Acute Kidney Injury and Hyponatremia in Ultra-Trail Racing: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Study Search and Selection
2.2. Inclusion Criteria
- Case studies, observational studies, longitudinal or prospective studies.
- Adult population of both sexes.
- Races longer than 42 km with one or more stages, held in a natural setting.
- Analysis of biomarkers related to the prevalence of AKI.
- Analysis of biomarkers related to EAH.
- Articles written in English or Spanish.
2.3. Exclusion Criteria
- Patients with previous chronic renal pathology.
- AKI or EAH was not assessed.
- Combined sports (triathlon or biathlon), short duration (<42 km) or track-like environments without elevation gain (>500 m).
2.4. Data Extraction
2.5. Overall Quality of Included Studies
2.6. Risk of Bias Assessment and Methodological Quality
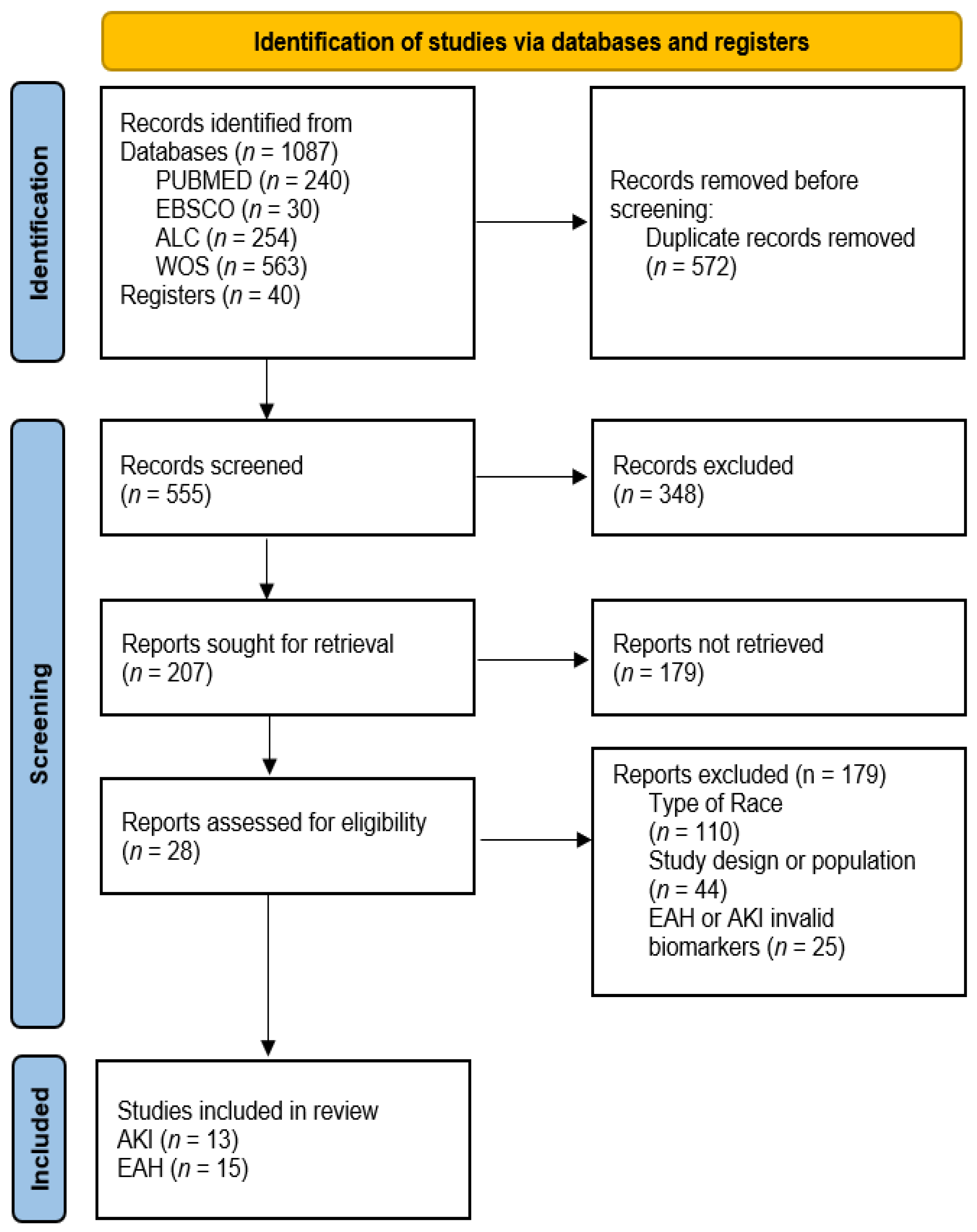
3. Results
3.1. Types of Ultra-Trail Races and Participant Data
3.2. AKI
3.3. EAH
3.4. Concurrence of AKI and EAH
3.5. Level of Evidence from Included Individual Studies
4. Discussion
4.1. AKI
4.2. EAH
- Types of ultra-trail races and development of EAH
- Hydration and body weight
- Environmental race conditions
4.3. AKI and EAH
4.3.1. Overhydration and Rhabdomyolysis
4.3.2. Race Elevation Gain and Increased AKI and EAH
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Cejka, N.; Rüst, C.A.; Lepers, R.; Onywera, V.; Rosemann, T.; Knechtle, B. Participation and performance trends in 100-km ultra-marathons worldwide. J. Sports Sci. 2014, 32, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrensperger, L.; Knechtle, B.; Rüst, C.A.; Rosemann, T. Participation and performance trends in 6-hour ultra-marathoners—A retrospective data analysis of worldwide participation from 1991–2010. J. Hum. Sport Exerc. 2013, 8, 905–924. [Google Scholar] [CrossRef] [Green Version]
- Runsignup. Annual Industry Report [Internet]. Volume 9, Annual Trend Race Report. 2020. Available online: extension://bfdogplmndidlpjfhoijckpakkdjkkil/pdf/viewer.html?file=https%3A%2F%2Fd368g9lw5ileu7.cloudfront.net%2Fdocuments%2FraceTrends2020_v20210128.pdf (accessed on 1 February 2022).
