Effects of Visual Cue Deprivation Balance Training with Head Control on Balance and Gait Function in Stroke Patients
Abstract
:1. Introduction
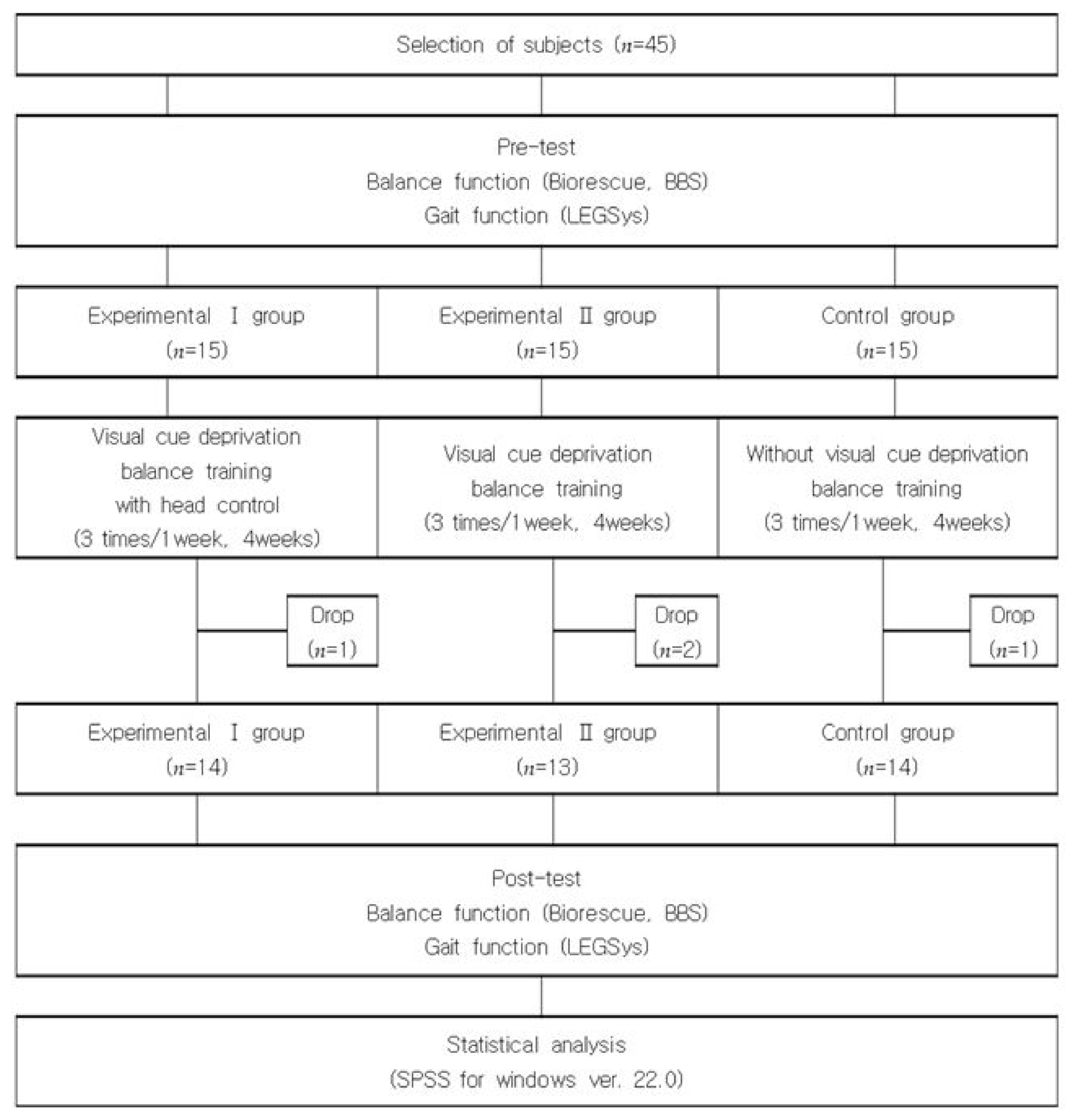
2. Materials and Methods
2.1. Participants
2.2. Study Procedure
2.3. Static Balance Test
2.4. Dynamic Balance Test
2.5. Gait Function Test
2.6. Statistics Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoff, S.; Hanke, T.A.; Evans, C.C. Community mobility after stroke: A systematic review. Top. Stroke Rehabil. 2018, 25, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Tasseel-Ponche, S.; Yelnik, A.P.; Bonan, I.V. Motor strategies of postural control after hemispheric stroke. Neurophysiol. Clin. Clin. Neurophysiol. 2015, 45, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Bonan, I.V.; Colle, F.M.; Guichard, J.P.; Vicaut, E.; Eisenfisz, M.; Tran Ba Huy, P.; Yelnik, A.P. Reliance on visual information after stroke. Part I: Balance on dynamic posturography. Arch. Phys. Med. Rehabil. 2004, 85, 268–273. [Google Scholar] [CrossRef]
- Laufer, Y.; Sivan, D.; Schwarzmann, R.; Sprecher, E. Standing balance and functional recovery of patients with right and left hemiparesis in the early stages of rehabilitation. Neurorehabilit. Neural Repair 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Mercer, V.S.; Freburger, J.K.; Chang, S.H.; Purser, J.L. Measurement of paretic-lower-extremity loading and weight transfer after stroke. Phys. Ther. 2009, 89, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Geurts, A.C.; de Haart, M.; van Nes, I.J.; Duysens, J. A review of standing balance recovery from stroke. Gait Posture 2005, 22, 267–281. [Google Scholar] [CrossRef]
- Mirelman, A.; Shema, S.; Maidan, I.; Hausdorff, J.M. Gait. Handb. Clin. Neurol. 2018, 159, 119–134. [Google Scholar] [CrossRef]
- Klöpfer-Krämer, I.; Brand, A.; Wackerle, H.; Müßig, J.; Kröger, I.; Augat, P. Gait analysis-Available platforms for outcome assessment. Injury 2020, 51 (Suppl. 2), S90–S96. [Google Scholar] [CrossRef]
