Abstract
Metformin (MTF) occupies a major and fundamental position in the therapeutic management of type 2 diabetes mellitus (T2DM). Gender differences in some effects and actions of MTF have been reported. Women are usually prescribed lower MTF doses compared to men and report more gastrointestinal side effects. The incidence of cardiovascular events in women on MTF has been found to be lower to that of men on MTF. Despite some promising results with MTF regarding pregnancy rates in women with PCOS, the management of gestational diabetes, cancer prevention or adjunctive cancer treatment and COVID-19, most robust meta-analyses have yet to confirm such beneficial effects.
1. Introduction: Metformin—Gender Medicine
Metformin (MTF) occupies a major and fundamental position in the therapeutic management of type 2 diabetes mellitus (T2DM) [1,2,3]. Sex pertains to “the different biological and physiological characteristics of males and females, such as reproductive organs, chromosomes or hormones”, whereas gender pertains to “the socially constructed characteristics of women and men—such as norms, roles and relationships of and between groups of women and men", quoting the relevant definitions from the Council of Europe (https://www.coe.int/en/web/gender-matters/sex-and-gender, accessed on 7 March 2022). Gender medicine is the medical discipline that integrates any effect of sex and/or gender on the overall level of health (prevention, diagnosis and treatment/management of diseases), taking into account biological as well as social sex differences [4]. Its aim is to improve health for any gender. Gender medicine may be a neglected dimension of medicine. Research results are accumulating, pointing to sex/gender-related differences in prescribing, as well as in the pharmacokinetics, the pharmacodynamics, the efficacy and side effects of various medications. In this concise review we will attempt to present the impact of sex/gender on the therapeutics of MTF. For practical purposes, in the following text, we will have to refer to human studies with the terms “sex” and “gender” interchangeably, acknowledging that this may not be correct; we will refer to animal studies with “sex” only.
2. Pharmacokinetics, Pharmacodynamics and Metabolism of MTF
MTF is a weak base, and is very polar and extremely soluble in water [5]. It is absorbed from the small intestine, leading to a peak in concentration in one to two hours after oral intake. Its bioavailability is from 50% to 60% [6]. MTF is weakly bound to proteins. Its plasma half-life is estimated to be 1.5 to 5.0 h and it is practically unmetabolized after being distributed mainly in the liver, kidneys and intestine [7,8,9]. Excretion occurs via the kidneys, with a clearance of 933–1317 mL/min, involving glomerular filtration and tubular secretion [9]. The mechanisms of cellular action of MTF are still poorly understood. Various molecular responses are elicited by MTF and apparently some are influenced by sex hormones (Figure 1) [10,11,12,13]. The normoglycemic effect of MTF results mainly from a decrease in hepatic glucose production by inhibition of gluconeogenesis and by an action on glucose-6-phosphatase [2]. In addition to this action on the liver, which results mainly in a decrease in fasting blood sugar, MTF also potentiates the effect of insulin on muscle glucose uptake.
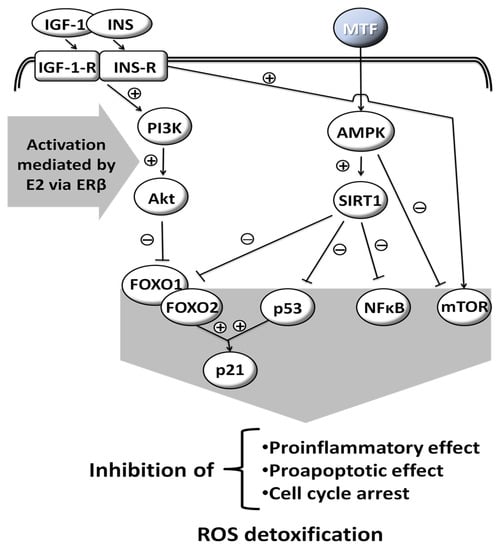
Figure 1.
Molecular responses that are elicited by MTF; some are influenced by sex hormones. Insulin-like Growth Factor-1, INS: Insulin, IGF-1-R: Insulin-like Growth Factor-1-Receptor, INS-R: Insulin Receptor, PI3K: Phosphoinositide 3-kinase, Akt: Serine/threonine protein kinase B, AMPK: AMP-activated protein kinase, SIRT1: Sirtuin 1, FOXO1: Forkhead box protein O1, FOXO2: Forkhead box protein O2, p53: Tumor protein p53, NFκB: Nuclear factor kappa-light-chain-enhancer of activated B cells, mTOR: Mammalian target of rapamycin, p21: Cyclin-dependent kinase inhibitor 1, ROS: Reactive oxygen species, E2: Estradiol, ERβ: Estrogen receptor β, (+): activation, (−): inhibition, drawn with data from [10,11,12,13].
3. Gender-Specific Use of MTF
3.1. MTF in Women with Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a common endocrine disease (with variable prevalence worldwide, ranging from 6% to 26%) [14], which is characterized by anovulation, clinical or biochemical hyperandrogenemia and polycystic ovary morphology [15]. Hyperinsulinemia as a result of tissue insulin resistance, is central to PCOS [16]. Insulin resistance is observed in 45–65% of patients with PCOS and is associated with excessive phosphorylation of insulin receptors. Hyperinsulinemia, impaired glucose tolerance, dyslipidemia and hypertension affect 40–45% of patients with PCOS [17]. Hyperinsulinemia adversely affects the hypothalamic-pituitary-ovarian axis, resulting in altered endocrine control, menstrual irregularity and infertility [15].
Many interventional studies have demonstrated the positive effect of MTF on both the reproductive as well as metabolic aspects of the syndrome [15]. However, the mechanisms, by which MTF exerts its effects in treating PCOS, are only partially understood. The rationale of MTF use in PCOS is based on the fact that hyperinsulinemia is the basis of the syndrome and adversely affects ovarian function. Insulin boosts 17OH-progesterone activity causing ovarian stroma hypertrophy, follicular atresia and anovulation [15]. Therefore, MTF directly or indirectly improves steroidogenesis (this has been noted by in vitro studies of granuloma cell response to follicle stimulating hormone (FSH) and insulin growth factor 1 (IGF-1) [18]).
In older studies, MTF use was associated with normalization of all insulin resistance parameters, in all PCOS patients grouped according to body mass index (BMI), and with degree of insulin resistance [19,20]. It was also shown that MTF led to improvement in BMI, diastolic blood pressure and high-density lipoprotein (HDL) cholesterol levels, decreasing the prevalence of metabolic syndrome in women with PCOS by 34.3% to 21.4%, in a dose-dependent fashion [21]. The usual MTF dosage described in the literature starts from 500 mg/day and reaches up to 850 mg three times daily for a duration of at least six weeks [22,23]. In 2013, the American Endocrine Society issued guidelines on the management of PCOS which included MTF as a treatment [24]. Specifically, MTF was recommended for women with PCOS and T2DM or insulin resistance, after failure of lifestyle change, diet and exercise, as a daily routine. It was not advised as a first line treatment of skin manifestations of PCOS (hair loss, acne), complications of the syndrome in pregnancy or for obesity [15]. MTF can also be given to women with menstrual disorders in which contraceptive treatment has failed or in women who wish to have children, as a second choice of treatment. There is no straightforward answer to whether all women with PCOS should undergo MTF treatment [25]. Proponents of MTF consider it a necessary drug for women with PCOS not only to prevent its long-term complications (in the context of insulin resistance) but also because MTF has been shown to improve all of the syndrome’s parameters. The first line treatment that includes diet and physical exercise is a time-consuming process that requires the compliance of women on a strict, long-term schedule, and relapse is very common.