- Scheer, V.; Basset, P.; Giovanelli, N.; Vernillo, G.; Millet, G.P.; Costa, R.J.S. Defining Off-road Running: A Position Statement from the Ultra Sports Science Foundation. Int. J. Sports Med. 2020, 41, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Urbaneja, J.S.; Farias, E.I. Trail running in Spain. Origin, evolution and current situation; natural areas. Retos 2018, 2041, 123–128. [Google Scholar]
- Balducci, P.; Clémençon, M.; Trama, R.; Blache, Y.; Hautier, C. Performance Factors in a Mountain Ultramarathon. Int. J. Sports Med. 2017, 38, 819–826. [Google Scholar] [CrossRef]
- Scheer, V. Participation trends of ultra endurance events. Sports Med. Arthrosc. 2019, 27, 3–7. [Google Scholar] [CrossRef]
- Vernillo, G.; Savoldelli, A.; La Torre, A.; Skafidas, S.; Bortolan, L.; Schena, F. Injury and Illness Rates during Ultratrail Running. Int. J. Sports Med. 2016, 37, 565–569. [Google Scholar]
- Hoffman, M.D.; Krishnan, E. Health and exercise-related medical issues among 1212 ultramarathon runners: Baseline findings from the Ultrarunners Longitudinal TRAcking (ULTRA) Study. PLoS ONE 2014, 9, e83867. [Google Scholar] [CrossRef] [Green Version]
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential Long-Term Health Problems Associated with Ultra-Endurance Running: A Narrative Review. Sports Med. 2021, 52, 725–740. [Google Scholar] [CrossRef]
- Dawadi, S.; Basyal, B.; Subedi, Y. Morbidity Among Athletes Presenting for Medical Care During 3 Iterations of an Ultratrail Race in the Himalayas. Wilderness Environ. Med. 2020, 31, 437–440. [Google Scholar] [CrossRef]
- Scheer, B.V.; Murray, A. Al Andalus Ultra Trail: An observation of medical interventions during a 219-km, 5-day ultramarathon stage race. Clin. J. Sport Med. 2011, 21, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Lecina, M.; Castellar, C.; Pradas, F.; López-Laval, I. 768-km Multi-Stage Ultra-Trail Case Study-Muscle Damage, Biochemical Alterations and Strength Loss on Lower Limbs. Int. J. Environ. Res. Public Health 2022, 19, 876. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Esch, B.T.A.; Shave, R.; Warburton, D.E.R.; Gaze, D.; George, K. Cardiovascular consequences of completing a 160-km ultramarathon. Med. Sci. Sports Exerc. 2009, 41, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sansoni, V.; Vernillo, G.; Perego, S.; Barbuti, A.; Merati, G.; Schena, F.; La Torre, A.; Banfi, G.; Lombardi, G. Bone turnover response is linked to both acute and established metabolic changes in ultra-marathon runners. Endocrine 2017, 56, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Lipman, G.S.; Ellis, M.A.; Lewis, E.J.; Waite, B.L.; Lissoway, J.; Chan, G.K.; Krabak, B.J. A Prospective Randomized Blister Prevention Trial Assessing Paper Tape in Endurance Distances (Pre-TAPED). Wilderness Environ. Med. 2014, 25, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Chlíbková, D.; Rosemann, T.; Posch, L.; Matoušek, R.; Knechtle, B. Pre- and post-race hydration status in hyponatremic and non-hyponatremic ultra-endurance athletes. Chin. J. Physiol. 2016, 59, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.J.S.; Knechtle, B.; Tarnopolsky, M.; Hoffman, M.D. Nutrition for ultramarathon running: Trail, track, and road. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Brge, J.; Knechtle, B.; Knechtle, P.; Gndinger, M.; Rst, A.C.; Rosemann, T. Maintained serum sodium in male ultra-marathoners the role of fluid intake, vasopressin, and aldosterone in fluid and electrolyte regulation. Horm. Metab. Res. 2011, 43, 646–652. [Google Scholar]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol. Dial. Transplant. 2014, 29, i1–i39. [Google Scholar] [CrossRef] [Green Version]
- Hew-Butler, T.; Rosner, M.H.; Fowkes-Godek, S.; Dugas, J.P.; Hoffman, M.D.; Lewis, D.P.; Maughan, R.J.; Miller, K.C.; Montain, S.J.; Rehrer, N.J.; et al. Statement of the 3rd international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Br. J. Sports Med. 2015, 49, 1432–1446. [Google Scholar] [CrossRef] [Green Version]
- Rosner, M.H.; Kirven, J. Exercise-associated hyponatremia. Clin. J. Am. Soc. Nephrol. 2007, 2, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Suppression of information on the prevalence and prevention of exercise-associated hyponatraemia. Br. J. Sports Med. 2011, 45, 1238–1242. [Google Scholar] [CrossRef]
