dis. Med. Microbiol. 2012, 23, 137–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, K.A.; Yunker, W.K. Kawasaki disease is associated with sensorineural hearing loss: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1216–1220. [Google Scholar] [CrossRef]
- Toomaj, K.; Akbariasbagh, P.; Karimi Yazdi, A.; Aghighi, Y.; Reza Raeeskarami, S.; Eslambol Nassaj, F.; Alamdari, S. The Prevalence of Sensorineural Hearing Loss in Patients with Kawasaki Disease after Treatment. Audit. Vestib. Res. 2016, 25, 119–126. [Google Scholar]
- Yuan, Y.; Lu, N. Facial nerve palsy presenting as rare neurological complication of Kawasaki disease. Medicine 2019, 98, e16888. [Google Scholar] [CrossRef]
- Kocabas, A.; Kardelen, F.; Aldemir-Kocabaş, B.; Akçurin, G.; Ertug, H. Facial Nerve Palsy and Kawasaki Disease. Indian J. Pediatr. 2013, 81, 186–188. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, H.J. Clinical implications of thrombocytosis in acute phase Kawasaki disease. Eur. J. Pediatr. 2021, 180, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Satou, G.M.; Giamelli, J.; Gewitz, M.H. Kawasaki Disease. Cardiol. Rev. 2007, 15, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bansal, A.; Gupta, A.; Kumar, R.M.; Mittal, B. Kawasaki Disease. A Decade of Experience from North India. Int. Heart J. 2005, 46, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yu, S.-F.; Bai, Y.-X.; Liang, Y.-Y.; Su, X.-W.; Pan, J.-Y. Kawasaki disease in children: Epidemiology, clinical symptoms and diagnostics of 231 cases in 10 years. Exp. Ther. Med. 2015, 10, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, Y.; Habibi, D.; Kahbazi, M.; Dorreh, F.; Lotfi, M. Clinical Characteristics of Kawasaki Disease in Markazi Province, Iran. J. Compr. Pediatr. 2020, 11. [Google Scholar] [CrossRef]
- Mizuta, M.; Shimizu, M.; Inoue, N.; Kasai, K.; Nakagishi, Y.; Takahara, T.; Hamahira, K.; Yachie, A. Serum ferritin levels as a useful diagnostic marker for the distinction of systemic juvenile idiopathic arthritis and Kawasaki disease. Mod. Rheumatol. 2016, 26, 929–932. [Google Scholar] [CrossRef]
- Maggio, M.C.; Corsello, G.; Prinzi, E.; Cimaz, R. Kawasaki disease in Sicily: Clinical description and markers of disease severity. Ital. J. Pediatr. 2016, 42, 92. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, H.H.; Fan, G.Z.; Chen, W.X.; Hu, P. The clinical implications of serum ferritin in Kawasaki disease: A helpful biomarker for evaluating therapeutic responsiveness, coronary artery involvement and the tendency of macrophage activation syndrome. Arch. Med. Sci. 2021, 18, 267–274. [Google Scholar] [CrossRef]
- Kong, W.-X.; Ma, F.-Y.; Fu, S.-L.; Wang, W.; Xie, C.-H.; Zhang, Y.-Y.; Gong, F.-Q. Biomarkers of intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. World J. Pediatr. 2019, 15, 168–175. [Google Scholar] [CrossRef]
- Motomura, H.; Fukunaga, H.; Nakagaki, M.; Hashimoto, K.; Hasuwa, T.; Moriuchi, H. Abstract 94: Diagnostic value of D-dimer and fibrinogen degradation products In Kawasaki disease. Circulation 2015, 131. [Google Scholar] [CrossRef]