Another element that comes to add to the beneficial action of MTF is in the treatment of adolescent girls with obesity and hyperandrogenemia. It seems that 50% of adolescent women with hyperandrogenemia have already developed resistance to progesterone-mediated gonadotropin-releasing hormone (GnRH) pulse suppression. The abnormal regulation of GnRH and luteinizing hormone (LH) secretion by the persistence of increased frequency of GnRH pulses is already present in adolescent girls with hyperandrogenemia before menarche [26]. Thus, the correction of androgen overproduction in PCOS is deemed to be necessary. The administration of MTF for at least three months improves glucose tolerance, lowers testosterone levels and plasma insulin and reduces adrenal overproduction of androgens in obese adolescents [27]. On the other hand, there are many who dispute the efficacy and benefits of MTF as a permanent treatment for women with PCOS. Insulin resistance is common but is not the main feature of PCOS [28,29]. Insulin sensitivity varies with the phenotype of the syndrome. MTF seems to improve the effects of hyperandrogenemia, though to a lesser degree than that of antiandrogens or oral contraceptive pills (OCP); therefore, MTF—according to some experts—cannot be used as a first-line treatment for these cases. Some also argue that insulin resistance cannot be reliably diagnosed from surrogate indicators. Insulin levels reflect its secretion as well as its clearance and do not adequately predict its action [30].
In the most recent and extensive relevant meta-analysis, treatment with MTF only was found to improve rates of live births when it was compared to no treatment (odds ratio [OR] = 1.59, 95% confidence interval [CI] = 1.00-2.51; 4 studies with total n = 435; the authors of the meta-analysis, however, found the quality of the data to be of low quality) [31]. Twenty-two percent to 40% of women experienced gastrointestinal side effects. Rates of ovulation and clinical pregnancy with MTF alone were higher compared to those with placebo (with OR of 2.64 [95% CI = 1.85-3.75] and 1.98 [95% CI = 1.47-2.65], respectively) [31]. In the same meta-analysis, no firm conclusions were formulated regarding MTF versus clomiphene citrate treatment, although the combination of both medications may increase ovulation rates and rates of clinical pregnancy [31]. The reproductive effects of MTF may be lower in obese compared to non-obese women. The same meta-analysis did not find an effect of MTF, when used alone, on the BMI of women with PCOS when compared to placebo; a probable reduction in BMI was noted for the combination of MTF with clomiphene citrate [31]. Furthermore, no firm conclusions were reached regarding MTF and testosterone, glucose or insulin levels in women with PCOS.
3.2. MTF in Women with Gestational Diabetes
When used in pregnancy, MTF does not show appreciable changes in its bioavailability, because any changes in the latter are being offset by changes in MTF’s clearance [32]. Although the mainstay of drug therapy for gestational diabetes (GDM) is insulin, MTF is used in pregnancy with increasing frequency, though it is still limited to a maximum of 5–6% of all medications for GDM, depending on the country in which it is assessed [33]. The use of MTF in pregnancy is considered to be safe overall, with favorable effects on maternal weight gain, the incidence of preeclampsia, the dosage in concomitant insulin administration, and the rate of fetal macrosomia and neonatal hypoglycemia; it may increase the rate of small-for-gestational-age infants [34,35,36,37,38,39,40]. Apparently, MTF lowers proinflammatory cytokines (tumor necrosis factor alpha (TNF-α), interleukin [IL]-1-alpha and IL-1-beta and IL-6) in serum, placenta and omental tissues [41]. Interestingly, although in a very critical assessment of metanalyses regarding MTF, most were deemed to be of low quality, the exception being those in obstetric/gynecological settings [42]. There are caveats in the use of MTF in pregnancy: it was found—in vitro, in human embryonic stem cells—to decrease the differentiation of pancreatic beta cells [43], and in mice to decrease or arrest early embryonic development [44].
4. Sex/Gender Differences Using MTF
4.1. Prescribing/Administering MTF for Diabetes
There may be a difference in T2DM prevalence by sex/gender [45]; this difference depends on the definition of diabetes per se: men tend to have higher fasting plasma glucose more often, whereas women tend to have abnormalities in the oral glucose tolerance test (both modalities are used in the diagnosis of the disease) [46]. Although MTF is widely prescribed worldwide as a first-choice medication for T2DM [47], few studies have seen the light regarding use by gender. Although women are more concerned than men about their body image [48], and MTF may show a modest effect on weight loss [49], women are usually prescribed lower MTF doses compared to men (and they report more gastrointestinal side effects) [50]. In a Dutch study, although women reported/experienced more gastrointestinal drug reactions during the first months of MTF treatment, the rate of the latter dropped in a way analogous to that in men [50]. Furthermore, after nine months of treatment, women were given a significantly lower daily dose. The caveat is that the researchers did not correct for body weight or body mass index (which is different between men and women) [51]. In one study, women in Austria were more apt to be prescribed MTF compared to men [52], whereas in another study, women in New Zealand were more apt to discontinue this treatment due to mainly gastrointestinal side effects [53]. In a recent report, with data from the Metformin and AcaRbose in Chinese as the initial Hypoglycemic treatment (MARCH) study, fasting and 2-h postprandial glucose were lower in women on MTF compared to men on MTF after 24 and 48 weeks of treatment. In the same study, an increase in insulin secretion was noted in women treated with MTF, whereas no appreciable change was noted in men on MTF [54]. Interestingly, in a fairly recent study, which was hampered by the small number of participating women in it (n = 13), the participants could not distinguish between MTF and placebo and did not report more gastrointestinal side effects compared to placebo [55]. While serum creatinine can be used as a criterion for use or non-use of MTF, a few years ago the focus shifted towards the glomerular filtration rate [measured (GFR) or calculated (eGFR)] as a criterion [56,57]. With such a criterion, more patients can be given MTF compared to those that can be given it using serum creatinine as a criterion, provided that eGFR is over 45 mL/min/1.73 m2. Additionally, patients already on MTF with eGFR in the 30–44 mL/min/1.73 m2 range can continue treatment at a maximum dose of 1 g/day. This change in criteria can have a profound effect on increasing the size of the target group of patients to be administered MTF, since gender differences in GFR/eGFR can lead to variance in prescription patterns [56]. Women have lower GFR compared to men and show a higher decline in this parameter of kidney function with advancing age. Thus, in this light, fewer women—particularly older ones—may be given MTF compared to men [56,58].
4.2. MTF and Vitamin B12/Homocysteine
The long-term administration of MTF significantly lowers vitamin B12 levels [59,60,61]. Vitamin B12 deficiency with MTF is rarely symptomatic; it is linked to a reduction in the intestinal absorption of cobalamin and can be reversed by the discontinuation of MTF or with oral B12 supplementation. Men have lower vitamin B12 levels compared to women [62]. In a study of patients with T2DM (without a control group), higher doses of MTF and male sex were factors associated with lower levels of vitamin B12 [63]. Nevertheless, the effect of MTF on B12 by sex/gender, to the best of our knowledge, has not been assessed adequately; this is of interest given the sex/gender differences presented above. Additionally, MTF may conditionally elevate or reduce homocysteine levels, which is critical for people with obesity [64,65].
4.3. MTF and Cardiovascular Disease
MTF is considered to be associated with some degree of cardioprotection [66,67]; the latter is apparently the net result of its beneficial actions on endothelial and smooth muscle cells, blood lipids and systemic chronic inflammation [68,69]. In experimental models, MTF was beneficial with regards to myocardial reperfusion, fibrosis and inflammation in post-experimental myocardial ischemia [70,71]. In an older, small scale, study, MTF was noted to have a favorable effect on cardiac metabolism in women (increasing myocardial glucose uptake and lowering fat metabolism), in contrast to having an unfavorable one (with opposite effects) in men [72]. In a study of 167,254 (46% women) patients with T2DM who were already using MTF and started newer anti-diabetic medications, the incidence of cardiovascular events, after a median observation time of 4.5 years, in women was lower compared to that of men (14.7 versus 16.7 per 1000-person-year) [73]. Nevertheless, a systematic review of MTF’s overall actions has not been conclusive regarding micro- and macrovascular complications in patients with T2DM [74].