- Cuthill, J.A.; Ellis, C.; Inglis, A. Hazards of ultra-marathon running in the Scottish highlands: Exercise-associated hyponatraemia. Emerg. Med. J. 2009, 26, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Stuempfle, K.J. Hydration strategies, weight change and performance in a 161 km ultramarathon. Res. Sports Med. 2014, 22, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Sahay, M.; Sahay, R. Hyponatremia: A practical approach. Indian J. Endocrinol. Metab. 2014, 18, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Scotney, B.; Reid, S. Body Weight, Serum Sodium Levels, and Renal Function in an Ultra-Distance Mountain Run. Clin. J. Sport Med. 2015, 25, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Boulter, J.; Noakes, T.D.; Hew-Butler, T. Acute renal failure in four Comrades Marathon runners ingesting the same electrolyte supplement: Coincidence or causation? S. Afr. Med. J. 2011, 101, 876–878. [Google Scholar]
- Lopes, J.A.; Jorge, S.; Seijas, M.; Baccino, C.; Nin, N.; Lorente, J.A.; Lee, S.W.; Baek, S.H.; Ahn, S.Y.; Na, K.Y.; et al. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Med. Intensiva 2016, 11, 85–98. [Google Scholar]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Crowe, J.; Timón, R.; Olcina, G.J. Exertional rhabdomyolysis and acute kidney injury in endurance sports: A systematic review. Eur. J. Sport Sci. 2020, 21, 261–274. [Google Scholar] [CrossRef]
- Seijas, M.; Baccino, C.; Nin, N.; Lorente, J.A. Definition and biomarkers of acute renal damage: New perspectives. Med. Intensiva 2014, 38, 376–385. [Google Scholar] [CrossRef]
- Sawhney, S.; Fraser, S.D. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Adv. Chronic Kidney Dis. 2017, 24, 194–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, P.K.; Hsu, R.K.; Liu, K.D. Management of Acute Kidney Injury: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 72, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, L.; Walter, E.; Venn, R.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L. Acute kidney injury associated with endurance events—Is it a cause for concern? A systematic review. BMJ Open Sport Exerc. Med. 2017, 3, e000093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.; de Jonge, R.; van der Cammen, T.; Zietse, R.; Hoorn, E.J. Acute kidney injury in patients presenting with hyponatremia. J. Nephrol. 2011, 24, 749–755. [Google Scholar] [CrossRef]
- Lee, S.W.; Baek, S.H.; Ahn, S.Y.; Na, K.Y.; Chae, D.W.; Chin, H.J.; Kim, S. The effects of pre-existing hyponatremia and subsequent-developing acute kidney injury on in-hospital mortality: A retrospective cohort study. PLoS ONE 2016, 11, e0162990. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Rosenbrand, K.; Van Croonenborg, J.; Wittenberg, J. Guideline Development. Stud. Health Technol. Inf. 2008, 139, 3–21. [Google Scholar]
- National Heart Lung and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 January 2022).
- Lipman, G.S.; Krabak, B.J.; Waite, B.L.; Logan, S.B.; Menon, A.; Chan, G.K. A prospective cohort study of acute kidney injury in multi-stage ultramarathon runners: The biochemistry in endurance runner study (BIERS). Res. Sports Med. 2014, 22, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Poussel, M.; Touzé, C.; Allado, E.; Frimat, L.; Hily, O.; Thilly, N.; Rousseau, H.; Vauthier, J.-C.; Chenuel, B. Ultramarathon and Renal Function: Does Exercise-Induced Acute Kidney Injury Really Exist in Common Conditions? Front. Sports Act. Living 2020, 1, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Physiological and pathophysiological responses to ultramarathon running in non-elite runners. Front. Physiol. 2019, 10, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouffroy, R.; Lebreton, X.; Mansencal, N.; Anglicheau, D. Acute kidney injury during an ultra-distance race. PLoS ONE 2019, 14, e0222544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, M.D.; Weiss, R.H. Does acute kidney injury from an ultramarathon increase the risk for greater subsequent injury? Clin. J. Sport Med. 2016, 26, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, C.; Kaux, J.F.; Dulgheru, R.; Seidel, L.; Pincemail, J.; Cavalier, E.; Melon, P. The impact of an ultra-trail on the dynamic of cardiac, inflammatory, renal and oxidative stress biological markers correlated with electrocardiogram and echocardiogram. Acta Cardiol. 2020, 76, 739–747. [Google Scholar] [CrossRef]
- Lipman, G.S.; Krabak, B.J.; Rundell, S.D.; Shea, K.M.; Badowski, N.; Little, C. Incidence and Prevalence of Acute Kidney Injury during Multistage Ultramarathons. Clin. J. Sport Med. 2016, 26, 314–319. [Google Scholar] [CrossRef]