- Dominguez, S.R.; Martin, B.; Heizer, H.; Jone, P.-N.; Tong, S.; Davidson, J.; Anderson, M.S.; Glodé, M.P. Procalcitonin (PCT) and Kawasaki Disease: Does PCT Correlate With IVIG-Resistant Disease, Admission to the Intensive Care Unit, or Development of Coronary Artery Lesions? J. Pediatr. Infect. Dis. Soc. 2016, 5, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Choi, H.J.; Kim, Y.H. Clinical usefulness of serum procalcitonin level in distinguishing between Kawasaki disease and other infections in febrile children. Korean J. Pediatr. 2017, 60, 112–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshikawa, H.; Nomura, Y.; Masuda, K.; Koriya, C.; Arata, M.; Hazeki, D.; Yanagimoto, K.; Ueno, K.; Eguchi, T.; Kawano, Y. Serum Procalcitonin Value is Useful for Predicting Severity of Kawasaki Disease. Pediatr. Infect. Dis. J. 2012, 31, 523–525. [Google Scholar] [CrossRef] [PubMed]
- ElAdawy, M.; Dominguez, S.R.; Anderson, M.S.; Glodé, M.P. Abnormal Liver Panel in Acute Kawasaki Disease. Pediatr. Infect. Dis. J. 2011, 30, 141–144. [Google Scholar] [CrossRef]
- Soleimani, G.; Bojd, S.S.; Tajik, M.; Shahri, E.S.; Rashidi, S. Paraclinical Evolutions Regarding Liver and Renal Abnormalities of Kawasaki Disease in the Southeast of Iran. J. Compr. Pediatr. 2014, 5. [Google Scholar] [CrossRef]
- Mammadov, G.; Liu, H.H.; Chen, W.X.; Fan, G.Z.; Li, R.X.; Liu, F.F.; Samadli, S.; Wang, J.J.; Wu, Y.F.; Luo, H.H.; et al. Hepatic dysfunction secondary to Kawasaki disease: Characteristics, etiology and predictive role in coronary artery abnormalities. Clin. Exp. Med. 2020, 20, 21–30. [Google Scholar] [CrossRef]
- Cao, L.; Tang, Y.-J.; Gang, M.; Ma, J.; Qian, W.-G.; Xu, Q.-Q.; Lv, H.-T. AST-to-ALT ratio and coronary artery lesions among patients with Kawasaki disease. World J. Pediatr. 2021, 17, 659–668. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Liang, C.-D.; Wang, C.-L.; Yu, H.-R.; Yang, K.D. 629 Serum Albumin Level Predicts Initial Intravenous Immunoglobulin Treatment Failure in Kawasaki Disease. Pediatr. Res. 2010, 68, 322. [Google Scholar] [CrossRef][Green Version]
- Huang, Z.; Tan, X.-H.; Wang, H.; Pan, B.; Lv, T.-W.; Tian, J. A New Diagnostic Model to Distinguish Kawasaki Disease from Other Febrile Illnesses in Chongqing: A Retrospective Study on 10,367 Patients. Front. Pediatr. 2020, 8. [Google Scholar] [CrossRef]
- Sano, T.; Kurotobi, S.; Matsuzaki, K.; Yamamoto, T.; Maki, I.; Miki, K.; Kogaki, S.; Hara, J. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur. J. Pediatr. 2006, 166, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Burns, J.C.; Beiser, A.S.; Loscalzo, J. Altered lipid profile after Kawasaki syndrome. Circulation 1991, 84, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Salo, E.; Pesonen, E.; Viikari, J. Serum cholesterol levels during and after Kawasaki disease. J. Pediatr. 1991, 119, 557–561. [Google Scholar] [CrossRef]
- Mostafavi, S.-N.; Barzegar, E.; Manssori, N.S.; Kelishadi, R. First Report on the Lipid Profile Late after Kawasaki Disease in Iranian Children. Int. J. Prev. Med. 2014, 5, 820–824. [Google Scholar] [PubMed]
