A Case Report on Dysgraphia in a Patient Receiving Blinatumomab: Complex Characters Are Easy to Find in a Handwriting Test
Abstract
:1. Introduction
2. Case Presentation
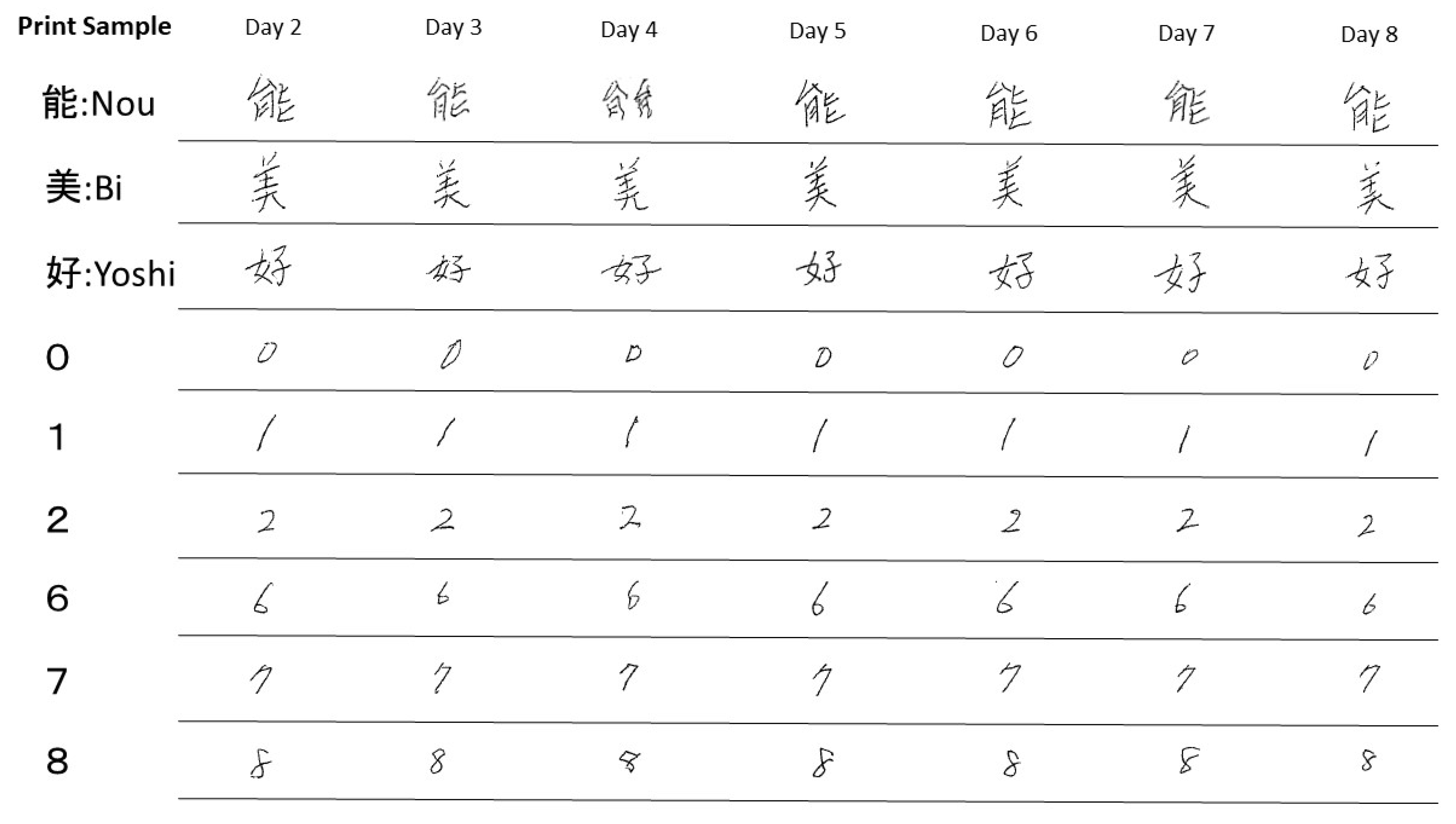
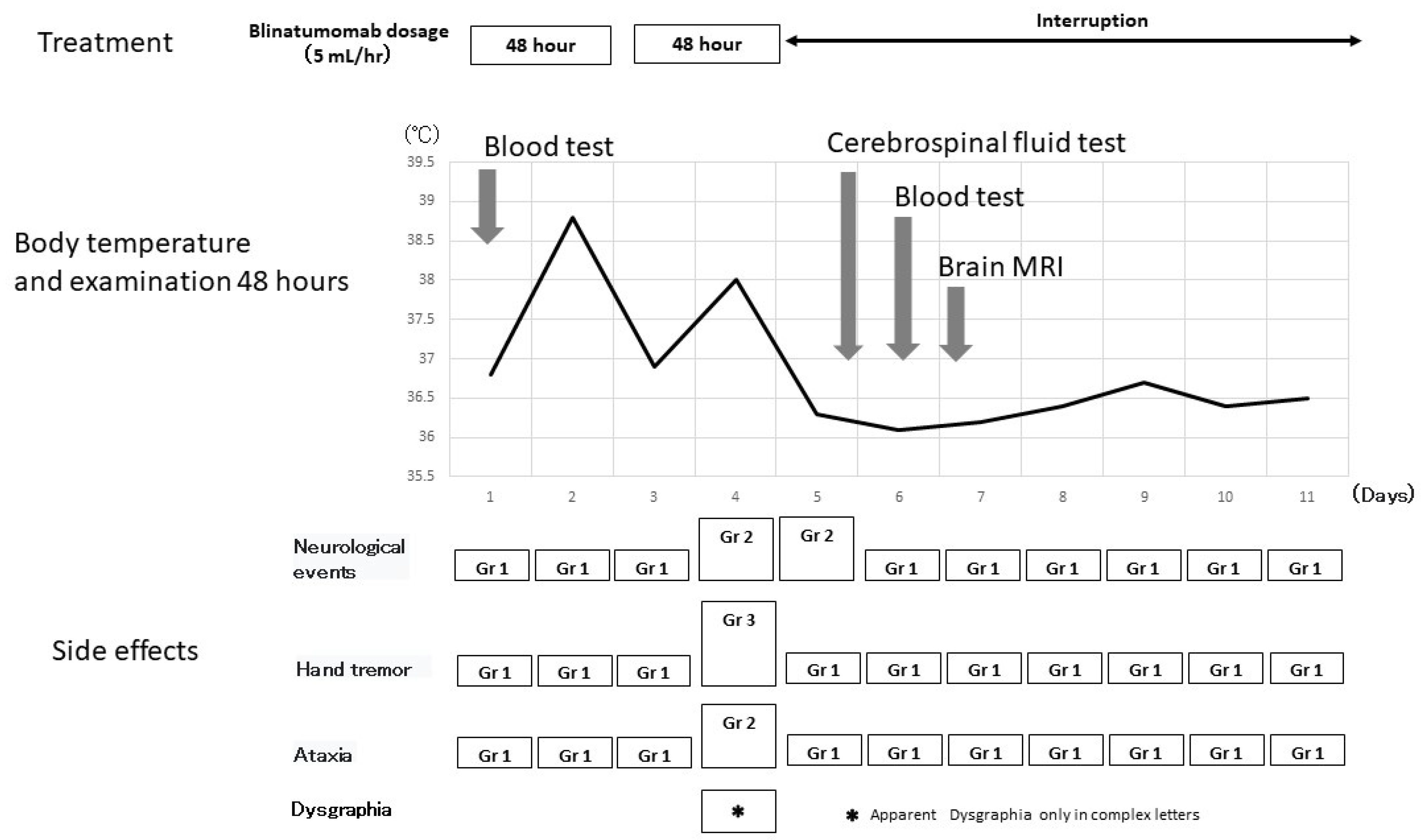
2.1. Methods
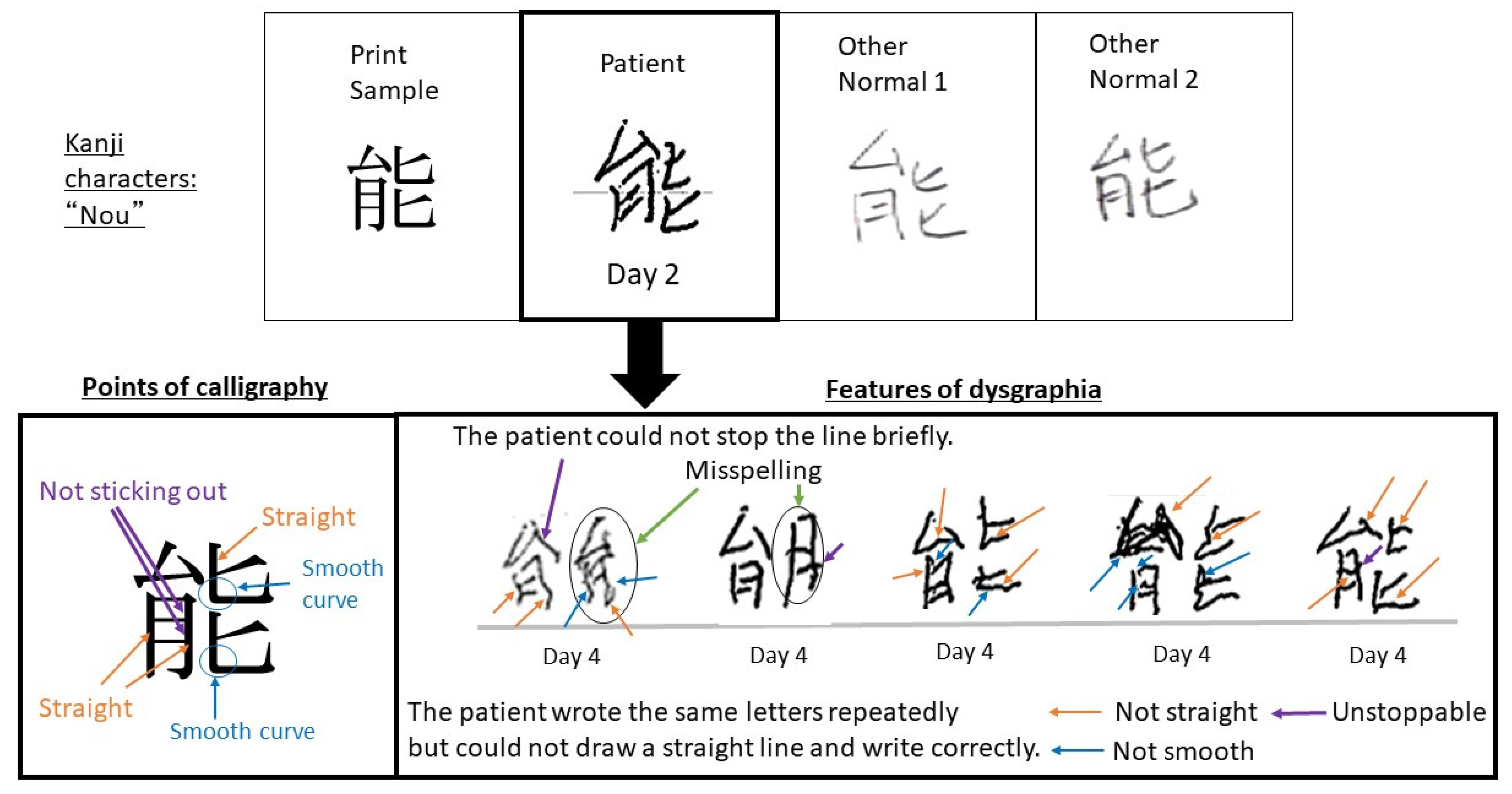
2.2. Progress after Hospitalization
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazarus, H.M.; Richards, S.M.; Chopra, R.; Litzow, M.R.; Burnett, A.K.; Wiernik, P.H.; Franklin, I.M.; Tallman, M.S.; Cook, L.; Buck, G.; et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: Results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood 2006, 108, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, X.; Boiron, J.M.; Huguet, F.; Dombret, H.; Bradstock, K.; Vey, N.; Kovacsovics, T.; Delannoy, A.; Fegueux, N.; Fenaux, P.; et al. Outcome of treatment in adults with acute lymphoblastic leukemia: Analysis of the LALA-94 trial. J. Clin. Oncol. 2004, 22, 4075–4086. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.M.; Buck, G.; Burnett, A.K.; Chopra, R.; Wiernik, P.H.; Richards, S.M.; Lazarus, H.M.; Franklin, I.M.; Litzow, M.R.; Ciobanu, N.; et al. Induction therapy for adults with acute lymphoblastic leukemia: Results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005, 106, 3760–3767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gokbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gokbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foa, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Hofmeister, R.; Brischwein, K.; Brandl, C.; Crommer, S.; Bargou, R.; Itin, C.; Prang, N.; Baeuerle, P.A. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int. J. Cancer 2005, 115, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Brischwein, K.; Parr, L.; Pflanz, S.; Volkland, J.; Lumsden, J.; Klinger, M.; Locher, M.; Hammond, S.A.; Kiener, P.; Kufer, P.; et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J. Immunother. 2007, 30, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Offner, S.; Hofmeister, R.; Romaniuk, A.; Kufer, P.; Baeuerle, P.A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol. Immunol. 2006, 43, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Dreier, T.; Lorenczewski, G.; Brandl, C.; Hoffmann, P.; Syring, U.; Hanakam, F.; Kufer, P.; Riethmuller, G.; Bargou, R.; Baeuerle, P.A. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int. J. Cancer 2002, 100, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Loffler, A.; Kufer, P.; Lutterbuse, R.; Zettl, F.; Daniel, P.T.; Schwenkenbecher, J.M.; Riethmuller, G.; Dorken, B.; Bargou, R.C. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000, 95, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Boissel, N.; Chevallier, P.; Ottmann, O.; Gokbuget, N.; Topp, M.S.; Fielding, A.K.; Rambaldi, A.; Ritchie, E.K.; Papayannidis, C.; et al. Complete Hematologic and Molecular Response in Adult Patients With Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia Following Treatment With Blinatumomab: Results From a Phase II, Single-Arm, Multicenter Study. J. Clin. Oncol. 2017, 35, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- von Stackelberg, A.; Locatelli, F.; Zugmaier, G.; Handgretinger, R.; Trippett, T.M.; Rizzari, C.; Bader, P.; O’Brien, M.M.; Brethon, B.; Bhojwani, D.; et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2016, 34, 