4.4. MTF and Andrology/Urology
In small (and—apparently—underpowered) studies, the effects of MTF solely on men have been observed. This medication has been reported to be of benefit in non-diabetic men with erectile dysfunction who had not responded to sildenafil [75]. The mechanisms are obscure: they may be direct, via endothelium-dependent vasodilatation or attenuation of sympathetic nerve activity, and indirect, via MTF’s effect on blood pressure [75]. Indirect proof of the low power of studies is that erectile dysfunction, low sex drive and low testosterone (total, free and bioavailable) have also been attributed to MTF use in men with T2DM [76]. Furthermore, the use of MTF for T2DM, in men with prostate cancer, has been associated with lower prostate-specific antigen levels and improved survival [75,77,78,79].
4.5. Musculoskeletal Effects of MTF
From in vitro studies, a role for MTF has been proposed in the stimulation of osteogenesis; in vivo studies are less conclusive [80]. Furthermore, MTF activates adenosine monophosphate-activated protein kinase (AMPK) signaling pathways. The activation of AMPK has been implicated in muscle repair [80]. Thus, it is not surprising that patients using MTF report less musculoskeletal pain vis-à-vis patients not on MTF [81]. The beneficial musculoskeletal effects of MTF were recently found to be more pronounced in women compared to men [81].
4.6. MTF and Experimentally-Induced Neurological Disease
An interesting dimorphism has been observed in mice regarding experimentally induced neuropathic pain and spinal cord microglial activation. MTF was shown to prevent and reverse neuropathic pain and spinal cord microglial activation only in male mice [82]. The researchers presume that the known activation of AMPK may be implicated, although no firm etiology for the sex difference in observations has been formulated. On the other hand, in another experimental study of brain injury in mice, MTF was beneficial for cognitive recovery in females but not males [83], pointing to a crucial relevant role for estradiol/testosterone [83].
4.7. MTF and Aging/Life Span (Experimental)
Dimorphic sex responses to MTF regarding life span have been described: chronic administration of MTF extended the lifespan of female mice and curtailed the lifespan of male mice [84]. Yet, more recent studies show that the positive effect of MTF on longevity is more prominent in male mice [85]. In the Mexican fruit fly the effect of MTF on longevity is dose-dependent, and is beneficial in higher doses for females and in lower doses for males [86].
4.8. MTF and Cancer
In older and newer studies, MTF in subjects with T2DM (in the older studies at low doses of 500 mg/day or less) was shown to be more beneficial vis-à-vis the incidence of colorectal cancer in women compared to men [87,88]. In men, MTF use may lower the risk of prostate cancer, but the effect—if any—is apparently slight and statistically non-significant [89]. In a Lithuanian cohort, the lowest risk for endometrial cancer was observed in diabetic women who used only MTF (with a standardized incidence ratio [SIR] of 1.69 and 95% confidence interval [CI] of 1.49 to 1.92) [90]. MTF was found to lower the markers of proliferation in endometrial cancer cells [91,92]. Regarding the treatment of endometrial hyperplasia (considered to be a precancerous entity) or of endometrial cancer with MTF, either alone or in combination with megestgrol/medroxyprogesterone acetate, there were some promising results stemming from small studies [93,94]. In an older meta-analysis, a beneficial effect of MTF on overall mortality in women with endometrial cancer was noted [95]. Nevertheless, in another meta-analysis, no beneficial effect of MTF was found regarding the progression of endometrial hyperplasia to cancer, histology, or rates of hysterectomy [96]. MTF is considered to lower the risk for breast cancer in subjects with diabetes [97]. Results of trials aiming at the prevention of breast cancer with MTF are pending [98]. A higher cumulative MTF dose decreases kidney cancer risk in T2DM patients [99]. An analysis of MTF by cumulative dose showed significantly lower mortality risk in the highest cumulative dose category (with hazard ratio [HR] of 0.76 and 95% CI of 0.58 to 0.99) [100].
4.9. MTF and the Microbiome
Subtle differences have been reported in the gut microbiome between the male and female offspring of MTF-treated mice [101], as well as after MTF treatment in adult mice [102]. Before treatment with MTF and a high-fat diet (HFD), female mice had a preponderance of Lactobacillus species, whereas male mice had a preponderance of Proteobacteria species. After ten weeks of HFD and MTF, the bacterial species were different in males and females: a more pronounced increase in Bacteroides was noted in female mice compared to male ones [102]. In this respect, Lee et al. [103] suggested that gut microbiota could be affected by hormone levels, subsequently influencing glucose and lipid metabolism [104]; one study demonstrated that progesterone promotes the growth of oral Bacteroides species [105]. Although various studies have demonstrated a positive relationship between abundance in Bacteroides species and the therapeutic effect of MTF, future studies should consider the influence of sex on the effect of hormones on Bacteroides [106]. Unfortunately, in a very recent human trial of MTF administration, which led to tangible changes in the participants’ gut microbiome, the authors give no details vis-à-vis sex/gender [107].
4.10. MTF and COVID-19
Currently, there is a global effort to fight and win against the new severe acute respiratory syndrome coronvirus-2 (SARS-CoV-2) pandemic and its related coronavirus disease 2019 (COVID-19); proper management of T2DM is of even greater importance, since the presence of diabetes is associated with the most severe forms of COVID-19 and related mortality [108,109], and glycemic control is crucial [110,111]. In addition, a significant increase of cardiometabolic complications has been reported in many geographical areas, highlighting the need of a comprehensive and multidisciplinary approach to this terrible pandemic [112,113]. Insights and lessons from this experience can guide us to better manage cardiometabolic risk and overcoming current challenges [114,115]. In this context, the action and the effects of distinct antidiabetic drugs, including MTF, have been extensively investigated over the last two years [116,117], as well as the impact of genetics, comorbidities and inflammation on gender differences in COVID-19 outcomes [118].
There are many studies investigating MTF and COVID-19, and in particular mortality from this disease; the studies point to a beneficial (lowering) effect on mortality, but most did not report results by sex/gender of MTF users [119,120,121,122]. A large-scale study of mortality attributed to COVID-19 vis-à-vis MTF therapy [123] used anonymized data of patients with T2DM and/or obesity from a healthcare provider in the USA. In this study, the researchers compared a cohort of 3923 patients with COVID-19 not on MTF (55% women) to 2333 patients with COVID-19 on MTF (48% women). From the subgroups analyses it was found that women treated with MTF had a lower OR regarding mortality of 0.79 (95% CI: 0.64-0.98). The authors acknowledge that in their data no information about adherence to treatment with MTF was available. Furthermore, they also acknowledge that men are at higher risk of dying from COVID-19 and that men treated with MTF did not show any advantage in survival. An analysis of 328 patients’ data from China showed that MTF use was associated with a lower incidence of acute respiratory distress syndrome (ARDS) in women, whereas such an association was not been observed in men [124]. The authors speculated that the observed beneficial effect of MTF on women may have been the combined result of female sex and that MTF provided protection against the production of proinflammatory cytokines such as IL-6, IL-10 or TNF-α, which are known to be produced in abundance in COVID-19 [124]. In a study of an ex vivo animal model, MTF was shown to enhance the integrity of the pulmonary endothelial barrier [125]. The proposed mechanism involves the activation of AMP-activated protein kinase α1 (AMPK-α1, which in turn induces the activation of myosin light chain 2 (MLC2) and the deactivation of cofilin (a binding protein that regulates actin filament dynamics and depolymerization), supporting endothelial integrity [125]. A research group from the United States conducted a retrospective electronic health record data analysis of 25,326 subjects and reached contradictory results [126]. They found that the OR of dying remained significantly lower in male subjects on MTF (OR 0.28; 95% CI 0.09–0.88; p = 0.029) [126]. Experts have put forth many theories to explain why mortality rates in men were more than two-fold higher than in women. These theoretical assumptions include the different plasma concentrations of sex steroids, the differences in adipose tissue distribution between men and women, differences in the levels of circulating pro-inflammatory cytokines, and the known differences in the innate and adaptive immune responses to viral infections between sexes [126,127,128].
