Diabetes-Modifying Antirheumatic Drugs: The Roles of DMARDs as Glucose-Lowering Agents
Abstract
1. Introduction
2. Disease-Modifying Antirheumatic Drugs (DMARDs)
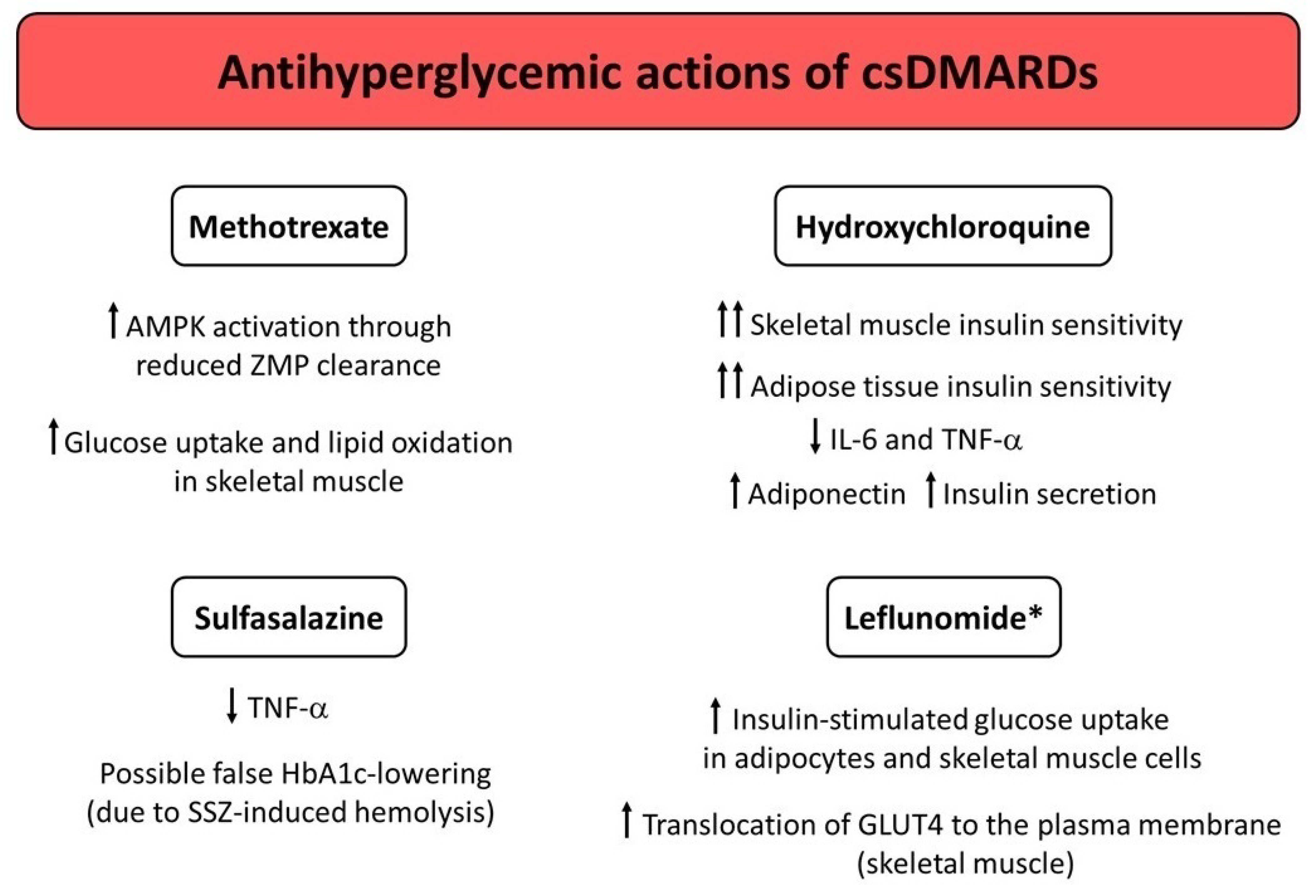
3. Conventional Synthetic DMARDs (csDMARDs)
3.1. Methotrexate (MTX)
3.2. Hydroxychloroquine (HCQ)
3.3. Sulfasalazine (SSZ)
3.4. Leflunomide (LEF)
4. Targeted Synthetic DMARDs (tsDMARDs)
4.1. Phosphodiesterase 4 (PDE4) Inhibitors
4.2. Janus Kinase (JAK) Inhibitors (JAKIs)
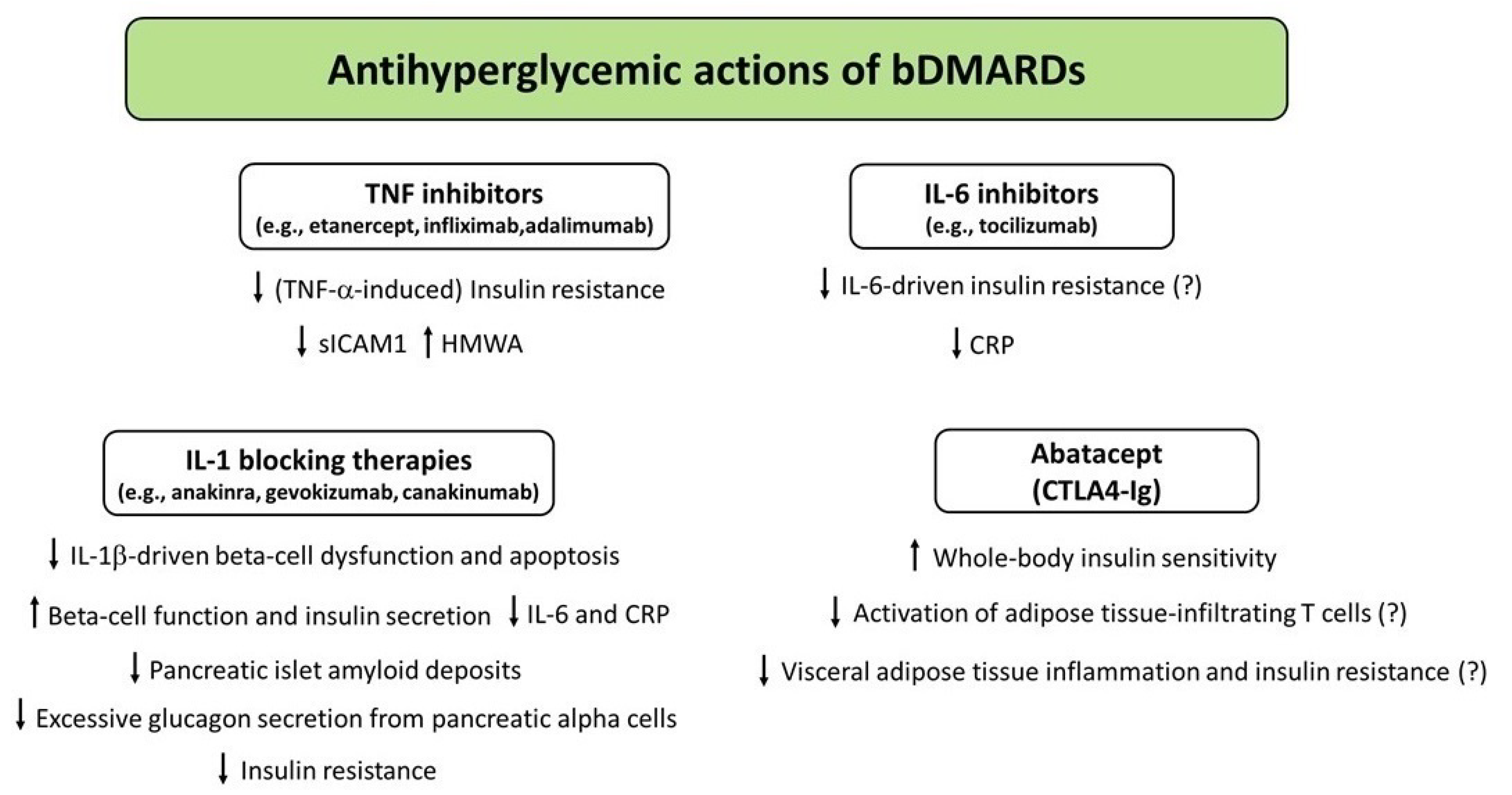
5. Biologic DMARDs (bDMARDs)
5.1. TNF Inhibitors
5.2. IL-1 Blocking Therapies
5.2.1. IL-1 Receptor Blockade in RA and T2D
5.2.2. IL-1β Pathway and CVD
5.3. IL-6 Inhibitors
5.4. Abatacept
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowler, J.M. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes Apr. 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Wright, A.K.; Kontopantelis, E.; Emsley, R.; Buchan, I.; Mamas, M.A.; Sattar, N.; Ashcroft, D.; Rutter, M.K. Cardiovascular Risk and Risk Factor Management in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2742–2753. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Shaw, L.C.; Grant, M.B. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol. 2012, 3, 170. [Google Scholar] [CrossRef]
- Donath, M.Y.; Dinarello, C.A.; Mandrup-Poulsen, T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019, 19, 734–746. [Google Scholar] [CrossRef]
- Nunemaker, C.S. Considerations for Defining Cytokine Dose, Duration, and Milieu That Are Appropriate for Modeling Chronic Low-Grade Inflammation in Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2846570. [Google Scholar] [CrossRef]
- Pickup, J.C.; Mattock, M.B.; Chusney, G.D.; Burt, D. NIDDM as a disease of the innate immune system: Association of Acute-Phase Reactants and Interleukin-6 with Metabolic Syndrome, X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F.H. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the Prospective Population-Based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef]
- Herder, C.; Illig, T.; Rathmann, W.; Martin, S.; Haastert, B.; Müller-Scholze, S.; Holle, R.; Thorand, B.; Koenig, W.; Wichmann, H.E.; et al. Inflammation and type 2 diabetes: Results from KORA Augsburg. Gesundheitswesen 2005, 67, S115–S121. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Brunner, E.J.; Rathmann, W.; Strassburger, K.; Tabák, A.G.; Schloot, N.C.; Witte, D.R. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: The Whitehall II Study. Diabetes Care 2009, 32, 421–423. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Liang, W.; Ye, D.D. The potential of adipokines as biomarkers and therapeutic agents for vascular complications in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2019, 48, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Böni-Schnetzler, M.; Meier, D.T. Islet inflammation in type 2 diabetes. Semin Immunopathol. 2019, 41, 501–513. [Google Scholar] [CrossRef]
- Eguchi, K.; Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef]
- Park, Y.M.; Myers, M.; Vieira-Potter, V.J. Adipose tissue inflammation and metabolic dysfunction: Role of Exercise. Mo. Med. 2014, 111, 65–72. [Google Scholar]
- Tam, L.S.; Tomlinson, B.; Chu, T.T.; Li, M.; Leung, Y.Y.; Kwok, L.W.; Li, T.K.; Yu, T.; Zhu, Y.-E.; Wong, K.-C.; et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—The role of inflammation. Rheumatology 2008, 47, 718–723. [Google Scholar] [CrossRef]
- Seriolo, B.; Ferrone, C.; Cutolo, M. Longterm anti-tumor necrosis factor-alpha treatment in patients with refractory rheumatoid arthritis: Relationship between Insulin Resistance and Disease Activity. J. Rheumatol. 2008, 35, 355–357. [Google Scholar] [PubMed]
- Ruscitti, P.; Ursini, F.; Cipriani, P.; Ciccia, F.; Liakouli, V.; Carubbi, F.; Guggino, G.; Berardicurti, O.; Grembiale, R.; Triolo, G.; et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: Results from a cross-sectional study. Medicine 2017, 96, e7896. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, P.I.; Karvounaris, S.A.; Boumpas, D.T. Metabolic syndrome in rheumatic diseases: Epidemiology, Pathophysiology, and Clinical Implications. Arthritis Res. Ther. 2008, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Ziade, N.; El Khoury, B.; Zoghbi, M.; Merheb, G.; Karam, G.A.; Mroue’, K.; Messaykeh, J. Prevalence and pattern of comorbidities in chronic rheumatic and musculoskeletal diseases: The Comord Study. Sci. Rep. 2020, 10, 7683. [Google Scholar] [CrossRef]
- Jiang, P.; Li, H.; Li, X. Diabetes mellitus risk factors in rheumatoid arthritis: A Systematic Review and Meta-Analysis. Clin. Exp. Rheumatol. 2015, 33, 115–121. [Google Scholar]
- Albrecht, K.; Luque Ramos, A.; Hoffmann, F.; Redeker, I.; Zink, A. High prevalence of diabetes in patients with rheumatoid arthritis: Results from a Questionnaire Survey Linked to Claims Data. Rheumatology 2018, 57, 329–336. [Google Scholar] [CrossRef]
- Dal Bello, G.; Gisondi, P.; Idolazzi, L.; Girolomoni, G. Psoriatic Arthritis and Diabetes Mellitus: A Narrative Review. Rheumatol. Ther. 2020, 7, 271–285. [Google Scholar] [CrossRef]
- Ruscitti, P.; Ursini, F.; Cipriani, P.; Liakouli, V.; Carubbi, F.; Berardicurti, O.; De Sarro, G.; Giacomelli, R. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: A 1-Year, Single-Centre, Longitudinal Study. PLoS ONE 2017, 12, e0181203. [Google Scholar] [CrossRef]