- Smania, N.; Picelli, A.; Gandolfi, M.; Fiaschi, A.; Tinazzi, M. Rehabilitation of sensorimotor integration deficits in balance impairment of patients with stroke hemiparesis: A before/after pilot study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2008, 29, 313–319. [Google Scholar] [CrossRef]
- Sackley, C.M.; Lincoln, N.B. Single blind randomized controlled trial of visual feedback after stroke: Effects on stance symmetry and function. Disabil. Rehabil. 1997, 19, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Barclay-Goddard, R.; Stevenson, T.; Poluha, W.; Moffatt, M.E.; Taback, S.P. Force platform feedback for standing balance training after stroke. Cochrane Database Syst. Rev. 2004, 2004, Cd004129. [Google Scholar] [CrossRef] [PubMed]
- Bonan, I.V.; Marquer, A.; Eskiizmirliler, S.; Yelnik, A.P.; Vidal, P.P. Sensory reweighting in controls and stroke patients. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2013, 124, 713–722. [Google Scholar] [CrossRef]
- Bonan, I.V.; Yelnik, A.P.; Colle, F.M.; Michaud, C.; Normand, E.; Panigot, B.; Roth, P.; Guichard, J.P.; Vicaut, E. Reliance on visual information after stroke. Part II: Effectiveness of a balance rehabilitation program with visual cue deprivation after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2004, 85, 274–278. [Google Scholar] [CrossRef]
- Yelnik, A.P.; Le Breton, F.; Colle, F.M.; Bonan, I.V.; Hugeron, C.; Egal, V.; Lebomin, E.; Regnaux, J.P.; Pérennou, D.; Vicaut, E. Rehabilitation of balance after stroke with multisensorial training: A single-blind randomized controlled study. Neurorehabilit. Neural Repair 2008, 22, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, M.C.; Park, H.S.; Cho, I.H.; Paik, J.K. Association of the Anxiety/Depression with Nutrition Intake in Stroke Patients. Clin. Nutr. Res. 2018, 7, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Danna-Dos-Santos, A.; Degani, A.M.; Latash, M.L. Anticipatory control of head posture. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007, 118, 1802–1814. [Google Scholar] [CrossRef] [Green Version]
- Berencsi, A.; Ishihara, M.; Imanaka, K. The functional role of central and peripheral vision in the control of posture. Hum. Mov. Sci. 2005, 24, 689–709. [Google Scholar] [CrossRef]