5. Conclusions
There are sex/gender differences with regards to glucose metabolism and the appearance of diabetes [129,130]. Pharmacogenetic studies have provided explanations—in part—for the variability and effectiveness in lowering glycemia with MTF [131]. To the best of our knowledge, sex/gender-wise, no such differences in the relevant genetic background have been shown to date. This may be a domain for future studies. Sex/gender differences in some effects and actions of MTF have been reported. Women may be prescribed lower MTF doses compared to men and report more gastrointestinal side effects. The incidence of cardiovascular events in women on MTF has been found to be lower to that of men on MTF. Despite some promising results with MTF regarding pregnancy rates in women with PCOS, for the management of gestational diabetes, cancer prevention or adjunctive cancer treatment and COVID-19, data from the most robust meta-analyses of clinical studies have yet to confirm such beneficial effects [35,38,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146]. A caveat is that any extrapolation of benefit to humans from animal or in vitro studies has to take into account the level of MTF concentration attained; the latter may differ considerably and be lower in humans [147]. Vitamin B12 deficiency with long-term administration of MTF is tangible and needs to be tackled. Inconsistencies in the studies have been noted and the field is open for new research before implementation in clinical practice. Any beneficial effect of MTF—other than on glycemia in patients with T2DM—should be scrutinized in the absence of T2DM.
Author Contributions
Conceptualization, I.I., M.R., L.Z.; methodology, I.I., L.Z.; writing—original draft preparation, I.I., M.R., L.Z.; writing—review and editing M.R., L.Z.; visualization, I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
I Ilias wishes to thank Alexandra-Stamatina Ilia for her assistance in the preparation of this article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Kaneto, H.; Kimura, T.; Obata, A.; Shimoda, M.; Kaku, K. Multifaceted Mechanisms of Action of Metformin Which Have Been Unraveled One after Another in the Long History. Int. J. Mol. Sci. 2021, 22, 2596. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, E.M.; Rueda, J.J.; Samson, S.L.; Hyman, D.J. Reducing the Burden of Diabetes Treatment: A Review of Low-cost Oral Hypoglycemic Medications. Curr. Diabetes Rev. 2020, 16, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V. Why Do We Need Gender Medicine? In Sex and Gender Aspects in Clinical Medicine; Oertelt-Prigione, S., Regitz-Zagrosek, V., Eds.; Springer: London, UK, 2012; pp. 1–4. [Google Scholar]
- Yendapally, R.; Sikazwe, D.; Kim, S.S.; Ramsinghani, S.; Fraser-Spears, R.; Witte, A.P.; La-Viola, B. A review of phenformin, metformin, and imeglimin. Drug Dev. Res. 2020, 81, 390–401. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Buse, J.B.; Kim, T.; Burns, C.; Skare, S.; Baron, A.; Fineman, M. Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial GLP-1 and PYY: Results from two randomised trials. Diabetologia 2016, 59, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Sundelin, E.; Jensen, J.B.; Jakobsen, S.; Gormsen, L.C.; Jessen, N. Metformin Biodistribution: A Key to Mechanisms of Action? J. Clin. Endocrinol. Metab. 2020, 105, 3374–3383. [Google Scholar] [CrossRef]
- Glossmann, H.H.; Lutz, O.M.D. Pharmacology of metformin—An update. Eur. J. Pharmacol. 2019, 865, 172782. [Google Scholar] [CrossRef]
- Mariano, F.; Biancone, L. Metformin, chronic nephropathy and lactic acidosis: A multi-faceted issue for the nephrologist. J. Nephrol. 2021, 34, 1127–1135. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.; Feng, Q.; Nie, C.; Li, T. Clinical perspectives and concerns of metformin as an anti-aging drug. Aging Med. 2020, 3, 266–275. [Google Scholar] [CrossRef]
- Hampsch, R.A.; Wells, J.D.; Traphagen, N.A.; McCleery, C.F.; Fields, J.L.; Shee, K.; Dillon, L.M.; Pooler, D.B.; Lewis, L.D.; Demidenko, E.; et al. AMPK Activation by Metformin Promotes Survival of Dormant ER(+) Breast Cancer Cells. Clin Cancer Res 2020, 26, 3707–3719. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Darko, K.O.; Tao, T.; Huang, Y.; Su, Q.; He, C.; Yin, T.; Liu, Z.; Yang, X. Combination of metformin with chemotherapeutic drugs via different molecular mechanisms. Cancer Treat. Rev. 2017, 54, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Hong, L.; Luo, C.; Li, Z.; Zhu, Y.; Huang, T.; Zhang, Y.; Yuan, H.; Hu, Y.; Wen, T.; et al. Metformin inhibits estrogen-dependent endometrial cancer cell growth by activating the AMPK-FOXO1 signal pathway. Cancer Sci. 2016, 107, 1806–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.; Broughton, K.S.; LeMieux, M.J. Cross-sectional Study on the Knowledge and Prevalence of PCOS at a Multiethnic University. Prog. Prev. Med. 2020. [Google Scholar] [CrossRef]
- Bulsara, J.; Patel, P.; Soni, A.; Acharya, S. A review: Brief insight into Polycystic Ovarian syndrome. Endocr. Metab. Sci. 2021, 3, 100085. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, K.; Naeem, M.; Rahimnajjad, N.A. Metformin: The hidden chronicles of a magic drug. Eur. J. Intern. Med. 2013, 24, 20–26. [Google Scholar] [CrossRef]
- Zhou, J.; Kumar, T.R.; Matzuk, M.M.; Bondy, C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol. Endocrinol. 1997, 11, 1924–1933. [Google Scholar] [CrossRef]
- Yasmin, E.; Glanville, J.; Barth, J.; Balen, A.H. Effect of dose escalation of metformin on clinical features, insulin sensitivity and androgen profile in polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 156, 67–71. [Google Scholar] [CrossRef]
- Tan, S.; Hahn, S.; Benson, S.; Dietz, T.; Lahner, H.; Moeller, L.C.; Schmidt, M.; Elsenbruch, S.; Kimmig, R.; Mann, K.; et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur. J. Endocrinol. 2007, 157, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Cheang, K.I.; Huszar, J.M.; Best, A.M.; Sharma, S.; Essah, P.A.; Nestler, J.E. Long-term effect of metformin on metabolic parameters in the polycystic ovary syndrome. Diab. Vasc. Dis. Res. 2009, 6, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Conlon, J.L.; Malcolm, S.; Monaghan, M. Diagnosis and treatment of polycystic ovary syndrome in adolescents. JAAPA 2021, 34, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Duleba, A.J. Medical management of metabolic dysfunction in PCOS. Steroids 2012, 77, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.C.; Dunaif, A. Should all women with PCOS be treated for insulin resistance? Fertil. Steril. 2012, 97, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apter, D.; Bützow, T.; Laughlin, G.A.; Yen, S.S. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: Relevance to the developmental phase of polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 1994, 79, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Arslanian, S.A.; Lewy, V.; Danadian, K.; Saad, R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: Amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J. Clin. Endocrinol. Metab. 