- Baker, J.F.; England, B.R.; George, M.; Cannon, G.; Sauer, B.; Ogdie, A.; Hamilton, B.C.; Hunter, C.; Duryee, M.J.; Thiele, G.; et al. Disease activity, cytokines, chemokines and the risk of incident diabetes in rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 566–572. [Google Scholar] [CrossRef]
- Ozen, G.; Pedro, S.; Holmqvist, M.E.; Avery, M.; Wolfe, F.; Michaud, K. Risk of diabetes mellitus associated with disease-modifying antirheumatic drugs and statins in rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 848–854. [Google Scholar] [CrossRef]
- Solomon, D.H.; Karlson, E.W.; Rimm, E.B.; Cannuscio, C.C.; Mandl, L.A.; Manson, J.E.; Stampfer, M.J.; Curhan, G.C. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003, 107, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- del Rincón, I.D.; Williams, K.; Stern, M.P.; Freeman, G.L.; Escalante, A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001, 44, 2737–2745. [Google Scholar] [CrossRef]
- Young, A.; Koduri, G.; Batley, M.; Kulinskaya, E.; Gough, A.; Norton, S.; Dixey, J. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology 2007, 46, 350–357. [Google Scholar] [CrossRef]
- Goodson, N.; Marks, J.; Lunt, M.; Symmons, D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann. Rheum. Dis. 2005, 64, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Zeller, C.B.; Appenzeller, S. Cardiovascular disease in systemic lupus erythematosus: The Role of Traditional and Lupus Related Risk Factors. Curr. Cardiol. Rev. 2008, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, E.; Baldini, C.; Schillaci, G.; Quartuccio, L.; Priori, R.; Carubbi, F.; Bini, V.; Alunno, A.; Bombardieri, S.; De Vita, S.; et al. Cardiovascular disease risk burden in primary Sjögren’s syndrome: Results of a Population-Based Multicentre Cohort Study. J. Intern. Med. 2015, 278, 185–192. [Google Scholar]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A Meta-Analysis of Observational Studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef]
- Meune, C.; Touzé, E.; Trinquart, L.; Allanore, Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: A Systematic Review and Meta-Analysis of Cohort Studies. Rheumatology 2009, 48, 1309–1313. [Google Scholar] [CrossRef]
- Lau, C.S.; Chia, F.; Harrison, A.; Hsieh, T.Y.; Jain, R.; Jung, S.M.; Kishimoto, M.; Kumar, A.; Leong, K.P.; Li, Z.; et al. APLAR rheumatoid arthritis treatment recommendations. Int. J. Rheum. Dis. 2015, 18, 685–713. [Google Scholar] [CrossRef]
- Bartels, C.M.; Buhr, K.A.; Goldberg, J.W.; Bell, C.L.; Visekruna, M.; Nekkanti, S.; Greenlee, R.T. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J. Rheumatol. 2014, 41, 680–687. [Google Scholar] [CrossRef]
- Goodson, N.J.; Wiles, N.J.; Lunt, M.; Barrett, E.M.; Silman, A.J.; Symmons, D.P. Mortality in early inflammatory polyarthritis: Cardiovascular Mortality is Increased in Seropositive Patients. Arthritis Rheum. 2002, 46, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Cano, G.; Del Rincón, I.; Pogosian, S.; Roldán, J.F.; Escalante, A. Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis Rheum. 2003, 48, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Maradit-Kremers, H.; Crowson, C.S.; Nicola, P.J.; Ballman, K.V.; Roger, V.L.; Jacobsen, S.J.; Gabriel, S.E. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A Population-Based Cohort Study. Arthritis Rheum. 2005, 52, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Sica, A.; Selmi, C. Frailty in Rheumatic Diseases. Front. Immunol. 2020, 11, 576134. [Google Scholar] [CrossRef]
- Del Rincón, I.; Williams, K.; Stern, M.P.; Freeman, G.L.; O’Leary, D.H.; Escalante, A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003, 48, 1833–1840. [Google Scholar] [CrossRef]
- Mäki-Petäjä, K.M.; Hall, F.C.; Booth, A.D.; Wallace, S.M.; Yasmin; Bearcroft, P.W.; Harish, S.; Furlong, A.; McEniery, C.M.; Brown, J.; et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006, 114, 1185–1192. [Google Scholar] [CrossRef]
- Kerekes, G.; Szekanecz, Z.; Dér, H.; Sándor, Z.; Lakos, G.; Muszbek, L.; Csipö, I.; Sipka, S.; Seres, I.; Paragh, G.; et al. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: A Multiparametric Analysis Using Imaging Techniques and Laboratory Markers of Inflammation and Autoimmunity. J. Rheumatol. 2008, 35, 398–406. [Google Scholar]
- Rho, Y.H.; Oeser, A.; Chung, C.P.; Milne, G.L.; Stein, C.M. Drugs Used in the Treatment of Rheumatoid Arthritis: Relationship between Current Use and Cardiovascular Risk Factors. Arch. Drug Inf. 2009, 2, 34–40. [Google Scholar] [CrossRef]
- Verma, A.K.; Bhatt, D.; Goyal, Y.; Dev, K.; Beg, M.M.A.; Alsahli, M.A.; Rahmani, A.H. Association of Rheumatoid Arthritis with Diabetic Comorbidity: Correlating Accelerated Insulin Resistance to Inflammatory Responses in Patients. J. Multidiscip. Healthc. 2021, 14, 809–820. [Google Scholar] [CrossRef]
- Velikova, T.V.; Kabakchieva, P.P.; Assyov, Y.S.; Georgiev, T. Targeting Inflammatory Cytokines to Improve Type 2 Diabetes. Control Biomed. Res. Int. 2021, 2021, 7297419. [Google Scholar] [CrossRef]
- Benjamin, O.; Bansal, P.; Goyal, A.; Lappin, S.L. Disease Modifying Anti-Rheumatic Drugs (DMARD); StatPearls: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507863/ (accessed on 16 March 2022).