- de Oliveira, C.B.; de Medeiros, I.R.; Frota, N.A.; Greters, M.E.; Conforto, A.B. Balance control in hemiparetic stroke patients: Main tools for evaluation. J. Rehabil. Res. Dev. 2008, 45, 1215–1226. [Google Scholar] [CrossRef]
- Han, K.-B.; Shin, W.-S. Effects of trunk position sense through visual cue deprivation balance training in subacute stroke. Korean Soc. Phys. Med. 2013, 8, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.-J.; Kim, Y.-W.; Kim, T.H. The Effects of Balance Training with Visual Cue Deprivation on Gait Function in Patients with Stroke. Korean Soc. Phys. Med. 2012, 7, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Godi, M.; Franchignoni, F.; Caligari, M.; Giordano, A.; Turcato, A.M.; Nardone, A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys. Ther. 2013, 93, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-W.; Kim, Y.-W. Effects of visual cue deprivation during sideways treadmill training on balance and walking in stroke patients. Phys. Ther. Korea 2014, 21, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Solomon, D.; Goldstein, J.E. Visual, Oculomotor and Vestibular Deficits. In Stroke Recovery and Rehabilitation; Springer: New York, NY, USA, 2009. [Google Scholar]
- Suh, H.R.; Hwang, J.-H.; Lee, S.-Y. The effect of visual information on gait parameters with induced ankle muscle fatigue. Phys. Ther. Rehabil. Sci. 2017, 6, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, S.D.; Song, H.S. The effects of a complex exercise program with the visual block on the walking and balance abilities of elderly people. J. Phys. Ther. Sci. 2014, 26, 2007–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenton, J.T.; Schwoebel, J.; Coslett, H.B. Mental motor imagery and the body schema: Evidence for proprioceptive dominance. Neurosci. Lett. 2004, 370, 19–24. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M. Attentional demands and postural control: The effect of sensory context. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M10–M16. [Google Scholar] [CrossRef]