2002, 87, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Wass, J.A.; McCarthy, M.I.; Franks, S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: Implications for the management of polycystic ovary syndrome. Clin. Endocrinol. 2007, 66, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, V.P.; Carmina, E.; Lobo, R.A. Not all women diagnosed with PCOS share the same cardiovascular risk profiles. Fertil. Steril. 2010, 94, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Hücking, K.; Watanabe, R.M.; Stefanovski, D.; Bergman, R.N. OGTT-derived measures of insulin sensitivity are confounded by factors other than insulin sensitivity itself. Obesity 2008, 16, 1938–1945. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, A.; Morley, L.C.; Tang, T.; Norman, R.J.; Balen, A.H. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 12, CD013505. [Google Scholar] [CrossRef]
- Liao, M.Z.; Flood Nichols, S.K.; Ahmed, M.; Clark, S.; Hankins, G.D.; Caritis, S.; Venkataramanan, R.; Haas, D.; Quinney, S.K.; Haneline, L.S.; et al. Effects of Pregnancy on the Pharmacokinetics of Metformin. Drug Metab. Dispos. 2020, 48, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Cesta, C.E.; Cohen, J.M.; Pazzagli, L.; Bateman, B.T.; Bröms, G.; Einarsdóttir, K.; Furu, K.; Havard, A.; Heino, A.; Hernandez-Diaz, S.; et al. Antidiabetic medication use during pregnancy: An international utilization study. BMJ Open Diabetes Res. Care 2019, 7, e000759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, O.A.H.; Syed, A.; Mohamed, A.M.; Chivese, T.; Clark, J.; Furuya-Kanamori, L.; Xu, C.; Toft, E.; Bashir, M.; Abou-Samra, A.B.; et al. Metformin is comparable to insulin for pharmacotherapy in gestational diabetes mellitus: A network meta-analysis evaluating 6046 women. Pharmacol. Res. 2021, 167, 105546. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Ozanne, S.E.; Aiken, C.E. Impact of metformin treatment during pregnancy on maternal outcomes: A systematic review/meta-analysis. Sci. Rep. 2021, 11, 9240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, W.; Chen, H.; Chen, Q. Comparison of Insulin, Metformin, and Glyburide on Perinatal Complications of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2021, 86, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Bidhendi Yarandi, R.; Amiri, M.; Ramezani Tehrani, F.; Behboudi-Gandevani, S. Effectiveness of antidiabetic agents for treatment of gestational diabetes: A methodological quality assessment of meta-analyses and network meta-analysis. J. Diabetes Investig. 2021, 12, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Guo, Q.; Ge, J.; Li, J.; Li, C.; Jing, Z. The efficacy and safety of metformin alone or as an add-on therapy to insulin in pregnancy with GDM or T2DM: A systematic review and meta-analysis of 21 randomized controlled trials. J. Clin. Pharm. Ther. 2021, 47, 168–177. [Google Scholar] [CrossRef]
- Benham, J.L.; Donovan, L.E.; Yamamoto, J.M. Metformin in Pregnancy for Women with Type 2 Diabetes: A Review. Curr. Diab. Rep. 2021, 21, 36. [Google Scholar] [CrossRef]
- Newman, C.; Dunne, F.P. Metformin for pregnancy and beyond; the pros and cons. Diabet. Med. 2021, 39, e14700. [Google Scholar] [CrossRef]
- Anness, A.R.; Baldo, A.; Webb, D.R.; Khalil, A.; Robinson, T.G.; Mousa, H.A. Effect of metformin on biomarkers of placental-mediated disease: A systematic review and meta-analysis. Placenta 2021, 107, 51–58. [Google Scholar] [CrossRef]
- Li, X.; Celotto, S.; Pizzol, D.; Gasevic, D.; Ji, M.M.; Barnini, T.; Solmi, M.; Stubbs, B.; Smith, L.; López Sánchez, G.F.; et al. Metformin and health outcomes: An umbrella review of systematic reviews with meta-analyses. Eur. J. Clin. Investig. 2021, 51, e13536. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Lim, L.Y.; Ding, S.S.L.; Amirruddin, N.S.; Hoon, S.; Chan, S.Y.; Teo, A.K.K. Metformin Perturbs Pancreatic Differentiation From Human Embryonic Stem Cells. Diabetes 2021, 70, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Nayak, G.; Salian, S.R.; Agarwal, P.; Suresh Poojary, P.; Rao, A.; Kumari, S.; Kalthur, S.G.; Shreya, A.B.; Mutalik, S.; Adiga, S.K.; et al. Antidiabetic drug metformin affects the developmental competence of cleavage-stage embryos. J. Assist. Reprod. Genet. 2020, 37, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A. Sex and Gender Differences in Endocrinology. In Sex and Gender Aspects in Clinical Medicine; Oertelt-Prigione, S., Regitz-Zagrosek, V., Eds.; Springer: London, UK, 2012; pp. 125–149. [Google Scholar]
- Mauvais-Jarvis, F. Epidemiology of Gender Differences in Diabetes and Obesity. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 3–8. [Google Scholar]
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926–1927. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Itakura, Y.; Minamizono, S.; Okayama, K.; Suzuki, Y.; Takemi, Y.; Nakanishi, A.; Eto, K.; Takahashi, H.; Kawata, Y.; et al. The Association of Body Image Self-Discrepancy with Female Gender, Calorie-Restricted Diet, and Psychological Symptoms among Healthy Junior High School Students in Japan. Front. Psychol. 2021, 12, 576089. [Google Scholar] [CrossRef]
- Grandone, A.; Di Sessa, A.; Umano, G.R.; Toraldo, R.; Miraglia Del Giudice, E. New treatment modalities for obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 535–549. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.T.; Denig, P.; Ekhart, C.; Mol, P.G.M.; van Puijenbroek, E.P. Sex Differences in Adverse Drug Reactions of Metformin: A Longitudinal Survey Study. Drug Saf. 2020, 43, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Flegal, K.M. Body mass index of healthy men compared with healthy women in the United States. Int. J. Obes. 2006, 30, 374–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkelmayer, W.C.; Stedman, M.R.; Pogantsch, M.; Wieninger, P.; Bucsics, A.; Asslaber, M.; Bauer, R.; Burkhardt, T.; Schautzer, A.; Brookhart, M.A. Guideline-conformity of initiation with oral hypoglycemic treatment for patients with newly therapy-dependent type 2 diabetes mellitus in Austria. Pharmacoepidemiol. Drug Saf. 2011, 20, 57–65. [Google Scholar] [CrossRef]
- Horsburgh, S.; Sharples, K.; Barson, D.; Zeng, J.; Parkin, L. Patterns of metformin monotherapy discontinuation and reinitiation in people with type 2 diabetes mellitus in New Zealand. PLoS ONE 2021, 16, e0250289. [Google Scholar] [CrossRef]