- Smolen, J.S.; van der Heijde, D.; Machold, K.P.; Aletaha, D.; Landewé, R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 2014, 73, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Ercikan-Abali, E.; Waltham, M.; Schnieders, B.; Hochhauser, D.; Li, W.W.; Fan, J.; Gorlick, R.; Goker, E.; Bertino, J.R. Molecular mechanisms of resistance to antifolates, a review. Acta Biochim. Pol. 1995, 42, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Perdan-Pirkmajer, K.; Pirkmajer, S.; Thevis, M.; Thomas, A.; Praprotnik, S.; Hočevar, A.; Rotar, Ž.; Gašperšič, N.; Sodin-Šemrl, S.; Žibert, J.; et al. Methotrexate reduces HbA1c concentration but does not produce chronic accumulation of ZMP in patients with rheumatoid or psoriatic arthritis. Scand. J. Rheumatol. 2016, 45, 347–355. [Google Scholar] [CrossRef]
- Baghdadi, L.R. Effect of methotrexate use on the development of type 2 diabetes in rheumatoid arthritis patients: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0235637. [Google Scholar]
- Mantravadi, S.; George, M.; Brensinger, C.; Du, M.; Baker, J.F.; Ogdie, A. Impact of tumor necrosis factor inhibitors and methotrexate on diabetes mellitus among patients with inflammatory arthritis. BMC Rheumatol. 2020, 4, 39. [Google Scholar] [CrossRef]
- Sotoudehmanesh, R.; Anvari, B.; Akhlaghi, M.; Shahraeeni, S.; Kolahdoozan, S. Methotrexate hepatotoxicity in patients with rheumatoid arthritis. Middle East J. Dig. Dis. 2010, 2, 104–109. [Google Scholar]
- Baghdadi, L.R.; Woodman, R.J.; Shanahan, E.M.; Wiese, M.D.; Mangoni, A.A. Genetic polymorphism of the methotrexate transporter ABCG2, blood pressure and markers of arterial function in patients with rheumatoid arthritis: Repeated Cross-Sectional Study. Pharmgenom. Pers. Med. 2018, 11, 205–210. [Google Scholar] [CrossRef]
- Chan, E.S.; Cronstein, B.N. Mechanisms of action of methotrexate. Bull. Hosp. Jt. Dis. 2013, 71, S5–S8. [Google Scholar]
- Hardie, D.G. AMPK: A Target for Drugs and Natural Products with Effects on Both Diabetes and Cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A New Strategy for the Management of Metabolic Hepatic Disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; Goodyear, L.J. AMP-activated protein kinase and muscle glucose uptake. Acta Physiol. Scand. 2003, 178, 337–345. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Pirkmajer, S.; Kulkarni, S.S.; Tom, R.Z.; Ross, F.A.; Hawley, S.A.; Hardie, D.G.; Zierath, J.R.; Chibalin, A.V. Methotrexate promotes glucose uptake and lipid oxidation in skeletal muscle via AMPK activation. Diabetes 2015, 64, 360–369. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for Rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Lotteau, V.; Teyton, L.; Peleraux, A.; Nilsson, T.; Karlsson, L.; Schmid, S.; Quaranta, V.; Peterson, A.P. Intracellular transport of class II MHC molecules directed by invariant chain. Nature 1990, 348, 600–605. [Google Scholar] [CrossRef]
- Kuznik, A.; Bencina, M.; Svajger, U.; Jeras, M.; Rozman, B.; Jerala, R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011, 186, 4794–4804. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An Old Drug Against Today’s Diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- van den Borne, B.E.; Dijkmans, B.A.; de Rooij, H.H.; le Cessie, S.; Verweij, C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997, 24, 55–60. [Google Scholar] [PubMed]
- Wondafrash, D.Z.; Desalegn, T.Z.; Yimer, E.M.; Tsige, A.G.; Adamu, B.A.; Zewdie, K.A. Potential Effect of Hydroxychloroquine in Diabetes Mellitus: A Systematic Review on Preclinical and Clinical Trial Studies. J. Diabetes Res. 2020, 2020, 5214751. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Ricordi, C.; Fabbri, A. Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: Risks and Benefits at the Time of COVID-19 Pandemic. J. Diabetes 2020, 12, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Wasko, M.C.M.; Hubert, H.B.; Lingala, V.B.; Elliott, J.R.; Luggen, M.E.; Fries, J.F.; Ward, M.M. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 2007, 298, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Bili, A.; Sartorius, J.A.; Kirchner, H.L.; Morris, S.J.; Ledwich, L.J.; Antohe, J.L.; Dancea, S.; Newman, E.D.; Wasko, M.C.M. Hydroxychloroquine use and decreased risk of diabetes in rheumatoid arthritis patients. J. Clin. Rheumatol. 2011, 17, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Massarotti, E.; Garg, R.; Liu, J.; Canning, C.; Schneeweiss, S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 2011, 305, 2525–2531. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, D.Y.; Lin, C.C.; Chen, Y.M.; Lai, K.L.; Lin, C.H. Association between use of disease-modifying antirheumatic drugs and diabetes in patients with ankylosing spondylitis, rheumatoid arthritis, or psoriasis/psoriatic arthritis: A Nationwide, Population-Based Cohort Study of 84,989 Patients. Ther. Clin. Risk Manag. 2017, 13, 583–592. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Lin, C.-H.; Lan, T.-H.; Chen, H.-H.; Chang, S.-N.; Wang, J.-S.; Hung, W.-T.; Lan, J.-L.; Chen, D.-Y. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: A Population-Based Cohort Study. Rheumatology 2015, 54, 1244–1249. [Google Scholar] [CrossRef]