- Martínez-Navarro, I.; Sanchez-Gómez, J.M.; Aparicio, I.; Priego-Quesada, J.I.; Pérez-Soriano, P.; Collado, E.; Hernando, B.; Hernando, C. Effect of mountain ultramarathon distance competition on biochemical variables, respiratory and lower-limb fatigue. PLoS ONE 2020, 15, e0238846. [Google Scholar] [CrossRef]
- Belli, T.; Macedo, D.V.; De Araújo, G.G.; Masselli dos Reis, I.G.; Menezes Scariot, P.P.; Lazarim, F.L.; Soares Nunes, L.A.; Brenzikofer, R.; Gobatto, C.A. Mountain ultramarathon induces early increases of muscle damage, inflammation, and risk for acute renal injury. Front. Physiol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Cairns, R.S.; Hew-Butler, T. Proof of concept: Hypovolemic hyponatremia may precede and augment creatine kinase elevations during an ultramarathon. Eur. J. Appl. Physiol. 2016, 116, 647–655. [Google Scholar] [CrossRef]
- Pradas, F.; Falcón, D.; Peñarrubia-Lozano, C.; Toro-Román, V.; Carrasco, L.; Castellar, C. Effects of ultratrail running on neuromuscular function, muscle damage and hydration status. Differences according to training level. Int. J. Environ. Res. Public Health 2021, 18, 5119. [Google Scholar] [CrossRef]
- Khodaee, M.; Irion, B.; Spittler, J.; Saeedi, A.; Hoffman, M.D. Characteristics of runners meeting acute kidney injury criteria following a 161-km ultramarathon. Transl. Sports Med. 2021, 4, 733–740. [Google Scholar] [CrossRef]
- Page, A.J.; Reid, S.A.; Speedy, D.B.; Mulligan, G.P.; Thompson, J. Exercise-associated hyponatremia, renal function, and nonsteroidal antiinflammatory drug use in an ultraendurance mountain run. Clin. J. Sport Med. 2007, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Chlíbková, D.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B.; Bednář, J. Maintained hydration status after a 24-h winter mountain running race under extremely cold conditions. Front. Physiol. 2019, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Hew-Butler, T.; Stuempfle, K.J. Exercise-associated hyponatremia and hydration status in 161-km ultramarathoners. Med. Sci. Sports Exerc. 2013, 45, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Winger, J.M.; Hoffman, M.D.; Hew-Butler, T.D.; Stuempfle, K.J.; Dugas, J.P.; Fogard, K.; Dugas, L.R. The effect of physiology and hydration beliefs on race behavior and postrace sodium in 161-km ultramarathon finishers. Int. J. Sports Physiol. Perform. 2013, 8, 536–541. [Google Scholar] [CrossRef]
- Bracher, A.; Knechtle, B.; Gnädinger, M.; Bürge, J.; Rüst, C.A.; Knechtle, P.; Rosemann, T. Fluid intake and changes in limb volumes in male ultra-marathoners: Does fluid overload lead to peripheral oedema? Eur. J. Appl. Physiol. 2012, 112, 991–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knechtle, B.; Gnädinger, M.; Knechtle, P.; Imoberdorf, R.; Kohler, G.; Ballmer, P.; Rosemann, T.; Senn, O. Prevalence of exercise-associated hyponatremia in male ultraendurance athletes. Clin. J. Sport Med. 2011, 21, 226–232. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Jordaan, E.; Stuempfle, K.J.; Speedy, D.B.; Siegel, A.J.; Noakes, T.D.; Soldin, S.J.; Verbalis, J.G. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J. Clin. Endocrinol. Metab. 2008, 93, 2072–2078. [Google Scholar] [CrossRef] [Green Version]
- Shephard, R.J. Low prevalence of exercise-associated hyponatremia in male 100km ultra-marathon runners in Switzerland. Yearb. Sports Med. 2012, 2012, 326–328. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Teixeira, A.; Rama, L.; Swancott, A.J.M.; Hardy, L.D.; Lee, B.; Camões-Costa, V.; Gill, S.; Waterman, J.P.; Freeth, E.C.; et al. Water and sodium intake habits and status of ultra-endurance runners during a multi-stage ultra-marathon conducted in a hot ambient environment: An observational field based study. Nutr. J. 2013, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejka, C.; Knechtle, B.; Knechtle, P.; Rüst, C.A.; Rosemann, T. An increased fluid intake leads to feet swelling in 100-km ultra-marathoners—An observational field study. J. Int. Soc. Sports Nutr. 2012, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knechtle, B.; Knechtle, P.; Wirth, A.; Alexander Rüst, C.; Rosemann, T. A faster running speed is associated with a greater body weight loss in 100-km ultra-marathoners. J. Sports Sci. 2012, 30, 1131–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, K.; Rauch, S.; Procter, E.; Grasegger, K.; Mrakic-Sposta, S.; Gatterer, H. Changes in Factors Regulating Serum Sodium Homeostasis during Two Ultra-Endurance Mountain Races of Different Distances: 69 km vs. 121 km. Front. Physiol. 2021, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Khodaee, M.; Saeedi, A.; Harris-Spinks, C.; Hew-Butler, T. Incidence of exercise-associated hyponatremia during a high-altitude 161-km ultramarathon. Phys. Act. Nutr. 2021, 25, 16–22. [Google Scholar] [CrossRef]