- Li, J.; Shan, Z.; Yang, W.; Liu, J.; Tian, H.; Zhou, Z.; Ji, Q.; Weng, J.; Jia, W.; Lu, J.; et al. Gender-differential effects on blood glucose levels between acarbose and metformin in Chinese patients with newly diagnosed type 2 diabetes: A sub-analysis of the MARCH trial. Endocr. J. 2021, 68, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Orloff, J.N.; Touhamy, S.H.; Truong, W.; Casper, A.; Shukla, A.P.; Igel, L.I.; Flory, J.H. Trial of restarting and tolerating metformin (TreatMet). Diabetes Obes. Metab. 2020, 22, 2189–2192. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Sang, Y.; Chang, A.R.; Dunning, S.C.; Coresh, J.; Inker, L.A.; Selvin, E.; Ballew, S.H.; Grams, M.E. The FDA Metformin Label Change and Racial and Sex Disparities in Metformin Prescription among Patients with CKD. J. Am. Soc. Nephrol. 2020, 31, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Orloff, J.; Min, J.Y.; Mushlin, A.; Flory, J. Safety and effectiveness of metformin in patients with reduced renal function: A systematic review. Diabetes Obes. Metab. 2021, 23, 2035–2047. [Google Scholar] [CrossRef]
- Moon, S.J.; Ahn, C.H.; Cho, Y.M. Effect of prescribing metformin according to eGFR instead of serum creatinine level: A study based on Korean National Health and Nutrition Examination Survey (KNHANES) 2009–2014. PLoS ONE 2017, 12, e0175334. [Google Scholar] [CrossRef] [Green Version]
- Chapman, L.E.; Darling, A.L.; Brown, J.E. Association between metformin and vitamin B(12) deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2016, 42, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef]
- Yang, W.; Cai, X.; Wu, H.; Ji, L. Associations between metformin use and vitamin B(12) levels, anemia, and neuropathy in patients with diabetes: A meta-analysis. J. Diabetes 2019, 11, 729–743. [Google Scholar] [CrossRef]
- Margalit, I.; Cohen, E.; Goldberg, E.; Krause, I. Vitamin B12 Deficiency and the Role of Gender: A Cross-Sectional Study of a Large Cohort. Ann. Nutr. Metab. 2018, 72, 265–271. [Google Scholar] [CrossRef]
- Alvarez, M.; Sierra, O.R.; Saavedra, G.; Moreno, S. Vitamin B12 deficiency and diabetic neuropathy in patients taking metformin: A cross-sectional study. Endocr. Connect. 2019, 8, 1324–1329. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; You, D.; Wang, H.; Yang, Y.; Zhang, D.; Lv, J.; Luo, S.; Liao, R.; Ma, L. Association between homocysteine and obesity: A meta-analysis. J. Evid. Based Med. 2021, 14, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Li, L.; Li, Q.; Ren, K.; Sun, X.; Li, J. Metformin Treatment and Homocysteine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monami, M.; Candido, R.; Pintaudi, B.; Targher, G.; Mannucci, E. Effect of metformin on all-cause mortality and major adverse cardiovascular events: An updated meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gunton, J.E. The Changing Landscape of Pharmacotherapy for Diabetes Mellitus: A Review of Cardiovascular Outcomes. Int. J. Mol. Sci. 2019, 20, 5853. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Das, A.; Chen, J.; Wu, P.; Li, X.; Fang, Z. Metformin in patients with and without diabetes: A paradigm shift in cardiovascular disease management. Cardiovasc. Diabetol. 2019, 18, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbara, K.C.; Mofo Mato, P.E.; Driver, C.; Nzuza, S.; Mkhombo, N.T.; Gcwensa, S.K.; McObothi, E.N.; Owira, P.M. Metformin turns 62 in pharmacotherapy: Emergence of non-glycaemic effects and potential novel therapeutic applications. Eur. J. Pharmacol. 2021, 898, 173934. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Kang, K.-K.; Chae, S.; Son, W.-C. Metformin Alleviates Left Ventricular Diastolic Dysfunction in a Rat Myocardial Ischemia Reperfusion Injury Model. Int. J. Mol. Sci. 2020, 21, 1489. [Google Scholar] [CrossRef] [Green Version]
- Loi, H.; Kramar, S.; Laborde, C.; Marsal, D.; Pizzinat, N.; Cussac, D.; Roncalli, J.; Boal, F.; Tronchere, H.; Oleshchuk, O.; et al. Metformin Attenuates Postinfarction Myocardial Fibrosis and Inflammation in Mice. Int. J. Mol. Sci. 2021, 22, 9393. [Google Scholar] [CrossRef]
- Lyons, M.R.; Peterson, L.R.; McGill, J.B.; Herrero, P.; Coggan, A.R.; Saeed, I.M.; Recklein, C.; Schechtman, K.B.; Gropler, R.J. Impact of sex on the heart’s metabolic and functional responses to diabetic therapies. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1584–H1591. [Google Scholar] [CrossRef] [Green Version]
- Raparelli, V.; Elharram, M.; Moura, C.S.; Abrahamowicz, M.; Bernatsky, S.; Behlouli, H.; Pilote, L. Sex Differences in Cardiovascular Effectiveness of Newer Glucose-Lowering Drugs Added to Metformin in Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e012940. [Google Scholar] [CrossRef]
- Gnesin, F.; Thuesen, A.C.B.; Kähler, L.K.A.; Madsbad, S.; Hemmingsen, B. Metformin monotherapy for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 6, CD012906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.H. The Effect of Metformin on Male Reproductive Function and Prostate: An Updated Review. World J. Men’s Health 2021, 40, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Erectile Dysfunction and Low Sex Drive in Men with Type 2 DM: The Potential Role of Diabetic Pharmacotherapy. J. Clin. Diagn. Res. 2016, 10, FC21–FC26. [Google Scholar] [CrossRef] [PubMed]
- Atalay, E.; Demir, A.; Eroglu, H.A. The Influences of Metformin on Prostate in Terms of PSA Level and Prostate Volume. Urol. J. 2020, 18, 181–185. [Google Scholar] [CrossRef]
- Kincius, M.; Patasius, A.; Linkeviciute-Ulinskiene, D.; Zabuliene, L.; Smailyte, G. Reduced risk of prostate cancer in a cohort of Lithuanian diabetes mellitus patients. Aging Male 2020, 23, 1333–1338. [Google Scholar] [CrossRef]
- Linkeviciute-Ulinskiene, D.; Patasius, A.; Kincius, M.; Zabuliene, L.; Smailyte, G. Preexisting diabetes, metformin use and long-term survival in patients with prostate cancer. Scand. J. Urol. 2020, 54, 401–407. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Fowlkes, J.L.; Popescu, I.; Thrailkill, K.M. Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab. Res. Rev. 2019, 35, e3100. [Google Scholar] [CrossRef]
- Carvalho, E.S.A.P.; Harmer, A.R.; Ferreira, M.L.; Ferreira, P.H. The effect of the anti-diabetic drug metformin on musculoskeletal pain: A cross-sectional study with 21,889 individuals from the UK biobank. Eur. J. Pain 2021, 25, 1264–1273. [Google Scholar] [CrossRef]
- Inyang, K.E.; Szabo-Pardi, T.; Wentworth, E.; McDougal, T.A.; Dussor, G.; Burton, M.D.; Price, T.J. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol. Res. 2019, 139, 1–16. [Google Scholar] [CrossRef]
- Ruddy, R.M.; Adams, K.V.; Morshead, C.M. Age- and sex-dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke. Sci. Adv. 2019, 5, eaax1912. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, V.N.; Piskunova, T.S.; Popovich, I.G.; Zabezhinski, M.A.; Tyndyk, M.L.; Egormin, P.A.; Yurova, M.V.; Rosenfeld, S.V.; Semenchenko, A.V.; Kovalenko, I.G.; et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging 2010, 2, 945–958. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Shen, W.; Liu, Z.; Sheng, S.; Xiong, W.; He, R.; Zhang, X.; Ma, L.; Ju, Z. Effect of Metformin on Cardiac Metabolism and Longevity in Aged Female Mice. Front. Cell Dev. Biol. 2020, 8, 626011. [Google Scholar] [CrossRef]