- Nichols, D.S.; Glenn, T.M.; Hutchinson, K.J. Changes in the mean center of balance during balance testing in young adults. Phys. Ther. 1995, 75, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Saucedo, F.; Yang, F. Effects of visual deprivation on stability among young and older adults during treadmill walking. Gait Posture 2017, 54, 106–111. [Google Scholar] [CrossRef]
- Song, M.-J.; Lee, J.-H.; Shin, W.-S. Minimal Clinically Important Difference of Berg Balance Scale scores in people with acute stroke. Phys. Ther. Rehabil. Sci. 2018, 7, 102–108. [Google Scholar] [CrossRef]
- Freitas, S.M.; Duarte, M.; Latash, M.L. Two kinematic synergies in voluntary whole-body movements during standing. J. Neurophysiol. 2006, 95, 636–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanamiya, Y.; Ota, S.; Sato, D. Ankle and hip balance control strategies with transitions. In Proceedings of the 2010 IEEE International Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010; pp. 3446–3451. [Google Scholar]
- Mackey, D.C.; Robinovitch, S.N. Mechanisms underlying age-related differences in ability to recover balance with the ankle strategy. Gait Posture 2006, 23, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Nashner, L.M.; Peters, J.F. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol. Clin. 1990, 8, 331–349. [Google Scholar] [CrossRef]
- Hallemans, A.; Beccu, S.; Van Loock, K.; Ortibus, E.; Truijen, S.; Aerts, P. Visual deprivation leads to gait adaptations that are age- and context-specific: II. Kinematic parameters. Gait Posture 2009, 30, 307–311. [Google Scholar] [CrossRef]
- Plummer, P.; Behrman, A.L.; Duncan, P.W.; Spigel, P.; Saracino, D.; Martin, J.; Fox, E.; Thigpen, M.; Kautz, S.A. Effects of stroke severity and training duration on locomotor recovery after stroke: A pilot study. Neurorehabilit. Neural Repair 2007, 21, 137–151. [Google Scholar] [CrossRef]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Handelzalts, S.; Melzer, I.; Soroker, N. Analysis of brain lesion impact on balance and gait following stroke. Front. Hum. Neurosci. 2019, 149–157. [Google Scholar] [CrossRef]