- Sehovic, E.; Knechtle, B.; Rüst, C.A.; Rosemann, T. 12-hour ultra-marathons-Increasing worldwide participation and dominance of Europeans. J. Hum. Sport Exerc. 2013, 8, 932–953. [Google Scholar] [CrossRef] [Green Version]
- Spittler, J.; Oberle, L. Current Trends in Ultramarathon Running. Curr. Sports Med. Rep. 2019, 18, 387–393. [Google Scholar] [CrossRef]
- Rüst, C.A.; Knechtle, B.; Rosemann, T.; Lepers, R. Analysis of performance and age of the fastest 100-mile ultra-marathoners worldwide. Clinics 2013, 68, 605–611. [Google Scholar] [CrossRef]
- Hernández-Sánchez, S.; Valenzuela, P.L.; Morales, J.S.; Carrero, J.J.; Lucia, A.; Ruiz, J.R. Ultraendurance exercise in a renal transplant recipient: A case study. Int. J. Sports Physiol. Perform. 2020, 15, 1039–1042. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Bagga, A.; Bakkaloglu, A.; Bonventre, J.V.; et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wołyniec, W.; Ratkowski, W.; Kasprowicz, K.; Jastrzȩbski, Z.; MaŁgorzewicz, S.; Witek, K.; Grzywacz, T.; Zmijewski, P.; Renke, M. Glomerular filtration rate is unchanged by ultramarathon. J. Strength Cond. Res. 2018, 32, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Little, C.E.; Lipman, G.S.; Migliaccio, D.; Young, D.S.; Krabak, B.J. Accuracy of Estimated Creatinine in Multistage Ultramarathon Runners. Wilderness Environ. Med. 2019, 30, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.K.; Chiu, Y.H.; Tsai, Y.F.; Tai, L.C.; Hou, P.C.; How, C.K.; Yang, C.C.; Kao, W.F. Clinical impact of speed variability to identify ultramarathon runners at risk for acute kidney injury. PLoS ONE 2015, 10, e0133146. [Google Scholar] [CrossRef]
- Knechtle, B.; Duff, B.; Schulze, I.; Kohler, G. A multi-stage ultra-endurance run over 1,200 km leads to a continuous accumulation of total body water. J. Sports Sci. Med. 2008, 7, 357–364. [Google Scholar]
- Krabak, B.J.; Lipman, G.S.; Waite, B.L.; Rundell, S.D. Exercise-Associated Hyponatremia, Hypernatremia, and Hydration Status in Multistage Ultramarathons. Wilderness Environ. Med. 2017, 28, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Fallon, K.E.; Sivyer, G.; Sivyer, K.; Dare, A. The biochemistry of runners in a 1600 km ultramarathon. Br. J. Sports Med. 1999, 33, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Sawatzki, M. Hyponatremia among runners in the Boston Marathon. Praxis 2006, 95, 1691. [Google Scholar] [CrossRef]
- Rust, C. Body mass change and ultraendurance performance: A decrease in boody mass is associated with an increased running speed in male 100-km ultramarathoners. J. Strength Cond. Res. 2012, 26, 1505–1516. [Google Scholar] [CrossRef] [Green Version]
- Lipman, G.S.; Burns, P.; Phillips, C.; Jensen, J.; Little, C.; Jurkiewicz, C.; Jarrett, B.; Walker, A.; Mansfield, N.; Krabak, B.J. Effect of Sodium Supplements and Climate on Dysnatremia During Ultramarathon Running. Clin. J. Sport Med. 2021, 31, e327–e334. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Stellingwerff, T.; Costa, R.J.S. Considerations for ultra-endurance activities: Part 2–hydration. Res. Sport. Med. 2019, 27, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Scheer, B.V.; Valero Burgos, E. The Hidden Danger of Endurance Races: Analgesic Use Among Ultramarathon Runners. Br. J. Sports Med. 2013, 47, e3. [Google Scholar] [CrossRef]
- Žákovská, A.; Knechtle, B.; Chlíbková, D.; Miličková, M.; Rosemann, T.; Nikolaidis, P.T. The effect of a 100-km ultra-marathon under freezing conditions on selected immunological and hematological parameters. Front. Physiol. 2017, 8, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipman, G.S.; Hew-Butler, T.; Phillips, C.; Krabak, B.; Burns, P. Prospective Observational Study of Weight-based Assessment of Sodium Supplements on Ultramarathon Performance (WASSUP). Sport. Med.-Open 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.A. Creatine kinase, exertional rhabdomyolysis, and exercise-associated hyponatremia in ultra-endurance athletes: A critically appraised paper. Int. J. Athl. Ther. Train. 2016, 21, 13–15. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Pino-Ortega, J.; Gómez-Carmona, C.; Gutiérrez-Vargas, R.; Timón, R.; Olcina, G. External workload indicators of muscle and kidney mechanical injury in endurance trail running. Int. J. Environ. Res. Public Health 2019, 16, 3909. [Google Scholar] [CrossRef] [Green Version]