- Aceves-Aparicio, E.; Pérez-Staples, D.; Arredondo, J.; Corona-Morales, A.; Morales-Mávil, J.; Díaz-Fleischer, F. Combined Effects of Methoprene and Metformin on Reproduction, Longevity, and Stress Resistance in Anastrepha ludens (Diptera: Tephritidae): Implications for the Sterile Insect Technique. J. Econ. Entomol. 2021, 114, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Hsu, C.-C.; Wahlqvist, M.L.; Tsai, H.-N.; Chang, Y.-H.; Huang, Y.-C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xiao, J.; Zhao, Y.; Du, S.; Du, J. Effect of metformin on the mortality of colorectal cancer patients with T2DM: Meta-analysis of sex differences. Int. J. Colorectal. Dis. 2020, 35, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, B.; Sarokhani, D.; Najafi, F.; Motedayen, M.; Dehkordi, A.H. The Relationship Between Prostate Cancer and Metformin Consumption: A Systematic Review and Meta-analysis Study. Curr. Pharm. Des. 2019, 25, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabuliene, L.; Kaceniene, A.; Steponaviciene, L.; Linkeviciute-Ulinskiene, D.; Stukas, R.; Arlauskas, R.; Vanseviciute-Petkeviciene, R.; Smailyte, G. Risk of Endometrial Cancer in Women with Diabetes: A Population-Based Retrospective Cohort Study. J. Clin. Med. 2021, 10, 3453. [Google Scholar] [CrossRef] [PubMed]
- Pabona, J.M.P.; Burnett, A.F.; Brown, D.M.; Quick, C.M.; Simmen, F.A.; Montales, M.T.E.; Liu, S.J.; Rose, T.; Alhallak, I.; Siegel, E.R.; et al. Metformin Promotes Anti-tumor Biomarkers in Human Endometrial Cancer Cells. Reprod. Sci. 2020, 27, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Petchsila, K.; Prueksaritanond, N.; Insin, P.; Yanaranop, M.; Chotikawichean, N. Effect of Metformin for Decreasing Proliferative Marker in Women with Endometrial Cancer: A Randomized Double-blind Placebo-Controlled Trial. Asian Pac. J. Cancer Prev. 2020, 21, 733–741. [Google Scholar] [CrossRef]
- Davis, S.R.; Robinson, P.J.; Jane, F.; White, S.; Brown, K.A.; Piessens, S.; Edwards, A.; McNeilage, J.; Woinarski, J.; Chipman, M.; et al. The benefits of adding metformin to tamoxifen to protect the endometrium-A randomized placebo-controlled trial. Clin. Endocrinol. 2018, 89, 605–612. [Google Scholar] [CrossRef]
- Garzon, S.; Uccella, S.; Zorzato, P.C.; Bosco, M.; Franchi, M.P.; Student, V.; Mariani, A. Fertility-sparing management for endometrial cancer: Review of the literature. Minerva Med. 2021, 112, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, F.R.; Pasupuleti, V.; Gianuzzi, X.; Palma-Ardiles, G.; Hernandez-Fernandez, W.; Hernandez, A.V. Systematic review and meta-analysis of the effect of metformin treatment on overall mortality rates in women with endometrial cancer and type 2 diabetes mellitus. Maturitas 2017, 101, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.S.; Oliver, T.R.; Shiwani, H.; Sanner, J.R.; Mulvaney, C.A.; Atiomo, W. Metformin for endometrial hyperplasia. Cochrane Database Syst. Rev. 2017, 10, CD012214. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Negalha, G.; Azevedo, A.; Martel, F. Metformin and Breast Cancer: Molecular Targets. J. Mammary Gland Biol. Neoplasia 2019, 24, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.C.; Dietze, E.C.; Jovanovic-Talisman, T.; McCune, J.S.; Seewaldt, V.L. Metformin and Chemoprevention: Potential for Heart-Healthy Targeting of Biologically Aggressive Breast Cancer. Front. Public Health 2020, 8, 509714. [Google Scholar] [CrossRef] [PubMed]
- Undzyte, G.; Patasius, A.; Linkeviciute-Ulinskiene, D.; Zabuliene, L.; Stukas, R.; Dulskas, A.; Smailyte, G. Increased kidney cancer risk in diabetes mellitus patients: A population-based cohort study in Lithuania. Aging Male 2020, 23, 1241–1245. [Google Scholar] [CrossRef]
- Dulskas, A.; Patasius, A.; Linkeviciute-Ulinskiene, D.; Zabuliene, L.; Smailyte, G. Cohort Study of Antihyperglycemic Medication and Pancreatic Cancer Patients Survival. Int. J. Environ. Res. Public Health 2020, 17, 6016. [Google Scholar] [CrossRef]
- Salomäki-Myftari, H.; Vähätalo, L.H.; Ailanen, L.; Pietilä, S.; Laiho, A.; Hänninen, A.; Pursiheimo, J.P.; Munukka, E.; Rintala, A.; Savontaus, E.; et al. Neuropeptide Y Overexpressing Female and Male Mice Show Divergent Metabolic but Not Gut Microbial Responses to Prenatal Metformin Exposure. PLoS ONE 2016, 11, e0163805. [Google Scholar] [CrossRef]
- Silamiķele, L.; Silamiķelis, I.; Ustinova, M.; Kalniņa, Z.; Elbere, I.; Petrovska, R.; Kalniņa, I.; Kloviņš, J. Metformin Strongly Affects Gut Microbiome Composition in High-Fat Diet-Induced Type 2 Diabetes Mouse Model of Both Sexes. Front. Endocrinol. 2021, 12, 626359. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef] [Green Version]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6 (Suppl. 1), 60–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornman, K.S.; Loesche, W.J. Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect. Immun. 1982, 35, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Differding, M.K.; Zhang, M.; Maruthur, N.M.; Juraschek, S.P.; Miller, E.R., 3rd; Appel, L.J.; Yeh, H.C. Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes Care 2021, 44, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.P.; Kempler, P.; Stulnig, T.; Rizvi, A.A.; Rizzo, M. Diabetes and COVID-19: What 2 Years of the Pandemic Has Taught Us. Metab. Syndr. Relat. Disord. 2021. [Google Scholar] [CrossRef]
- Ceriello, A.; Stoian, A.P.; Rizzo, M. COVID-19 and diabetes management: What should be considered? Diabetes Res. Clin. Pract. 2020, 163, 108151. [Google Scholar] [CrossRef]
- Ilias, I.; Jahaj, E.; Kokkoris, S.; Zervakis, D.; Temperikidis, P.; Magira, E.; Pratikaki, M.; Vassiliou, A.G.; Routsi, C.; Kotanidou, A.; et al. Clinical Study of Hyperglycemia and SARS-CoV-2 Infection in Intensive Care Unit Patients. Vivo 2020, 34, 3029–3032. [Google Scholar] [CrossRef]
- Michalakis, K.; Ilias, I. COVID-19 and hyperglycemia/diabetes. World J. Diabetes 2021, 12, 642–650. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Janez, A.; Rizzo, M. Cardiometabolic Alterations in the Interplay of COVID-19 and Diabetes: Current Knowledge and Future Avenues. Int. J. Mol. Sci. 2021, 22, 2311. [Google Scholar] [CrossRef]
- Al Mahmeed, W.; Al-Rasadi, K.; Banerjee, Y.; Ceriello, A.; Cosentino, F.; Galia, M.; Goh, S.Y.; Kempler, P.; Lessan, N.; Papanas, N.; et al. Promoting a Syndemic Approach for Cardiometabolic Disease Management During COVID-19: The CAPISCO International Expert Panel. Front. Cardiovasc. Med. 2021, 8, 787761. [Google Scholar] [CrossRef]