4381–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topp, M.S.; Gokbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Kiyoi, H.; Morris, J.D.; Oh, I.; Maeda, Y.; Minami, H.; Miyamoto, T.; Sakura, T.; Iida, H.; Tuglus, C.A.; Chen, Y.; et al. Phase 1b/2 study of blinatumomab in Japanese adults with relapsed/refractory acute lymphoblastic leukemia. Cancer Sci. 2020, 111, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Available online: https://www.ich.org/ (accessed on 13 May 2022).
- Common Terminology Standard for Adverse Events. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 13 May 2022).
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, T.; Litzow, M.R. No free rides: Management of toxicities of novel immunotherapies in ALL, including financial. Blood Adv. 2018, 2, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.; Rezania, K.; Kelly, T.; Bishop, M.R. Clinical predictors of chimeric antigen receptor T-cell therapy neurotoxicity: A single-center study. Immunotherapy 2021, 13, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Sakura, T.; Hayakawa, F.; Sugiura, I.; Murayama, T.; Imai, K.; Usui, N.; Fujisawa, S.; Yamauchi, T.; Yujiri, T.; Kakihana, K.; et al. High-dose methotrexate therapy significantly improved survival of adult acute lymphoblastic leukemia: A phase III study by JALSG. Leukemia 2018, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kebriaei, P.; Cutler, C.; de Lima, M.; Giralt, S.; Lee, S.J.; Marks, D.; Merchant, A.; Stock, W.; van Besien, K.; Stelljes, M. Management of important adverse events associated with inotuzumab ozogamicin: Expert panel review. Bone Marrow Transplant. 2018, 53, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Item | Reference Ranges | Day 6 | Item | Reference Ranges | Day 6 |