- Chen, T.H.; Lai, T.Y.; Wang, Y.H.; Chiou, J.Y.; Hung, Y.M.; Wei, J.C. Hydroxychloroquine was associated with reduced risk of new-onset diabetes mellitus in patients with Sjögren syndrome. QJM 2019, 112, 757–762. [Google Scholar] [CrossRef]
- Quatraro, A.; Consoli, G.; Magno, M.; Caretta, F.; Nardozza, A.; Ceriello, A.; Giugliano, D. Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus. A new job for an old drug? Ann. Intern. Med. 1990, 112, 678–681. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Thorpe, K.E.; Taylor, D.W.; Haynes, R.B. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas—A randomized trial. Diabetes Res. Clin. Pract. 2002, 55, 209–219. [Google Scholar] [CrossRef]
- Hsia, S.H.; Duran, P.; Lee, M.L.; Davidson, M.B. Randomized controlled trial comparing hydroxychloroquine with pioglitazone as third-line agents in type 2 diabetic patients failing metformin plus a sulfonylurea: A Pilot Study. Diabetes 2020, 12, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Rekedal, L.R.; Massarotti, E.; Garg, R.; Bhatia, R.; Gleeson, T.; Lu, B.; Solomon, D.H. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Care Res. 2010, 62, 3569–3573. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sánchez-García, A.; Linden-Torres, E. Effect of hydroxychloroquine on glucose control in patients with and without diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Eur. J. Clin. Pharmacol. 2021, 77, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Real-World Clinical Effectiveness and Tolerability of Hydroxychloroquine 400 Mg in Uncontrolled Type 2 Diabetes Subjects who are not Willing to Initiate Insulin Therapy (HYQ-Real-World Study). Curr. Diabetes Rev. 2019, 15, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Chandurkar, N.; Thomas, N.; Viswanathan, V.; Deshpande, A.; Gupta, O.; Shah, A.; Kakrani, A.; Bhandari, S.; Thulasidharan, N.; et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: A Double Blind, Randomized Comparison with Pioglitazone. Curr. Med. Res. Opin. 2014, 30, 1257–1266. [Google Scholar] [CrossRef]
- Purkait, I.; Pareek, A.; Panneerselvam, A.; Mukhopadhyay, M.K.; Kumar, S.; Chandratreya, S.A. 1189-P: Effectiveness of Hydroxychloroquine (HCQ) 400 mg in Uncontrolled T2D Patients on Dual Therapy of Metformin and Sulfonylurea: A Real-World Experience in India. Diabetes 2019, 68, 1189. [Google Scholar] [CrossRef]
- Das, A.K.; Kalra, S.; Tiwaskar, M.; Bajaj, S.; Seshadri, K.; Chowdhury, S.; Sahay, R.; Indurkar, S.; Unnikrishnan, A.G.; Phadke, U.; et al. Expert Group Consensus Opinion: Role of Anti-inflammatory Agents in the Management of Type-2 Diabetes (T2D). J. Assoc. Physicians India 2019, 67, 65–74. [Google Scholar]
- Chakravarti, H.N.; Nag, A. Efficacy and safety of hydroxychloroquine as add-on therapy in uncontrolled type 2 diabetes patients who were using two oral antidiabetic drugs. J. Endocrinol. Investig. 2021, 44, 481–492. [Google Scholar] [CrossRef]
- Wasko, M.C.M.; McClure, C.K.; Kelsey, S.F.; Huber, K.; Orchard, T.; Toledo, F.G.S. Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: A randomised trial. Diabetologia 2015, 58, 2336–2343. [Google Scholar] [CrossRef]
- Powrie, J.K.; Smith, G.D.; Shojaee-Moradie, F.; Sonksen, P.H.; Jones, R.H. Mode of action of chloroquine in patients with non-insulin-dependent diabetes mellitus. Am. J. Physiol. Metab. 1991, 260, E897–E904. [Google Scholar] [CrossRef] [PubMed]
- Toledo, F.G.S.; Miller, R.G.; Helbling, N.L.; Zhang, Y.; DeLany, J.P. The effects of hydroxychloroquine on insulin sensitivity, insulin clearance and inflammation in insulin-resistant adults: A Randomized Trial. Diabetes Obes. Metab. 2021, 23, 1252–1261. [Google Scholar] [CrossRef]
- Jorge, A.; Lu, N.; Choi, H.; Esdaile, J.M.; Lacaille, D.; Avina-Zubieta, J.A. Hydroxychloroquine Use and Cardiovascular Events Among Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis. Arthritis Care Res. 2021, 2021, 24850. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zhang, Y.; Kwong, J.S.-W.; Li, L.; Zhang, Y.; Xu, C.; Li, Q.; Sun, X.; Tian, H.; et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2018, 12, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Floris, A.; Piga, M.; Mangoni, A.A.; Bortoluzzi, A.; Erre, G.L.; Cauli, A. Protective Effects of Hydroxychloroquine against Accelerated Atherosclerosis in Systemic Lupus Erythematosus. Mediat. Inflamm. 2018, 2018, 3424136. [Google Scholar]
- Schmidt-Tanguy, A.; Voswinkel, J.; Henrion, D.; Subra, J.F.; Loufrani, L.; Rohmer, V.; Ifrah, N.; Belizna, C. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J. Thromb. Haemost. 2013, 11, 1927–1929. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zhou, Z.-C.; Niu, R.; Su, Y.-T.; Sun, Y.; Liu, H.-L.; Teng, J.-L.; Ye, J.-N.; Shi, H.; Yang, C.-D.; et al. Hydroxychloroquine Improves Obesity-Associated Insulin Resistance and Hepatic Steatosis by Regulating Lipid Metabolism. Front. Pharmacol. 2019, 10, 855. [Google Scholar] [CrossRef]
- La Montagna, G.; Cacciapuoti, F.; Buono, R.; Manzella, D.; Mennillo, G.A.; Arciello, A.; Valentini, G.; Paolisso, G. Insulin resistance is an independent risk factor for atherosclerosis in rheumatoid arthritis. Diabetes Vasc. Dis. Res. 2007, 4, 130–135. [Google Scholar] [CrossRef]
- Choi, J.; Fenando, A. Sulfasalazine; StatPearls: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557809/. (accessed on 16 March 2022).