- Stoian, A.P.; Banerjee, Y.; Rizvi, A.A.; Rizzo, M. Diabetes and the COVID-19 Pandemic: How Insights from Recent Experience Might Guide Future Management. Metab. Syndr. Relat. Disord. 2020, 18, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Ilias, I.; Zabuliene, L. Hyperglycemia and the novel COVID-19 infection: Possible pathophysiologic mechanisms. Med. Hypotheses 2020, 139, 109699. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, Y.; Pantea Stoian, A.; Silva-Nunes, J.; Sonmez, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. The role of GLP-1 receptor agonists during COVID-19 pandemia: A hypothetical molecular mechanism. Expert Opin. Drug Saf. 2021, 20, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.S.; Papanas, N.; Pantea Stoian, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Use of Novel Antidiabetic Agents in Patients with Type 2 Diabetes and COVID-19: A Critical Review. Diabetes Ther. 2021, 12, 3037–3054. [Google Scholar] [CrossRef]
- Anca, P.S.; Toth, P.P.; Kempler, P.; Rizzo, M. Gender differences in the battle against COVID-19: Impact of genetics, comorbidities, inflammation and lifestyle on differences in outcomes. Int. J. Clin. Pract. 2021, 75, e13666. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J. Med. Virol. 2021, 93, 695–697. [Google Scholar] [CrossRef]
- Lukito, A.A.; Pranata, R.; Henrina, J.; Lim, M.A.; Lawrensia, S.; Suastika, K. The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 2177–2183. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Yan, P.; Sun, T.; Zeng, Z.; Li, S. Metformin in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 704666. [Google Scholar] [CrossRef]
- Kan, C.; Zhang, Y.; Han, F.; Xu, Q.; Ye, T.; Hou, N.; Sun, X. Mortality Risk of Antidiabetic Agents for Type 2 Diabetes with COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 708494. [Google Scholar] [CrossRef]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Healthy Longev. 2021, 2, e34–e41. [Google Scholar] [CrossRef]
- Jiang, N.; Chen, Z.; Liu, L.; Yin, X.; Yang, H.; Tan, X.; Wang, J.; Li, H.; Tian, M.; Lu, Z.; et al. Association of metformin with mortality or ARDS in patients with COVID-19 and type 2 diabetes: A retrospective cohort study. Diabetes Res. Clin. Pract. 2021, 173, 108619. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.A.; Akhter, M.S.; Kubra, K.-T.; Siejka, A.; Barabutis, N. Metformin in acute respiratory distress syndrome: An opinion. Exp. Gerontol. 2021, 145, 111197. [Google Scholar] [CrossRef] [PubMed]
- Crouse, A.B.; Grimes, T.; Li, P.; Might, M.; Ovalle, F.; Shalev, A. Metformin Use Is Associated with Reduced Mortality in a Diverse Population with COVID-19 and Diabetes. Front. Endocrinol. 2020, 11, 600439. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Aging, Male Sex, Obesity, and Metabolic Inflammation Create the Perfect Storm for COVID-19. Diabetes 2020, 69, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018, 187, 20–23. [Google Scholar] [CrossRef]
- Gannon, M.; Kulkarni, R.N.; Tse, H.M.; Mauvais-Jarvis, F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol. Metab. 2018, 15, 82–91. [Google Scholar] [CrossRef]
- Nasykhova, Y.A.; Tonyan, Z.N.; Mikhailova, A.A.; Danilova, M.M.; Glotov, A.S. Pharmacogenetics of Type 2 Diabetes-Progress and Prospects. Int. J. Mol. Sci. 2020, 21, 6842. [Google Scholar] [CrossRef]
- Brancher, S.; Ribeiro, A.E.; Toporcov, T.N.; Weiderpass, E. The role of metformin on lung cancer survival: The first systematic review and meta-analysis of observational studies and randomized clinical trials. J. Cancer Res. Clin. Oncol. 2021, 147, 2819–2836. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, X.; Sun, C.; Kim, N.H.; Kailas, S.; Qureshi, F.; Karadsheh, Z.; Wu, Y.; Hu, L.; Zhou, Z.; et al. Is metformin use associated with a reduced risk of oesophageal cancer? A systematic review and meta-analysis. Postgrad. Med. J. 2021. [Google Scholar] [CrossRef]
- Harewood, R.; Disney, R.; Kinross, J.; von Wagner, C.; Cross, A.J. Medication use and risk of proximal colon cancer: A systematic review of prospective studies with narrative synthesis and meta-analysis. Cancer Causes Control 2021, 32, 1047–1061. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Jang, H.J.; Lee, J. The addition of metformin to systemic anticancer therapy in advanced or metastatic cancers: A meta-analysis of randomized controlled trials. Int. J. Med. Sci. 2020, 17, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, Y.; Xie, J.; Chen, L.; Zhu, G. The efficacy and safety of metformin combined with simvastatin in the treatment of polycystic ovary syndrome: A meta-analysis and systematic review. Medicine 2021, 100, e26622. [Google Scholar] [CrossRef] [PubMed]
- Lusica, P.M.M.; Eugenio, K.P.Y.; Sacdalan, D.B.L.; Jimeno, C.A. A systematic review and meta-analysis on the efficacy and safety of metformin as adjunctive therapy among women with metastatic breast cancer. Cancer Treat. Res. Commun. 2021, 29, 100457. [Google Scholar] [CrossRef] [PubMed]
- Takhwifa, F.; Aninditha, T.; Setiawan, H.; Sauriasari, R. The potential of metformin as an antineoplastic in brain tumors: A systematic review. Heliyon 2021, 7, e06558. [Google Scholar] [CrossRef]
- Wu, Y.; Tu, M.; Huang, Y.; Liu, Y.; Zhang, D. Association of Metformin With Pregnancy Outcomes in Women with Polycystic Ovarian Syndrome Undergoing In Vitro Fertilization: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2011995. [Google Scholar] [CrossRef]
- Bao, L.X.; Shi, W.T.; Han, Y.X. Metformin versus insulin for gestational diabetes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2021, 34, 2741–2753. [Google Scholar] [CrossRef]
- Cao, Q.; Hu, Y.; Fu, J.; Huang, X.; Wu, L.; Zhang, J.; Huang, W. Gestational metformin administration in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized control studies. J. Obstet. Gynaecol. Res. 2021, 47, 4148–4157. [Google Scholar] [CrossRef]
- Herath, M.P.; Beckett, J.M.; Hills, A.P.; Byrne, N.M.; Ahuja, K.D.K. Gestational Diabetes Mellitus and Infant Adiposity at Birth: A Systematic Review and Meta-Analysis of Therapeutic Interventions. J. Clin. Med. 2021, 10, 835. [Google Scholar] [CrossRef]
- Kgosidialwa, O.; Bogdanet, D.; Egan, A.; Newman, C.; O’Shea, P.M.; Biesty, L.; McDonagh, C.; O’Shea, C.; Devane, D.; Dunne, F. A systematic review on outcome reporting in randomised controlled trials assessing treatment interventions in pregnant women with pregestational diabetes. BJOG 2021, 128, 1894–1904. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Lucerón-Lucas-Torres, M.; Sanabria-Martínez, G.; Poyatos-León, R.; Rodríguez-Martín, B.; Martínez-Vizcaíno, V. Exercise versus Metformin to Improve Pregnancy Outcomes among Overweight Pregnant Women: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2021, 10, 3490. [Google Scholar] [CrossRef] [PubMed]
- Stochino-Loi, E.; Major, A.L.; Gillon, T.E.R.; Ayoubi, J.M.; Feki, A.; Bouquet de Joliniere, J. Metformin, the Rise of a New Medical Therapy for Endometriosis? A Systematic Review of the Literature. Front. Med. 2021, 8, 581311. [Google Scholar] [CrossRef] [PubMed]
- Tso, L.O.; Costello, M.F.; Albuquerque, L.E.T.; Andriolo, R.B.; Macedo, C.R. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2020, 12, CD006105. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021, 12, 718942. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).