| EGI (n = 14) | EGII (n = 13) | CG (n = 14) | p | |
|---|---|---|---|---|
| Gender (Male/Female) | 7/7 | 5/8 | 8/6 | 0.619 |
| Age (year) | 63.26 ± 12.4 | 68.01 ± 10.22 | 64.39 ± 11.55 | 0.708 |
| Height (cm) | 161.65 ± 13.72 | 154.09 ± 5.09 | 163.93 ± 9.27 | 0.178 |
| Weight (kg) | 63.32 ± 7.33 | 58.65 ± 9.21 | 63.65 ± 12.71 | 0.532 |
| On set (month) | 25.41 ± 25.32 | 23.30 ± 19.56 | 21.87 ± 19.32 | 0.689 |
| Affected side (Left/Right) | 6/8 | 7/6 | 9/5 | 0.522 |
| Etiology (Hemorrhage/Infarction) | 6/8 | 5/8 | 3/11 | 0.448 |
| EGI (n = 14) a | EGII (n = 13) b | CG (n = 14) c | Group × Time | Post-hoc | |||
|---|---|---|---|---|---|---|---|
| F | η2 | ||||||
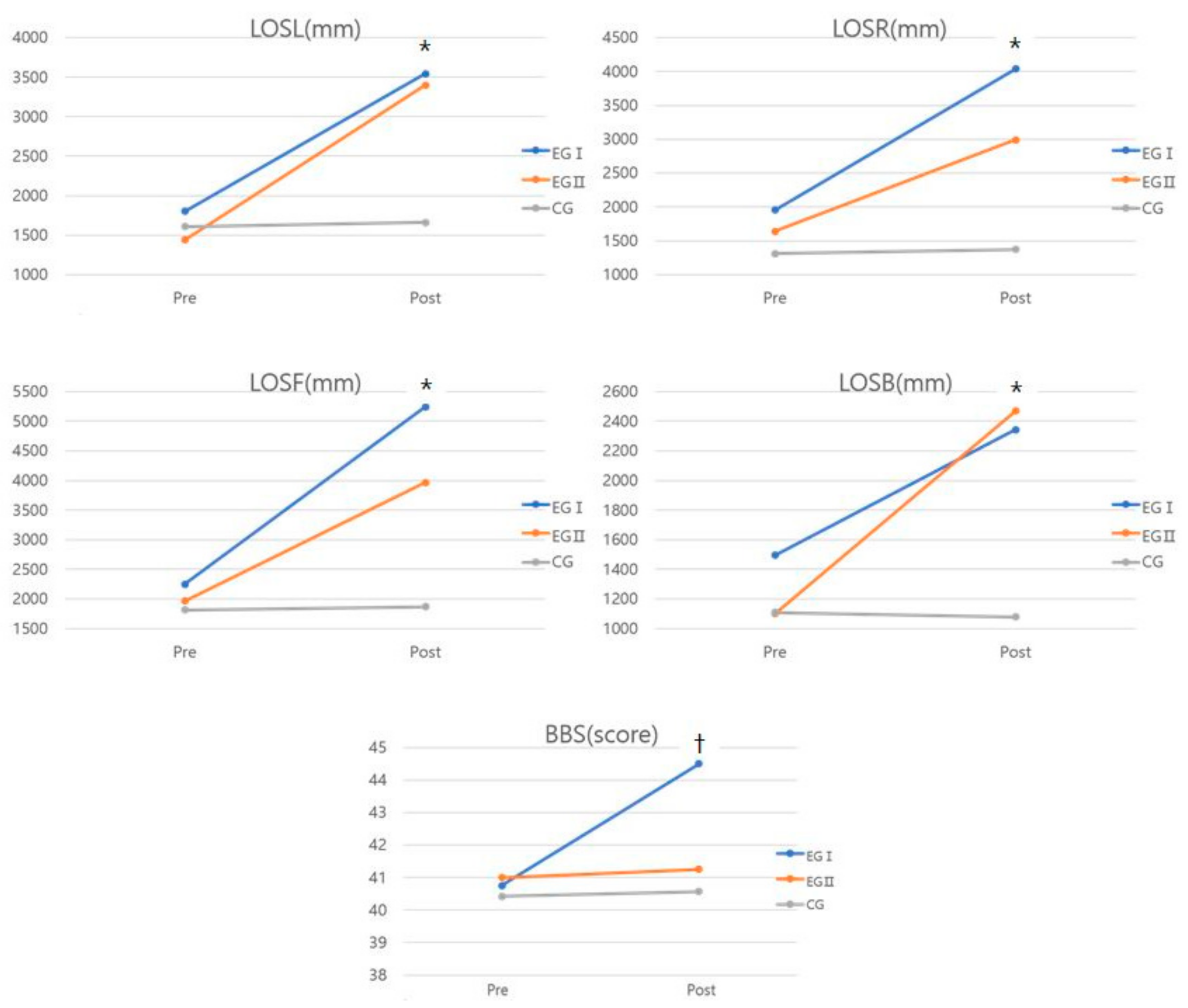
| LOSL (mm) | Pre | 1801.09 ± 969.98 (1240.75–2361.05) | 1439.31 ± 633.83 (879.20–1999.42) | 1608.69 ± 589.59 (1009.71–2207.67) | 11.103 * | 0.525 | a, b > c |
| Post | 3544.31 ± 1801.69 (2487.10–4601.52) | 3401.02 ± 1500.08 (2343.98–4458.06) | 1662.82 ± 660.78 (532.71–2792.93) | ||||
| p | 0.002 * | 0.001 * | 0.578 | ||||
| LOSR (mm) | Pre | 1955.29 ± 824.37 (1463.59–2446.99) | 1640.68 ± 707.59 (1149.02–2132.34) | 1310.03 ± 322.891 (784.35–1835.71) | 8.315 * | 0.455 | a, b > c |
| Post | 4040.52 ± 2042.62 (2954.04–5127.01) | 2989.59 ± 1386.23 (1903.17–4076.01) | 1372.25 ± 358.52 (210.54–2533.96) | ||||
| p | 0.003 * | 0.005 * | 0.055 | ||||
| LOSF (mm) | Pre | 2248.39 ± 976.38 (1647.50–2849.25) | 1962.57 ± 858.31 (1361.88–2563.62) | 1814.48 ± 490.77 (1172.07–2456.79) | 9.057 * | 0.475 | a, b > c |
| Post | 5244.41 ± 2769.67 (3809.13–6679.62) | 3967.52 ± 1709.42 (2532.25–5402.75) | 1867.87 ± 517.09 (333.51–3402.20) | ||||
| p | 0.004 * | 0.001 * | 0.293 | ||||
| LOSB (mm) | Pre | 1495.78 ± 830.27 (1045.51–1946.26) | 1099.53 ± 441.62 (649.11–1549.91) | 1108.31 ± 459.07 (626.81–1589.79) | 8.709 * | 0.467 | a, b > c |
| Post | 2342.29 ± 1165.36 (1580.38–3104.15) | 2469.38 ± 1236.35 (1707.54–3231.25) | 1078.16 ± 435.631 (263.67–1892.63) | ||||
| p | 0.004 * | 0.005 * | 0.475 | ||||