- Lemire, M.; Remetter, R.; Hureau, T.J.; Kouassi, B.Y.L.; Lonsdorfer, E.; Geny, B.; Isner-Horobeti, M.E.; Favret, F.; Dufour, S.P. High-intensity downhill running exacerbates heart rate and muscular fatigue in trail runners. J. Sports Sci. 2021, 39, 815–825. [Google Scholar] [CrossRef]
- Nance Jessica, R.; Mammen, A.L. Diagnostic Evaluation of Rhabdomyolysis. Muscle Nerve 2015, 176, 139–148. [Google Scholar]
| Author; Year; Reference | Population; Number; Sex; Age | Race; Length, Stages, Elevation | Temperature (Range); Humidity (% Relative) | AKI Biomarkers Pre, Post and Rec SCR (mg/dL) eGFR (mL/min/m²) | EAH Biomarkers [Na+] (mmol/L); Body Weight Loss | AKI Criteria (Stage; n; %) EAH Criteria (Stage; n; %) |
|---|---|---|---|---|---|---|
| Lipman, 2014 [43] | 30; | 250 km; | - | ↑SCR | - | RIFLE; |
| 7 F, 23 M; | 6; | Pre 1 ± 0.25; | Risk (8, 57%) | |||
| 39.6 ± 10 yrs | - | Post 1.4 ± 1.3 | Injury (1, 7%) | |||
| Poussel, 2020 [44] | 24; 1 F, 23 M; 36.5 yrs | 120 km; 1; 5700+ m | 8.6–11.1 °C; 89–99% | ↑SCR Pre 8.6 Post 8.9 ↓ eGFR Post (−29.2%) | - | KDIGO; Risk (1, 4.2%) Injury (0, 0%) |
| Hoppel, 2019 [45] | 8; 0 F, 8 M; 41.5 yrs | 67 km; 1; 4500+ m | 17–37 °C; 54% | ↑SCR Pre 0.9 ± 0.05 Post 1.54 ± 0.29 Rec 1.03 ± 0.14 ↓ eGFR Pre 106.23 ± 0.05 Post 58.53 ± 14.33 Rec 93.23 ± 13.06 | ↓[Na+] Pre 140.13 ± 1.64 Post 137.5 ± 42 Rec 139.5 ± 2.83 BDWL (−0.8 kg) | AKIN; Stage 1 (0, 0%) Stage 2 (2, 25%) EAH; Mil (3, 26.66%) Mod (1, 12.5%) |
| Jouffroy, 2019 [46] | 21; -; 43 ± 7 yrs | 80 km; 1; 1500+ m | - | ↑SCR Pre 0.89 ± 16.1 Post 0.68.8 ± 21.4 ↓ eGFR Pre 90 ± 14 Post 119 ± 33 | ↓[Na+] Pre 143 ± 5 Post 141 ± 7 Rec(9 d) 141 ± 5 - | KDIGO; Risk (1, 6%) Injury (0, 0%) |
| Hoffmann, 2016 [47] | 627; 187 F, 440 M; 41.36 yrs | 161 km; 1; 5500+, 7000− m | - | ↑SCR Group Risk 1.46 (1.39–1.61) Group Injury 1.98 (1.85–2.57) | - | RIFLE; Risk (227, 36.2%) Injury (31, 4.9%) |
| Pradas, 2021 [53] | 20; 0 F, 20 M; 42.45 yrs | 108 km; 1; 5800+ m | 14.4 ± 4.4 °C; 57 ± 16.1% | ↑SCR amateur Pre 90.1 ± 13.1 Post 120.8 ± 23.4 ↑SCR high lvl Pre 85 ± 10 Post 133 ± 11 | ↓[Na+] amateur Pre 140 ± 2 Post 140 ± 2 ↓[Na+] high lvl Pre 140 ± 1 Post 141 ± 3 | RIFLE; Risk (0, 0%) Injury (0, 0%) EAH; Mil (0, 0%) |
| Khodaee, 2021 [54] | 37; 8 F, 29 M; 43 yrs | 161 km; 1; 2800+ m | 2–22 °C; 57% | ↑SCR Pre 0.99 ± 0.15 Post 68.8 ± 21.4 | - | RIFLE Risk (18, 49%) Injury (0, 0%) |
| Le Goff, 2020 [48] | 33; 0 F3 M; 45.8 ± 8.7 yrs | 64.2 km; 1; 1400+ m | - | ↑SCR Pre 9.14 ± 1.05, Post 12.46 ± 2.09 Rec (3 h) 11.03 ± 2.0 | - | AKIN; Risk (0, 0%) |
| Lipman, 2016 [49] | 128; 36 F, 92 M; 39.6 ± 9 yrs | 250 km; 6; Nr | - | ↑SCR Pre 0.72 ± 0.13 Post 1.28 ± 0.34 | - | RIFLE; Risk (62, 48.4%) Injury (36, 28.1%) |
| M. Navarro, 2020 [50] | 65 km race 4 F,15 M; 107 km race 17 F, 26 M 41 ± 6 yrs | 107 km and 65 km; 1; 5604+, 4356− m | - | ↑SCR ↑107 km >↑65 km Post, Post 24 h ↓ eGFR 65 km = 107 km Post, Post 24 h | ↓[Na+] 65 km race BDWL (−6, +9) kg | RIFLE; Risk (32, 51.6%) Injury (0, 0%) EAH: Mil (3, 4.83%) Mod (2, 3.22%) Sev (0, 0%) |
| Belli, 2018 [51] | 6; 0 F,6 M; 47 ± 5 yrs | 217 kms; 1; 12,200 m; | - | ↑SCR Pre 1.00 ± 0.03, Post 1.33 ± 0.08 ↓ eGFR Pre 89 ± 5 Post 65 ± 5 | ↓[Na+] Nr; BDWL (−4.1 ± 0.7%) | RIFLE Risk (0, 0%) |
| Cairns, 2016 [52] | 15; 3 F,12 M; (40.7–50.6) yrs | 100 km y 174 km; - | 17.3–21.6 °C - | ↓ eGFR EAH mod-31, −43 y-8 ↓ eGFR EAH mil −26,−25 y-7 ↓ eGFR No EAH −11,−20 y-1 | - | RIFLE; Risk (0, 0%) EAH; Mil (3, 20%) Mod (7, 46%) |
| Boulter, 2011 [28] | 4; 0 F,4 M; 35 ± 6 yrs | 89 km; 1; - | 14-(−24 °C); 63% | ↑SCR Post (656–1139) | ↓[Na+] Post (131–136) BDWL (−6 a +9) kg | RIFLE; Risk (0, 0%) Injury (4, 100%) |
| Author; Year; Reference | Population; Number; Sex; Age | Race; Length, Stages, Elevation | Temperature (Range); Humidity (% Relative) | AKI Biomarkers Pre, Post and Rec SCR (mg/dL) eGFR(mL/min/m2) | EAH Biomarkers [Na+] (mmol/L); Body Weight | AKI Criteria (Stage; n; %) EAH (Stage; n; %) |
|---|---|---|---|---|---|---|