|---|---|---|---|---|---|
| WBC | 3300–8600 | 2150/μL | TP | 6.6–8.1 | 5.3 g/dL |
| Neut. | 40–77 | 59.1% | Alb | 4.1–5.1 | 3.5 g/dL |
| Lym. | 16–44 | 30.1% | T-Bil | 0.4–1.5 | 0.5 mg/dL |
| Eos. | 1–7 | 1.6% | AST | 13–30 | 20 U/L |
| Baso. | 0–1 | 0.1% | ALT | 7–23 | 20 U/L |
| Mono. | 4–9 | 8.1% | LDH | 124–222 | NA |
| Blast. | NA | ALP | 38–113 | NA | |
| RBC | 3.86–4.92 × 106 | 4.31 × 106/μL | γ-GTP | 9–32 | 39 U/L |
| Hb | 11.6–14.8 | 13.2 g/dL | BUN | 8–20 | 26 mg/dL |
| Hct | 35.1–44.4 | 40.8% | Cre | 0.46–0.79 | 0.45 mg/dL |
| MCV | 83.6–98.2 | 94.6 fL | UA | 2.6–5.5 | 3.0 mg/dL |
| Ret | 0.8–2.1 | 1.3% | Na | 138–145 | 142 mmol/L |
| PLT | 15.8–34.8 × 104 | 10.4 × 104/μL | K | 3.6–4.8 | 3.7 mmol/L |
| Cl | 101–108 | 103 mmol/L | |||
| Ca | 8.8–10.1 | 8.8 mg/dL | |||
| CRP | 0.00–0.14 | 0.03 mg/dL |
| Drugs | Content and Frequency of Side Effects Associated with Peripheral Neuropathy (Drug Package Insert) | Prescription Intent |
|---|---|---|
| Dexamethasone sodium phosphate | No description | Prevention and Treatment of side effects |
| Hydrocortisone Sodium Succinate | Myopathy (Frequency unknown) | Treatment of side effects |
| Methylprednisolone Sodium Succinate | Myopathy (Frequency unknown) convulsions (Frequency unknown) | Treatment of side effects |
| Betamethasone sodium phosphate | No description | Treatment of side effects |
| Acetaminophen | No description | Clothes fever |
| Fluconazole | tremor (Frequency unknown) | Regular oral administration |
| Rabeprazole Sodium | Numbness in the limbs (Less than 0.1%) Weakness limbs (Less than 0.1%) Weakness (Less than 0.1%) | Regular oral administration |