- Wahl, C.; Liptay, S.; Adler, G.; Schmid, R.M. Sulfasalazine: A Potent and Specific Inhibitor of Nuclear Factor Kappa B. J. Clin. Investig. 1998, 101, 1163–1174. [Google Scholar] [CrossRef]
- Haas, R.M.; Li, P.; Chu, J.W. Glucose-Lowering Effects of Sulfasalazine in Type 2 Diabetes. Diabetes Care 2005, 28, 2238–2239. [Google Scholar] [CrossRef]
- Mitchell, K.; Mukhopadhyay, B. Drug-Induced Falsely Low A1C: Report of a Case Series From a Diabetes Clinic. Clin. Diabetes 2018, 36, 80–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tack, C.J.; Wetzels, J.F. Decreased HbA1c Levels Due to Sulfonamide-Induced Hemolysis in Two IDDM Patients. Diabetes Care 1996, 19, 775–776. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kesson, C.M.; Whitelaw, J.W.; Ireland, J.T. Drug-induced haemolysis and fast haemoglobin A1 in diabetes mellitus. BMJ 1979, 2, 1037–1038. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, Y.-C.; Wang, C.-S.; Wang, Y.-C.; Hsu, Y.-L.; Chuang, L.-M. Falsely decreased HbA1c in a type 2 diabetic patient treated with dapsone. J. Formos. Med. Assoc. 2012, 111, 109–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- N’Dow, S.M.S.; Donnelly, L.A.; Pearson, E.R.; Rena, G. In a cohort of individuals with type 2 diabetes using the drug sulfasalazine, HbA 1c lowering is associated with haematological changes. Diabet. Med. 2020, 38, e14463. [Google Scholar] [CrossRef]
- Padda, I.S.; Goyal, A. Leflunomide; StatPearls: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557799/ (accessed on 16 March 2022).
- Fox, R.I.; Herrmann, M.L.; Frangou, C.G.; Wahl, G.M.; Morris, R.E.; Strande, V.; Kirschbaum, B.J. Mechanism of Action for Leflunomide in Rheumatoid Arthritis. Clin. Immunol. 1999, 93, 198–208. [Google Scholar] [CrossRef]
- Doscas, M.E.; Williamson, A.J.; Usha, L.; Bogachkov, Y.; Rao, G.S.; Xiao, F.; Wang, Y.; Ruby, C.; Kaufman, H.; Zhou, J.; et al. Inhibition of p70 S6 Kinase (S6K1) Activity by A77 1726 and Its Effect on Cell Proliferation and Cell Cycle Progress. Neoplasia 2014, 16, 824–834. [Google Scholar] [CrossRef]
- Chen, J.; Sun, J.; Doscas, M.E.; Ye, J.; Williamson, A.J.; Li, Y.; Li, Y.; Prinz, R.A.; Xu, X. Control of hyperglycemia in male mice by leflunomide: Mechanisms of Action. J. Endocrinol. 2018, 237, 43–58. [Google Scholar] [CrossRef]
- Zerilli, T.; Ocheretyaner, E. Apremilast (Otezla): A New Oral Treatment for Adults With Psoriasis and Psoriatic Arthritis. Pharm. Ther. 2015, 40, 495–500. [Google Scholar]
- Schafer, P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef]
- Kumar, N.; Goldminz, A.M.; Kim, N.; Gottlieb, A.B. Phosphodiesterase 4-targeted treatments for autoimmune diseases. BMC Med. 2013, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Houslay, M.D.; Schafer, P.; Zhang, K.Y. Keynote review: Phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 2005, 10, 1503–1519. [Google Scholar] [CrossRef]
- Mazzilli, S.; Lanna, C.; Chiaramonte, C.; Cesaroni, G.M.; Zangrilli, A.; Palumbo, V.; Cosio, T.; Dattola, A.; Gaziano, R.; Galluzzo, M.; et al. Real life experience of apremilast in psoriasis and arthritis psoriatic patients: Preliminary Results on Metabolic Biomarkers. J. Dermatol. 2020, 47, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Korman, N.; Greggio, C.; Cirulli, J.; Chandran, V.; Khraishi, M.; Meht, N.N. Hemoglobin A1c and weight changes with apremilast in patients with psoriasis and psoriatic arthritis: Pooled Laboratory Analysis of the Phase 3 Esteem and Palace Trials. J. Am. Acad. Derm. 2018, 79, AB151. [Google Scholar]
- Lanna, C.; Cesaroni, G.M.; Mazzilli, S.; Bianchi, L.; Campione, E. Small Molecules, Big Promises: Improvement of Psoriasis Severity and Glucidic Markers with Apremilast: A Case Report. Diabetes Metab. Syndr. Obesity Targets Ther. 2019, 12, 2685–2688. [Google Scholar] [CrossRef]
- Puig, L.; Korman, N.; Greggio, C.; Cirulli, J.; Teng, L.; Chandran, V.; Khraishi, M.; Paris, M.; Mehta, N.N. Long-term hemoglobin A1c changes with apremilast in patients with psoriasis and psoriatic arthritis: Pooled Analysis of Phase 3 Esteem and Palace Trials and Phase 3b Liberate Trial. J. Am. Acad. Dermatol. 2019, 81, AB89. [Google Scholar]
- Wouters, E.F.M.; Bredenbroker, D.; Teichmann, P.; Brose, M.; Rabe, K.F.; Fabbri, L.M.; Göke, B. Effect of the Phosphodiesterase 4 Inhibitor Roflumilast on Glucose Metabolism in Patients with Treatment-Naive, Newly Diagnosed Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, E1720–E1725. [Google Scholar] [CrossRef]
- Heimann, E.; Jones, H.A.; Resjö, S.; Manganiello, V.C.; Stenson, L.; Degerman, E. Expression and Regulation of Cyclic Nucleotide Phosphodiesterases in Human and Rat Pancreatic Islets. PLoS ONE 2010, 5, e14191. [Google Scholar] [CrossRef]
- Pyne, N.; Furman, B.L. Cyclic nucleotide phosphodiesterases in pancreatic islets. Diabetologia 2003, 46, 1179–1189. [Google Scholar] [CrossRef]
- Muo, I.M.; MacDonald, S.D.; Madan, R.; Park, S.-J.; Gharib, A.M.; Martinez, P.E.; Walter, M.F.; Yang, S.B.; Rodante, J.A.; Courville, A.B.; et al. Early effects of roflumilast on insulin sensitivity in adults with prediabetes and overweight/obesity involve age-associated fat mass loss—Results of an exploratory study. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 743–759. [Google Scholar] [CrossRef]
- Armani, A.; Marzolla, V.; Rosano, G.M.; Fabbri, A.; Caprio, M. Phosphodiesterase type 5 (PDE5) in the adipocyte: A Novel Player in Fat Metabolism? Trends Endocrinol. Metab. 2011, 22, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Cada, D.J.; Demaris, K.; Levien, T.L.; Baker, D.E. Tofacitinib. Hosp. Pharm. 2013, 48, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Al-Salama, Z.T.; Scott, L.J. Baricitinib: A Review in Rheumatoid Arthritis. Drugs 2018, 78, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Dodington, D.W.; Desai, H.R.; Woo, M. JAK/STAT—Emerging Players in Metabolism. Trends Endocrinol. Metab. 2018, 29, 55–65. [Google Scholar] [CrossRef]
- Bako, H.Y.; Ibrahim, M.A.; Isah, M.S.; Ibrahim, S. Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci. 2019, 239, 117045. [Google Scholar] [CrossRef]
- Collotta, D.; Hull, W.; Mastrocola, R.; Chiazza, F.; Cento, A.S.; Murphy, C.; Verta, R.; Alves, G.F.; Gaudioso, G.; Fava, F.; et al. Baricitinib counteracts metaflammation, thus protecting against diet-induced metabolic abnormalities in mice. Mol. Metab. 2020, 39, 101009. [Google Scholar] [CrossRef]
- Marrero, M.B.; Banes-Berceli, A.K.; Stern, D.M.; Eaton, D. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am. J. Physiol. Physiol. 2006, 290, F762–F768. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Brosius, F.C.; Adler, S.G.; Kretzler, M.; Mehta, R.L.; Tumlin, J.A.; Tanaka, Y.; Handea, M.; Liu, J.; Slik, M.E.; et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a Phase 2 Randomized Controlled Clinical Trial. Nephrol. Dial. Transplant. 2018, 33, 1950–1959. [Google Scholar] [CrossRef]
- IIdriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-Function Relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-Mediated Inhibition of Insulin Receptor Tyrosine Kinase Activity in TNF-α- and Obesity-Induced Insulin Resistance. Science 1996, 271, 665–670. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; FitzGerald, O.; Helliwell, P.S.; Paul, C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is All Inflammation the Same? Semin. Arthritis Rheum. 2016, 46, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Coates, L.C.; Ash, Z.R.; Finzel, S.; Conaghan, P.G. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: Traditional Views, Novel Insights Gained from TNF Blockade, and Concepts for the Future. Arthritis Res. Ther. 2011, 13, S4. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Gravallese, E. Bone erosion in rheumatoid arthritis: Mechanisms, Diagnosis and Treatment. Nat. Rev. Rheumatol. 2012, 8, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.; Bansal, P.; Goyal, A. Tumor Necrosis Factor Inhibitors; StatPearls: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482425/ (accessed on 16 March 2022).