| Page, 2007 [55] | 123; 23 F, 97 M; - | 60 km; 1; 1340+ m | 8–14 °C; 60% | - | ↓[Na+] (130–134); BDWL +1.32 kg | EAH; Mil (5, 4%) |
| Scotney, 2015 [27] | 44; 8 F, 36 M; 39.5 yrs | 82 km; 1; 1000+ m | 6–15.6 °C; 79% | ↑SCR Post 96.6 ± 20 | ↓[Na+] (132–147); BDWL 1.75 ± 1.36 kg (2,42–1.85%) | AKI; Risk (0, 0%) EAH Mod (2, 5%) |
| Chlíbkovál, 2019 [56] | 20; 6 F, 14 M; 39.5 yrs | 24 horas; 1; 764+ m | (−7.9)–20.6 °C; 88.3% | - | ↓[Na+] (137–147); BDWL (−0.9%) | EAH Mil (0, 0%) |
| Hoffman, 2013 [57] | 669; 229 F, 440 M 41.36 yrs | 161 km; 1; 5500+, 7000− m | 20–38 °C; | - | ↓[Na+] (137–147); BDWL = 34.9% BDWL (0->3%) 46.6% BDWL (>3%)18.5% | EAH Mil (88, 13.2%) Mod (13, 1.9%) |
| Winger, 2013 [58] | 207; 40 F, 167 M; 43.0 ± 9.6 yrs | 161 km; 1; 5500+, 7000− m | - | - | ↓[Na+] Post (131–134); | EAH; Mil (12, 5.8%) |
| Bracher, 2012 [59] | 50; 0 F, 50 M; 47.8 yrs (45.4–50.3) | 100 km; 1; 645+ m; | 15.6–21.7 °C; 52–69% | ↑SCR Pre 77.8 (74.5–81.1) Post 100.4 (93.3–107.5) | ↓[Na+] Pre 136.6 (135.4–136.7) Post 100.4 (93.3–107.5) | AKI; Risk (0, 0%) EAH; Mil (0, 0%) |
| Knechtle, 2011 [60] | 120; -; 44.5 ± 7 yrs | 350 km; 7; 11,000+/− m | 15.6–21 °C; 62% | - | ↓[Na+] Pre 138.2 Post 138.49 BDWL (−0.2%) | EAH; Mil (7, 8%) |
| Hew-Butler, 2008 [61] | 82; 24 F, 58 M; 43.7 ± 1.1 yrs | 56 km; 1; 1000+ m | - | - | ↓[Na+] Pre 139 ± 0.3 Post 138± 10.4 BDWL (−3.8%) | EAH, Mil (0, 0%) |
| Cuthill, 2009 [24] | 4; 3 F,1 M; 41.5 yrs | 152.88 km; 1; 4267 m | - | ↑SCR Post 350.75 (114–761) | [Na+] Post 127.5 (120–134) | AKI; Injury (2, 50%) Failure (1, 25%) EAH; Mil (4, 100%) |
| Shepard, 2012 [62] | 145; 0 F, 145 M; | 100 km; 1; >500+ m | - | - | ↓[Na+] Post (130–135) | EAH; Mil (7, 4.8%) |
| Costa, 2013 [63] | 74; 28 F, 46 M; 41.8 yrs | 225 km; 5; 2200+ m | 32–40 °C; - | - | ↓[Na+] - BDWL (−1.2%) | EAH; Mil (8, 42%) |
| Cejka, 2012 [64] | 76; 0 F, 76 M; 47.1 yrs | 100 km; 1; 645+ m | 12–21 °C; - | - | [Na+] Pre 137.0 (2.7) Post 138.6 (2.6) BDWL (−1.8%) | EAH; Mil (4, 5.3%) |
| Knechtle, 2012 [65] | 219; 219 M, 0 F; 46.2 ± 9.3 yrs | 100 km; 1; 1050+ m | 15–8 °C; - | - | [Na+] Pre 137.7 ± 2.3 Post 138.6 ± 2.7 BDWL | EAH; Mil (0, 0%) |
| Schenk, 2021 [66] | 69 km race 0 F,11 M; 121 km race 0 F, 7 M 41.2 yrs | 121 km and 69 km; 1; 7554+ m, 4260+ m | 22–30 °C; - | - | ↓[Na+] 69 km Pre 142.9 ± 1.9 Post 143.7 ± 2.1 ↓[Na+] 121 km Pre 142.0 ± 1.7 Post 142.6 ± 4.2 BDWL 69 km −3.1%; 121 km −2.7% | EAH 69 km Mil (0, 0%) Mod (0, 0%) EAH 121 km Mil (0, 0%) Mod (0, 0%) |
| Khodaee, 2021 [67] | 84; 69 M, 15 F 42.1 ± 9 yrs | 161 km; 1; 2800+ m | 2–22 °C; 57% | ↓[Na+] Pre 138.4 ± 1.7 Post 135.8 ± 3 | EAH: Mil (17, 20%) Mod (1, 1.19%) |
| Race Category (Number of Studies) | Length (km) | Elevation (m) | Population; Gender; Age | AKI Cases (n and %) | |||
|---|---|---|---|---|---|---|---|
| Risk | Injury | Failure | Total | ||||
| Medium (3) | 65.33 ± 1.52 | 3630 ± 1950 | 60 (4 F,56 M) 42.98 ± 2.45 | 34 56.67% | 0 0% | 0 0% | 34 53.67% |
| Large (3) | 83.66 ± 4.75 | 1166 ± 288 | 69 (8 F,61 M) 39.60 ± 4.05 | 1 1.44% | 4 5.80% | 0 0% | 5 7.24% |
| Extra (10) | 136.22 ± 39.59 | 4851 ± 3440 | 826 (219 F, 607 M,) 42.92 ± 3.38 | 287 34.74% | 34 4.11% | 1 0.12% | 304 38.98% |
| Multistage (2) | 2500 ± 0 | 2000 ± 0 | 158 (43 F,115 M) 39.6 ± 2.1 | 70 44.30% | 37 23.41% | 0 0% | 108 68.35% |
| Total (18) | 127.82 ± 61.56 | 3560 ± 2951 | 1113 (274 F, 839 M) | 392 35.22% | 75 6.73% | 1 0.1% | 468 42.04% |
| Race Category (Number of Studies) | Length (km) | Elevation (m) | Population; Gender, Age | EAH Cases (n and %) | |||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Total | ||||
| Medium (7) | 61 ± 8.30 | 2501 ± 1974 | 482 (422 F, 60 M) 42.98 ± 2.45 | 11 2.28% | 2 0.41% | 0 0% | 13 2.69% |
| Large (3) | 83.66 ± 4.75 | 1166 ± 288 | 69 (8 F, 61 M) 39.06 ± 4.05 | 4 5.79% | 2 2.90% | 0 0% | 6 8.69% |
| Extra (11) | 124.63 ± 27.82 | 3746 ± 2613 | 1320 (307 F, 1013 M) 42.68 ± 2.95 | 142 10.75% | 19 1.43% | 0 0% | 161 12.19% |
| Multistage (2) | 287.50 ± 88.38 | 7067 ± 5562 | 194 (52 F, 142 M) 43.15 ± 1.90 | 15 7.73% | 0 0% | 0 0% | 15 7.73% |
| Total (19) | 114.08 ± 67.38 | 3319 ± 2792 | 2065 (427 F, 1638 M) 42.1 ± 1.90 | 172 7.73% | 23 1.11% | 0 0% | 195 9.44% |
| Race Category (Number of Studies) | Length (km) | Elevation (m) | Population; Gender; Age | AKI+EAH (n and %) |
|---|---|---|---|---|
| Medium (2) | 66 ± 1.41 | 4750 ± 353 | 27 (4 F, 23 M) 41.55 ± 0.07 | 5 18.51% |
| Large (3) | 83.7 ± 4.72 | 1166 ± 288.67 | 69 (8 F, 61 M) 39.60 ± 4.05 | 4 5.79% |