- Ou, C.-Y.; Tseng, Y.-F.; Lee, C.-L.; Chiou, Y.-H.; Hsieh, K.-S. Significant Relationship between Serum High-sensitivity C-Reactive Protein, High-density Lipoprotein Cholesterol Levels and Children with Kawasaki Disease and Coronary Artery Lesions. J. Formos. Med. Assoc. 2009, 108, 719–724. [Google Scholar] [CrossRef]
- Lazea, C.; Man, O.; Sur, L.M.; Serban, R.S.; Lazar, C. Unusual Presentation of Kawasaki Disease With Gastrointestinal And Renal Manifestations. Ther. Clin. Risk Manag. 2019, 15, 1411–1416. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Cai, X.; Liu, S.; Wang, Z.; Xie, Y. Atypical manifestations of cardiomegaly and nephrotic syndrome in Kawasaki disease. Medicine 2019, 98, e18117. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Liang, C.-D.; Wang, C.-L.; Yu, H.-R.; Hwang, K.-P.; Yang, K.D. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr. 2010, 99, 1578–1583. [Google Scholar] [CrossRef]
- Han, S.B.; Lee, S.-Y. Differentiating Kawasaki disease from urinary tract infection in febrile children with pyuria and C-reactive protein elevation. Ital. J. Pediatr. 2018, 44, 137. [Google Scholar] [CrossRef]
- Watanabe, T.; Abe, Y.; Sato, S.; Uehara, Y.; Ikeno, K.; Abe, T. Hyponatremia in Kawasaki disease. Pediatr. Nephrol. 2006, 21, 778–781. [Google Scholar] [CrossRef]
- Application of Serum Interleukin, Anti-Cardiolipin Antibody and Anti-Endothelial Cell Antibody Detection in Children with Kawasaki Disease. Chin. J. Postgrad. Med. 2017, 36, 970–974.
- Yoshizawa, H.; Nogami, K.; Matsumoto, T.; Tsujii, N.; Sakai, T.; Takase, T.; Tanaka, I.; Shima, M. Dynamic evaluation of hemostasis in the acute phase of Kawasaki disease using comprehensive coagulation functional assays. Thromb. Res. 2019, 174, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kim, Y.; Cha, S.-H.; Suh, J.-T.; Han, M.Y.; Lee, H.J. Adjuvant Laboratory Marker of Kawasaki Disease; NT-pro-BNP or Hs-CRP? Ann. Clin. Lab. Sci. 2011, 41, 360–363. [Google Scholar] [PubMed]
- Lee, S.H.; Song, E.S.; Yoon, S.; Hong, S.; Cho, H.J.; Yang, E.M.; Eom, G.H.; Kang, G.; Cho, Y.K. Usefulness of Age-Stratified N-Terminal Prohormone of Brain Natriuretic Peptide for Diagnosing Kawasaki Disease. Dis. Markers 2017, 2017, 6263121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Wei, C.-P.; Wang, W.-D.; Zheng, X.-C.; Wang, Y.-J.; Ma, S.-C.; Xu, Y.-J. Serum brain natriuretic peptide in children with Kawasaki disease. World J. Emerg. Med. 2010, 1, 114–117. [Google Scholar]
- Shiraishi, M.; Fuse, S.; Mori, T.; Doyama, A.; Honjyo, S.; Hoshino, Y.; Hoshino, E.; Kawaguchi, A.; Kuroiwa, Y.; Hotsubo, T. N-Terminal Pro-Brain Natriuretic Peptide as a Useful Diagnostic Marker of Acute Kawasaki Disease in Children. Circ. J. 2013, 77, 2097–2101. [Google Scholar] [CrossRef]
- Checchia, P.A.; Borensztajn, J.; Shulman, S.T. Circulating Cardiac Troponin I Levels in Kawasaki Disease. Pediatr. Cardiol. 2001, 22, 102–106. [Google Scholar] [CrossRef]