- Ma, X.; Xu, S. TNF inhibitor therapy for rheumatoid arthritis. Biomed. Rep. 2013, 1, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef]
- Kanety, H.; Feinstein, R.; Papa, M.Z.; Hemi, R.; Karasik, A. Tumor Necrosis Factor α-induced Phosphorylation of Insulin Receptor Substrate-1 (IRS-1). J. Biol. Chem. 1995, 270, 23780–23784. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Stagakis, I.; Bertsias, G.; Karvounaris, S.; Kavousanaki, M.; Virla, D.; Raptopoulou, A.; Kardassis, D.; Boumpas, D.T.; Sidiropoulos, P.I. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res. Ther. 2012, 14, R141. [Google Scholar] [CrossRef]
- Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Panoulas, V.F.; Nightingale, P.; Koutedakis, Y.; Kitas, G.D. Anti-tumour necrosis factor alpha therapy improves insulin sensitivity in normal-weight but not in obese patients with rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R160. [Google Scholar] [CrossRef]
- Oguz, F.M.; Oguz, A.; Uzunlulu, M. The effect of infliximab treatment on insulin resistance in patients with rheumatoid arthritis. Acta Clin. Belg. 2007, 62, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Méndez-García, L.A.; Trejo-Millán, F.; Martínez-Reyes, C.P.; Manjarrez-Reyna, A.N.; Esquivel-Velázquez, M.; Melendez-Mier, G.; Islas-Andrade, S.; Rojas-Bernabé, A.; Kzhyshkowska, J.; Escobedo, G. Infliximab ameliorates tumor necrosis factor-alpha-induced insulin resistance by attenuating PTP1B activation in 3T3L1 adipocytes in vitro. Scand. J. Immunol. 2018, 88, e12716. [Google Scholar] [CrossRef] [PubMed]
- Kiortsis, D.N.; Mavridis, A.K.; Vasakos, S.; Nikas, S.N.; Drosos, A.A. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 2005, 64, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Ganguli, M.; Cox, K.; Means, B.; Gerling, I.; Solomon, S.S. Does therapy with anti-TNF-alpha improve glucose tolerance and control in patients with type 2 diabetes? Diabetes Care 2011, 34, e121. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.; Zanni, M.; Johnsen, S.; Rasheed, S.; Makimura, H.; Lee, H.; Khor, V.K.; Ahima, R.S.; Grinspoon, S.K. TNF-α Antagonism with Etanercept Decreases Glucose and Increases the Proportion of High Molecular Weight Adiponectin in Obese Subjects with Features of the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E146–E150. [Google Scholar] [CrossRef]
- Bernstein, L.E.; Berry, J.; Kim, S.; Canavan, B.; Grinspoon, S.K. Effects of Etanercept in Patients With the Metabolic Syndrome. Arch. Intern. Med. 2006, 166, 902–908. [Google Scholar] [CrossRef]
- Martínez-Abundis, E.; Drateln, C.R.-V.; Hernández-Salazar, E.; González-Ortiz, M. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch. Dermatol. Res. 2007, 299, 461–465. [Google Scholar] [CrossRef]
- Dominguez, H.; Storgaard, H.; Rask-Madsen, C.; Hermann, T.S.; Ihlemann, N.; Nielsen, D.B.; Spohr, C.; Kober, L.; Vaag, A.A.; Torp-Pedersen, C. Metabolic and Vascular Effects of Tumor Necrosis Factor-α Blockade with Etanercept in Obese Patients with Type 2 Diabetes. J. Vasc. Res. 2005, 42, 517–525. [Google Scholar] [CrossRef]
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology 2015, 54, 2134–2144. [Google Scholar] [CrossRef]
- Goldbach-Mansky, R. Blocking Interleukin-1 in Rheumatic Diseases. Ann. N. Y. Acad. Sci. 2009, 1182, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Stassi, G.; De Maria, R.; Trucco, G.; Rudert, W.; Testi, R.; Galluzzo, A.; Giordano, C.; Trucco, M. Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J. Exp. Med. 1997, 186, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Cipriani, P.; Di Benedetto, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Ciccia, F.; Alvaro, S.; Triolo, G.; Giacomelli, R. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: A Possible Implication for Therapeutic Decision in these Patients. Clin. Exp. Immunol. 2015, 182, 35–44. [Google Scholar]
- Marzban, L.; Park, K.; Verchere, C.B. Islet amyloid polypeptide and type 2 diabetes. Exp. Gerontol. 2003, 38, 347–351. [Google Scholar] [CrossRef]
- Fernández, M.S. Human IAPP amyloidogenic properties and pancreatic β-cell death. Cell Calcium. 2014, 56, 416–427. [Google Scholar] [CrossRef]
- Masters, S.L.; Dunne, A.; Subramanian, S.L.; Hull, R.L.; Tannahill, G.M.; Sharp, F.A.; Becker, C. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 2010, 11, 897–904. [Google Scholar] [CrossRef]
- Park, Y.J.; Warnock, G.L.; Ao, Z.; Safikhan, N.; Meloche, M.; Asadi, A.; Kieffer, T.J.; Marzban, L. Dual role of interleukin-1β in islet amyloid formation and its β-cell toxicity: Implications for Type 2 Diabetes and Islet Transplantation. Diabetes Metab. 2017, 19, 682–694. [Google Scholar] [CrossRef]
- Böni-Schnetzler, M.; Boller, S.; Debray, S.; Bouzakri, K.; Meier, D.T.; Prazak, R.; Kerr-Conte, J.; Pattou, F.; Ehses, J.A.; Schuit, F.C.; et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 2009, 150, 5218–5229. [Google Scholar] [CrossRef]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Vølund, A.; Ehses, J.A.; Seifert, B.; Mandrup-poulsen, T.; Donath, M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, D.L.; Choi, K.M.; Baik, S.H.; Choi, D.S. Serum insulin, proinsulin and proinsulin/insulin ratio in type 2 diabetic patients: As an Index of Beta-Cell Function or Insulin Resistance. Korean J. Intern. Med. 2000, 15, 195–201. [Google Scholar] [CrossRef]
- Choi, C.S.; Kim, C.H.; Lee, W.J.; Park, J.Y.; Hong, S.K.; Lee, K.U. Elevated plasma proinsulin/insulin ratio is a marker of reduced insulin secretory capacity in healthy young men. Horm. Metab. Res. 1999, 31, 267–270. [Google Scholar] [CrossRef]
- van Asseldonk, E.J.; Stienstra, R.; Koenen, T.B.; Joosten, L.A.; Netea, M.G.; Tack, C.J. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 2011, 96, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- van Poppel, P.C.; van Asseldonk, E.J.; Holst, J.J.; Vilsbøll, T.; Netea, M.G.; Tack, C.J. The interleukin-1 receptor antagonist anakinra improves first-phase insulin secretion and insulinogenic index in subjects with impaired glucose tolerance. Diabetes Obes. Metab. 2014, 16, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Hensen, J.; Howard, C.P.; Walter, V.; Thuren, T. Impact of interleukin-1β antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: Results of Secondary Endpoints from a Randomized, Placebo-Controlled Trial. Diabetes Metab. 2013, 39, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Cavelti-Weder, C.; Babians-Brunner, A.; Keller, C.; Stahel, M.A.; Kurz-Levin, M.; Zayed, H.; Solinger, A.M.; Mandrup-Poulsen, T.; Dinarello, C.A.; Donath, M.Y. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care 2012, 35, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Kataria, Y.; Ellervik, C.; Mandrup-Poulsen, T. Treatment of type 2 diabetes by targeting interleukin-1: A Meta-Analysis of 2921 patients. Semin. Immunopathol. 2019, 41, 413–425. [Google Scholar] [CrossRef]