| BBS (score) | Pre | 40.76 ± 2.81 (38.10–43.41) | 41.08 ± 2.28 (38.61–43.89) | 40.42 ± 4.65 (37.74–43.41) | 20.433 * | 0.672 | a > b, c |
| Post | 44.53 ± 2.55 (42.09–46.91) | 41.27 ± 2.75 (38.61–43.39) | 40.58 ± 4.96 (37.88–42.98) | ||||
| p | 0.000 * | 0.749 | 0.604 | ||||
| EGI (n = 14) a | EGII (n = 13) b | CG (n = 14) c | Group × Time | Post-hoc | |||
|---|---|---|---|---|---|---|---|
| F | η2 | ||||||
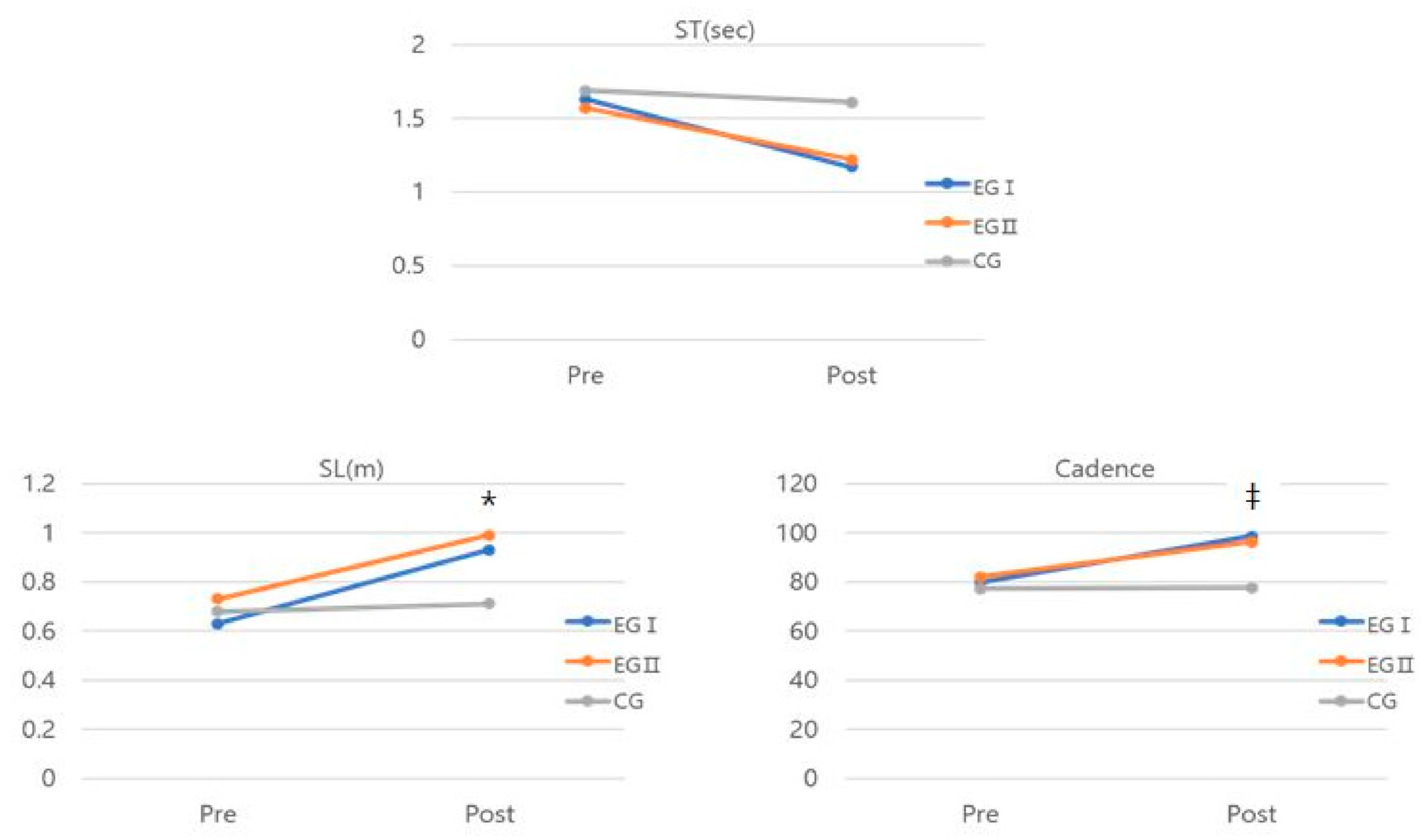
| ST (sec) | Pre | 1.64 ± 0.59 (1.27–2.01) | 1.57 ± 0.79 (1.22–1.94) | 1.69 ± 0.48 (1.31–2.08) | 2.967 | 0.230 | |
| Post | 1.16 ± 0.39 (0.95–1.41) | 1.22 ± 0.37 (1.00–1.44) | 1.61 ± 0.52 (1.39–1.85) | ||||
| p | 0.016 * | 0.014 * | 0.139 | ||||
| SL (m) | Pre | 0.63 ± 0.33 (0.48–0.78) | 0.73 ± 0.87 (0.59–0.89) | 0.68 ± 0.21 (0.55–0.87) | 17.608 * | 0.638 | a, b > c |
| Post | 0.93 ± 0.53 (0.80–1.07) | 0.99 ± 0.38 (0.86–1.13) | 0.71 ± 0.83 (0.55–0.83) | ||||
| p | 0.000 * | 0.003 * | 0.341 | ||||
| Cadence | Pre | 79.77 ± 18.65 (64.67–94.87) | 81.98 ± 23.01 (66.90–97.08) | 77.28 ± 20.65 (61.15–93.42) | 3.953 * | 0.286 | a > c |
| Post | 98.71 ± 11.45 (87.36–110.06) | 96.27 ± 5.01 (84.92–107.63) | 77.57 ± 8.17 (65.43–89.71) | ||||
| p | 0.011 * | 0.041 * | 0.705 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, S.-M.; Lee, D.-Y. Effects of Visual Cue Deprivation Balance Training with Head Control on Balance and Gait Function in Stroke Patients. Medicina 2022, 58, 629. https://doi.org/10.3390/medicina58050629
Nam S-M, Lee D-Y. Effects of Visual Cue Deprivation Balance Training with Head Control on Balance and Gait Function in Stroke Patients. Medicina. 2022; 58(5):629. https://doi.org/10.3390/medicina58050629
Chicago/Turabian StyleNam, Seung-Min, and Do-Youn Lee. 2022. "Effects of Visual Cue Deprivation Balance Training with Head Control on Balance and Gait Function in Stroke Patients" Medicina 58, no. 5: 629. https://doi.org/10.3390/medicina58050629
APA StyleNam, S.-M., & Lee, D.-Y. (2022). Effects of Visual Cue Deprivation Balance Training with Head Control on Balance and Gait Function in Stroke Patients. Medicina, 58(5), 629. https://doi.org/10.3390/medicina58050629