- Sato, Y.Z.; Molkara, D.P.; Daniels, L.B.; Tremoulet, A.H.; Shimizu, C.; Kanegaye, J.T.; Best, B.M.; Snider, J.V.; Frazer, J.R.; Maisel, A.; et al. Serum Brain Natriuretic Peptide in Children with Kawasaki Disease. Int. J. Cardiol. 2011, 164, 58–63. [Google Scholar] [CrossRef]
| Authors | Saundankar et al. (2014) [28] | Sanchez-Manubens et al. (2014) [58] | Ae et al. (2021) [59] | Pinto et al. (2017) [60] | G. B. Kim et al. (2017) [61] | Xie et al. (2020) [62] | Son et al. (2020) [63] |
|---|---|---|---|---|---|---|---|
| Country (n sample) | Australia n = 281 | Spain n = 398 | Japan n = 3714 | Italy n = 470 | South Korea n = 12,269 | China n = 4442 | Vietnam n = 167 |
| Coronary artery dilation/ecstasia #, n (%) | 47 (16.7) | N/A | 3343 (90) | N/A | 1328 (10.8) | 231 (5.2) | N/A |
| Coronary artery aneurysm/small aneurysm #, n (%) | 14 (5.0) | 53 (13.3) | 316 (9) | 40 (8.5) | 207 (1.7) | N/A | 49 (29.2) |
| Medium aneurysm, n (%) | N/A | N/A | N/A | N/A | N/A | 118 (2.7) | 60 (9.6) |
| Large/giant aneurysm, n (%) | 5 (1.8) | N/A | 49 (1) | N/A | 19 (0.16) | 31 (0.7) | N/A |
| Myocarditis, n (%) | N/A | 4 (1) | N/A | N/A | N/A | N/A | N/A |
| Pericarditis, n (%) | N/A | 9 (2.3) | N/A | 18 (3.8) | N/A | N/A | N/A |
| Valvular lesion, n (%) | N/A | 28 (7) | N/A | 2 (0.4) | N/A | 653 (14.7) | 2 (1.2) |
| FBC Variables | Malik et al. (1996) [16] n = 7 | Xuan et al. (2020) [63] n = 168 | Singh et al. (2005) [94] n = 69 | Zhu et al. (2015) [95] n = 226 | Ghandi et al. (2020) [96] n = 69 | Mean (%) |
|---|---|---|---|---|---|---|
| Anaemia, n (%) | 4 (57.1) | 147 (87.5) | N/A | 89 (39.4) | 17 (24.6) | 52.2 |
| Leukocytosis, n (%) | 6 (85.7) | 142 (84.5) | 39 (56.5) | 138 (61.1) | 24 (34.8) | 64.5 |
| Thrombocytosis, n (%) | 5 (71.4) | 58 (34.5) | 36 (52.2) | 208 (92.0.) | 55 (79.7) | 66.0 |
| High Erythrocyte Sedimentation Rate (ESR), n (%) | 5 (71.4) | 94 (54.6) | 37 (75.5) | 172 (79.3) | 54 (78.3) | 71.8 |
| High C-Reactive Protein (CRP), n (%) | N/A | 128 (76.2) | 28 (62.2) | 133 (61.6) | 20 (29.0) | 57.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.; Cheah, C.S.; Suhaini, S.A.; Azidin, A.H.; Khoo, M.S.; Ismail, N.A.S.; Ali, A. Clinical Manifestations and Laboratory Findings of Kawasaki Disease: Beyond the Classic Diagnostic Features. Medicina 2022, 58, 734. https://doi.org/10.3390/medicina58060734
Lee W, Cheah CS, Suhaini SA, Azidin AH, Khoo MS, Ismail NAS, Ali A. Clinical Manifestations and Laboratory Findings of Kawasaki Disease: Beyond the Classic Diagnostic Features. Medicina. 2022; 58(6):734. https://doi.org/10.3390/medicina58060734
Chicago/Turabian StyleLee, Wendy, Chooi San Cheah, Siti Aisyah Suhaini, Abdullah Harith Azidin, Mohammad Shukri Khoo, Noor Akmal Shareela Ismail, and Adli Ali. 2022. "Clinical Manifestations and Laboratory Findings of Kawasaki Disease: Beyond the Classic Diagnostic Features" Medicina 58, no. 6: 734. https://doi.org/10.3390/medicina58060734
APA StyleLee, W., Cheah, C. S., Suhaini, S. A., Azidin, A. H., Khoo, M. S., Ismail, N. A. S., & Ali, A. (2022). Clinical Manifestations and Laboratory Findings of Kawasaki Disease: Beyond the Classic Diagnostic Features. Medicina, 58(6), 734. https://doi.org/10.3390/medicina58060734