- Giacomelli, R.; Ruscitti, P.; Alvaro, S.; Ciccia, F.; Liakouli, V.; Di Benedetto, P.; Guggino, G.; Berardicurti, O.; Carubbi, F.; Triolo, G.; et al. IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: May We Kill Two Birds with One Stone? Expert Rev. Clin. Immunol. 2016, 12, 849–855. [Google Scholar] [CrossRef]
- Abramson, S.B.; Amin, A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology 2002, 41, 972–980. [Google Scholar] [CrossRef]
- Strand, V.; Kavanaugh, A.F. The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology 2004, 43, iii10–iii16. [Google Scholar] [CrossRef]
- Herder, C.; Dalmas, E.; Böni-Schnetzler, M.; Donath, M.Y. The IL-1 Pathway in Type 2 Diabetes and Cardiovascular Complications. Trends Endocrinol. Metab. 2015, 26, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Masedu, F.; Alvaro, S.; Airò, P.; Battafarano, N.; Cantarini, L.; Cantatore, F.P.; Carlino, G.; D’Abrosca, V.; Frassi, M.; et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A Multicentre, Open-Label, Randomised Controlled Trial. PLoS Med. 2019, 16, e1002901. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Berardicurti, O.; Cipriani, P.; Giacomelli, R. Group Ts. Benefits of anakinra versus TNF inhibitors in rheumatoid arthritis and type 2 diabetes: Long-Term Findings from Participants Furtherly Followed-Up in the Track Study, a Multicentre, Open-Label, Randomised, Controlled Trial. Clin. Exp. Rheumatol. 2021, 39, 403–406. [Google Scholar] [PubMed]
- Ruscitti, P.; Ursini, F.; Cipriani, P.; Greco, M.; Alvaro, S.; Vasiliki, L.; Di Benedetto, P.; Carubbi, F.; Berardicurti, O.; Gulletta, E.; et al. IL-1 inhibition improves insulin resistance and adipokines in rheumatoid arthritis patients with comorbid type 2 diabetes: An Observational Study. Medicine 2019, 98, e14587. [Google Scholar] [CrossRef]
- Unger, R.H.; Cherrington, A.D. Glucagonocentric restructuring of diabetes: A Pathophysiologic and Therapeutic Makeover. J. Clin. Investig. 2012, 122, 4–12. [Google Scholar] [CrossRef]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Everett, B.M.; Donath, M.Y.; Pradhan, A.D.; Thuren, T.; Pais, P.; Nicolau, J.; Glynn, R.J.; Libby, P.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J. Am. Coll. Cardiol. 2018, 71, 2392–2401. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, B.; Peraldi, P.; Filloux, C.; Chavey, C.; Freidinger, K.; Hilton, D.J.; Hotamisligil, G.S.; Van Obberghen, E. SOCS-3 Inhibits Insulin Signaling and Is Up-regulated in Response to Tumor Necrosis Factor-α in the Adipose Tissue of Obese Mice. J. Biol. Chem. 2001, 276, 47944–47949. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Morishima, A.; Hirano, T.; Hishitani, Y.; Hagihara, K.; Shima, Y.; Narazaki, M.; Tanaka, T. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab: Figure. Ann. Rheum. Dis. 2011, 70, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Kiyohara, C.; Kashiwado, Y.; Sawabe, T.; Nagano, S.; Kimoto, Y.; Ayano, M.; Mitoma, H.; Akahoshi, M.; Arinobu, Y.; et al. Effects of tumor necrosis factor inhibitors and tocilizumab on the glycosylated hemoglobin levels in patients with rheumatoid arthritis; an observational study. PLoS ONE 2018, 13, e0196368. [Google Scholar] [CrossRef]
- Kohase, M.; May, L.T.; Tamm, I.; Vilcek, J.; Sehgal, P.B. A cytokine network in human diploid fibroblasts: Interactions of Beta-Interferons, Tumor Necrosis Factor, Platelet-Derived Growth Factor, and Interleukin-1. Mol. Cell. Biol. 1987, 7, 273–280. [Google Scholar]
- Loppnow, H.; Libby, P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin. J. Clin. Investig. 1990, 85, 731–738. [Google Scholar] [CrossRef]
- Sironi, M.; Breviario, F.; Proserpio, P.; Biondi, A.; Vecchi, A.; Van Damme, J.; Dejana, E.; Mantovani, A. IL-1 stimulates IL-6 production in endothelial cells. J. Immunol. 1989, 142, 549–553. [Google Scholar]
- Tosato, G.; Jones, K.D. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood 1990, 75, 1305–1310. [Google Scholar] [CrossRef]
- Le, J.M.; Vilcek, J. Interleukin 6: A Multifunctional Cytokine Regulating Immune Reactions and the Acute Phase Protein Response. Lab. Investig. 1989, 61, 588–602. [Google Scholar]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Fabra, R.; Trullenque, R.; Heinrich, P.C. Acute-phase response of human hepatocytes: Regulation of Acute-Phase Protein Synthesis by Interleukin-6. Hepatology. 1990, 12, 1179–1186. [Google Scholar] [CrossRef]
- Blair, H.A.; Deeks, E.D. Abatacept: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Martínez Calatrava, M.J.; Castañeda, S. Abatacept mechanism of action: Concordance with its Clinical Profile. Reumatol. Clin. 2012, 8, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Mauro, D.; Naty, S.; Gagliardi, D.; Grembiale, R.D. Improvement in insulin resistance after short-term treatment with abatacept: Case Report and Short Review. Clin. Rheumatol. 2012, 31, 1401–1402. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Russo, E.; Letizia Hribal, M.; Mauro, D.; Savarino, F.; Bruno, C.; Tripolino, C.; Rubino, M.; Naty, S.; Grembiale, R.D. Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: An Observational Study. Medicine 2015, 94, e888. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta 2014, 1842, 446–462. [Google Scholar] [CrossRef]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.E.; Dragun, D.; Skurk, T.; et al. T-lymphocyte infiltration in visceral adipose tissue: A Primary Event in Adipose Tissue Inflammation and the Development of Obesity-Mediated Insulin Resistance. Arterioscler Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Pollack, R.M.; Donath, M.Y.; LeRoith, D.; Leibowitz, G. Anti-inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications. Diabetes Care 2016, 39, S244–S252. [Google Scholar] [CrossRef]