| Extra (5) | 113 ± 21.90 | 3342 ± 2366 | 132 (23 F, 109 M) 43.59 ± 2.47 | 18 13.63% |
| Total (10) | 95 ± 25.2 | 2971 ± 2094 | 228 (35 F, 193 M) 41.99 ± 3.12 | 36 11.84% |
| Study | Quality Assessment Tool 1 [41] | Level of Evidence 2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | Q | [42] | |
| Hoffman, 2013 [57] | yes | yes | no | no | yes | yes | yes | yes | yes | yes | yes | na | na | no | Good | A |
| Winger, 2013 [58] | yes | yes | yes | nr | no | no | yes | yes | no | no | yes | na | no | no | Good | A |
| Bracher, 2012 [59] | yes | yes | yes | yes | no | yes | yes | na | yes | no | yes | na | yes | yes | Good | A |
| Knechtle, 2011 [60] | yes | yes | nr | na | yes | yes | yes | yes | yes | no | yes | nr | nr | yes | Good | A |
| Hew-Butler, 2008 [61] | yes | yes | nr | nr | yes | yes | yes | yes | yes | no | yes | na | yes | yes | Good | A |
| Lipman, 2014 [43] | yes | yes | yes | no | no | yes | yes | yes | yes | no | yes | na | yes | na | Fair | B |
| Poussel, 2020 [44] | yes | yes | yes | yes | yes | yes | yes | no | yes | no | yes | na | no | na | Fair | B |
| Hoppel, 2019 [45] | yes | yes | no | yes | no | yes | yes | na | yes | yes | yes | na | no | na | Fair | B |
| Jouffroy, 2019 [46] | yes | yes | yes | yes | no | yes | yes | na | yes | yes | yes | na | yes | yes | Fair | B |
| Hoffmann, 2015 [47] | yes | yes | no | no | no | no | no | yes | yes | no | yes | na | no | yes | Fair | B |
| Le Goff, 2015 [48] | yes | no | no | yes | no | yes | yes | na | yes | no | yes | na | nr | yes | Fair | B |
| Lipman, 2016 [49] | yes | yes | yes | no | no | yes | yes | yes | yes | no | yes | na | yes | na | Fair | B |
| M. Navarro, 2020 [50] | yes | yes | yes | na | yes | yes | yes | na | yes | yes | yes | na | yes | yes | Fair | B |
| Khodaee, 2021 [54] | yes | yes | yes | no | no | yes | yes | yes | yes | no | yes | na | yes | na | Fair | B |
| Khodaee, 2021 [67] | yes | yes | yes | no | no | yes | yes | yes | yes | no | yes | na | yes | na | Fair | B |
| Pradas, 2021 [53] | yes | yes | yes | yes | yes | yes | yes | no | yes | no | yes | na | no | na | Fair | B |
| Schenk, 2021 [66] | yes | yes | yes | yes | yes | yes | yes | no | yes | no | yes | na | no | na | Fair | B |
| Belli, 2018 [51] | yes | yes | no | yes | yes | yes | yes | na | yes | yes | yes | na | yes | yes | Fair | B |
| Cairns, 2016 [52] | yes | yes | yes | yes | no | no | yes | yes | yes | yes | yes | na | no | yes | Fair | B |
| Boulter, 2011 [28] | yes | yes | nr | no | na | na | yes | na | yes | na | yes | na | na | yes | Fair | B |
| Page, 2007 [55] | yes | yes | nr | yes | no | yes | yes | yes | yes | no | yes | na | nr | yes | Fair | B |
| Scotney, 2015 [27] | yes | yes | yes | yes | yes | no | yes | yes | yes | no | yes | na | yes | yes | Fair | B |
| Chlíbková1, 2019 [56] | yes | yes | yes | no | yes | yes | yes | no | yes | no | yes | na | no | yes | Fair | B |
| Cuthill, 2009 [24] | yes | yes | na | yes | na | na | yes | yes | yes | no | yes | na | na | yes | Poor | C |
| Shepard, 2012 [62] | yes | nr | nr | no | yes | yes | yes | no | nr | no | yes | na | nr | no | Poor | C |
| Costa, 2013 [63] | yes | yes | nr | no | no | yes | yes | yes | yes | yes | yes | na | yes | yes | Poor | C |
| Cejka, 2012 [64] | yes | yes | yes | nr | no | yes | yes | yes | yes | no | yes | na | nr | yes | Poor | C |
| Knechtle, 2012 [65] | yes | yes | nr | yes | no | yes | yes | yes | yes | no | yes | nr | yes | yes | Poor | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecina, M.; Castellar-Otín, C.; López-Laval, I.; Carrasco Páez, L.; Pradas, F. Acute Kidney Injury and Hyponatremia in Ultra-Trail Racing: A Systematic Review. Medicina 2022, 58, 569. https://doi.org/10.3390/medicina58050569
Lecina M, Castellar-Otín C, López-Laval I, Carrasco Páez L, Pradas F. Acute Kidney Injury and Hyponatremia in Ultra-Trail Racing: A Systematic Review. Medicina. 2022; 58(5):569. https://doi.org/10.3390/medicina58050569
Chicago/Turabian StyleLecina, Miguel, Carlos Castellar-Otín, Isaac López-Laval, Luis Carrasco Páez, and Francisco Pradas. 2022. "Acute Kidney Injury and Hyponatremia in Ultra-Trail Racing: A Systematic Review" Medicina 58, no. 5: 569. https://doi.org/10.3390/medicina58050569
APA StyleLecina, M., Castellar-Otín, C., López-Laval, I., Carrasco Páez, L., & Pradas, F. (2022). Acute Kidney Injury and Hyponatremia in Ultra-Trail Racing: A Systematic Review. Medicina, 58(5), 569. https://doi.org/10.3390/medicina58050569