| Enterococcus feacium Clostridium butyricum Bacillus subtilis | No description | Regular oral administration |
| Sulfamethoxazole trimethoprim | Numbness in the limbs (Less than 0.1%) Shivering limbs (Frequency unknown) Weakness (Frequency unknown) | Regular oral administration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, Y.; Shimasaki, T.; Ishigaki, Y.; Fujimoto, S.; Takahashi, Y.; Kimura, S.; Aijo, K.; Takayanagi, M.; Mizuta, S.; Masauji, T.; et al. A Case Report on Dysgraphia in a Patient Receiving Blinatumomab: Complex Characters Are Easy to Find in a Handwriting Test. Medicina 2022, 58, 733. https://doi.org/10.3390/medicina58060733
Yamamoto Y, Shimasaki T, Ishigaki Y, Fujimoto S, Takahashi Y, Kimura S, Aijo K, Takayanagi M, Mizuta S, Masauji T, et al. A Case Report on Dysgraphia in a Patient Receiving Blinatumomab: Complex Characters Are Easy to Find in a Handwriting Test. Medicina. 2022; 58(6):733. https://doi.org/10.3390/medicina58060733
Chicago/Turabian StyleYamamoto, Yasuto, Takeo Shimasaki, Yasuhito Ishigaki, Shino Fujimoto, Yoshimitsu Takahashi, Shiori Kimura, Keiko Aijo, Mami Takayanagi, Shuichi Mizuta, Togen Masauji, and et al. 2022. "A Case Report on Dysgraphia in a Patient Receiving Blinatumomab: Complex Characters Are Easy to Find in a Handwriting Test" Medicina 58, no. 6: 733. https://doi.org/10.3390/medicina58060733
APA StyleYamamoto, Y., Shimasaki, T., Ishigaki, Y., Fujimoto, S., Takahashi, Y., Kimura, S., Aijo, K., Takayanagi, M., Mizuta, S., Masauji, T., & Masaki, Y. (2022). A Case Report on Dysgraphia in a Patient Receiving Blinatumomab: Complex Characters Are Easy to Find in a Handwriting Test. Medicina, 58(6), 733. https://doi.org/10.3390/medicina58060733