- Izumi, K.; Murata, O.; Higashida-Konishi, M.; Kaneko, Y.; Oshima, H.; Takeuchi, T. Steroid-Sparing Effect of Tocilizumab and Methotrexate in Patients with Polymyalgia Rheumatica: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 2948. [Google Scholar] [CrossRef]
- Hwang, J.L.; Weiss, R.E. Steroid-induced diabetes: A clinical and molecular approach to understanding and treatment. Diabetes/Metab. Res. Rev. 2014, 30, 96–102. [Google Scholar] [CrossRef]
- Ito, S.; Ogishima, H.; Kondo, Y.; Sugihara, M.; Hayashi, T.; Chino, Y.; Goto, D.; Matsumoto, I.; Sumida, T. Early diagnosis and treatment of steroid-induced diabetes mellitus in patients with rheumatoid arthritis and other connective tissue diseases. Mod. Rheumatol. 2014, 24, 52–59. [Google Scholar] [CrossRef]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, D.; Morand, E.F. It hasn’t gone away: The Problem of Glucocorticoid Use in Lupus Remains. Rheumatology 2017, 56, i114–i122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B. Eular recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A Data-Driven Cluster Analysis of Six Variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Anjana, R.M.; Baskar, V.; Nair, A.T.N.; Jebarani, S.; Siddiqui, M.K.; Pradeepa, R.; Unnikrishnan, R.; Palmer, C.; Pearson, E.; Mohan, V. Novel subgroups of type 2 diabetes and their association with microvascular outcomes in an Asian Indian population: A Data-Driven Cluster Analysis: The INSPIRED Study. BMJ Open Diabetes Res. Care 2020, 8, e001506. [Google Scholar] [CrossRef]
- Wright, L.A.; Hirsch, I.B. Metrics Beyond Hemoglobin A1C in Diabetes Management: Time in Range, Hypoglycemia, and Other Parameters. Diabetes Technol. Ther. 2017, 19, S16–S26. [Google Scholar] [CrossRef]
- Ajjan, R.A. How Can We Realize the Clinical Benefits of Continuous Glucose Monitoring? Diabetes Technol. Ther. 2017, 19, S27–S36. [Google Scholar] [CrossRef]
- Amigues, I.; Pearlman, A.H.; Patel, A.; Reid, P.; Robinson, P.C.; Sinha, R.; Kim, A.H.; Youngstein, T.; Jayatilleke, A.; Konig, M. Coronavirus disease 2019: Investigational Therapies in the Prevention and Treatment of Hyperinflammation. Expert Rev. Clin. Immunol. 2020, 16, 1185–1204. [Google Scholar] [CrossRef]
- Goodarzi, P.; Mahdavi, F.; Mirzaei, R.; Hasanvand, H.; Sholeh, M.; Zamani, F.; Sohrabi, M.; Tabibzadeh, A.; Jeda, A.S.; Niya, M.H.K.; et al. Coronavirus disease 2019 (COVID-19): Immunological Approaches and Emerging Pharmacologic Treatments. Int. Immunopharmacol. 2020, 88, 106885. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.M.; Fabbri, A.; Infante, M. Cytokine storm modulation in COVID-19: A Proposed Role for Vitamin D and DPP-4 Inhibitor Combination Therapy (VIDPP-4i). Immunotherapy 2021, 13, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Cauchois, R.; Koubi, M.; Delarbre, D.; Manet, C.; Carvelli, J.; Blasco, V.B.; Jean, R.; Fouche, L.; Bornet, C.; Pauly, V.; et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 18951–18953. [Google Scholar] [CrossRef]
- Pasin, L.; Cavalli, G.; Navalesi, P.; Sella, N.; Landoni, G.; Yavorovskiy, A.G.; Likhvantsev, V.V.; Zangrillo, A.; Dagna, L.; Monti, G. Anakinra for patients with COVID-19: A Meta-Analysis of Non-Randomized Cohort Studies. Eur. J. Intern. Med. 2021, 86, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Group, R.C. Tocilizumab in patients admitted to hospital with COVID-19 (Recovery): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2021, 397, 1637–1645. [Google Scholar]
- Hasan, M.J.; Rabbani, R.; Anam, A.M.; Huq, S.M.R.; Polash, M.M.I.; Nessa, S.S.T.; Bachar, S.C. Impact of high dose of baricitinib in severe COVID-19 pneumonia: A Prospective Cohort Study in Bangladesh. BMC Infect. Dis. 2021, 21, 427. [Google Scholar] [CrossRef]
- Saghir, S.A.M.; AlGabri, N.A.; Alagawany, M.M.; Attia, Y.A.; Alyileili, S.R.; Elnesr, S.S.; Shafi, M.E.; Al-Shargi, O.Y.; Al-Balagi, N.; Alwajeeh, A.S.; et al. Chloroquine and Hydroxychloroquine for the Prevention and Treatment of COVID-19: A Fiction, Hope or Hype? An Updated Review. Ther. Clin. Risk Manag. 2021, 17, 371–387. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Alejandro, R.; Caprio, M.; Fabbri, A. Hydroxychloroquine in the COVID-19 pandemic era: In Pursuit of a Rational Use for Prophylaxis of SARS-CoV-2 Infection. Expert Rev. Anti Infect. Ther. 2020, 19, 5–16. [Google Scholar] [CrossRef]
- Drucker, D.J. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr. Rev. 2020, 41, bnaa011. [Google Scholar] [CrossRef]
- Noor, F.M.; Islam, M.M. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J. Community Health 2020, 45, 1270–1282. [Google Scholar] [CrossRef]
- Paoli, A.; Gorini, S.; Caprio, M. The dark side of the spoon—Glucose, ketones and COVID-19: A Possible Role for Ketogenic Diet? J. Transl. Med. 2020, 18, 441. [Google Scholar] [CrossRef]
- Drucker, D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The End of the Beginning. Cell Metab. 2021, 33, 479–498. [Google Scholar] [CrossRef]
- Schreiber, K.; Hendricks, O. First data on COVID-19 morbidity and mortality in patients with rheumatic disease from South Korea. Lancet Rheumatol. 2021, 3, e673–e675. [Google Scholar] [CrossRef]
- Ahmed, S.; Gasparyan, A.Y.; Zimba, O. Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol. Int. 2021, 41, 243–256. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infante, M.; Padilla, N.; Alejandro, R.; Caprio, M.; Della-Morte, D.; Fabbri, A.; Ricordi, C. Diabetes-Modifying Antirheumatic Drugs: The Roles of DMARDs as Glucose-Lowering Agents. Medicina 2022, 58, 571. https://doi.org/10.3390/medicina58050571
Infante M, Padilla N, Alejandro R, Caprio M, Della-Morte D, Fabbri A, Ricordi C. Diabetes-Modifying Antirheumatic Drugs: The Roles of DMARDs as Glucose-Lowering Agents. Medicina. 2022; 58(5):571. https://doi.org/10.3390/medicina58050571
Chicago/Turabian StyleInfante, Marco, Nathalia Padilla, Rodolfo Alejandro, Massimiliano Caprio, David Della-Morte, Andrea Fabbri, and Camillo Ricordi. 2022. "Diabetes-Modifying Antirheumatic Drugs: The Roles of DMARDs as Glucose-Lowering Agents" Medicina 58, no. 5: 571. https://doi.org/10.3390/medicina58050571
APA StyleInfante, M., Padilla, N., Alejandro, R., Caprio, M., Della-Morte, D., Fabbri, A., & Ricordi, C. (2022). Diabetes-Modifying Antirheumatic Drugs: The Roles of DMARDs as Glucose-Lowering Agents. Medicina, 58(5), 571. https://doi.org/10.3390/medicina58050571










