Prognostic Gene Predictors of Gestational Diabetes in Endometrium and Follicular Fluid of Women after Infertility
Abstract
1. Introduction
2. Results
Expression of Genes Inherent in Glucose Metabolism in Healthy and GD Patients
3. Discussion
4. Materials and Methods
4.1. Study Population and Data Collection
4.2. Isolation and Cultivation of Human Endometrium Derived Mesenchymal Stem Cells and Follicular Fluid
4.3. RNA Isolation and Gene Expression Analysis Using RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Medical Data of Births, 2019; Institute of Hygiene Health Information Centre the Lithuanian Academy of Sciences: Vilnius, Lithuania, 2020; Available online: https://www.hi.lt/uploads/pdf/leidiniai/Statistikos/Gimimu/gimimai_2019.pdf (accessed on 8 March 2022).
- Li, G.; Wei, T.; Ni, W.; Zhang, A.; Zhang, J.; Xing, Y.; Xing, Q. Incidence and Risk Factors of Gestational Diabetes Mellitus: A Prospective Cohort Study in Qingdao, China. Front. Endocrinol. 2020, 11, 636. [Google Scholar] [CrossRef]
- De Paula Ferreira, L.A.; de Azevedo Piccinato, C.; Cordioli, E.; Zlotnik, E. Pregestational Body Mass Index, Weight Gain during Pregnancy and Perinatal Outcome: A Retrospective Descriptive Study. Einstein 2019, 18, eAO4851. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High Prevalence of Type 2 Diabetes and Pre-Diabetes in Adult Offspring of Women with Gestational Diabetes Mellitus or Type 1 Diabetes: The Role of Intrauterine Hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational Diabetes and the Risk of Cardiovascular Disease in Women: A Systematic Review and Meta-Analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, D.; Carrozzino, D.; Fraticelli, F.; Fulcheri, M.; Vitacolonna, E. Quality of Life in Women with Gestational Diabetes Mellitus: A Systematic Review. J. Diabetes Res. 2017, 2017, 7058082. [Google Scholar] [CrossRef]
- Murray, S.R.; Reynolds, R.M. Short- and Long-Term Outcomes of Gestational Diabetes and Its Treatment on Fetal Development. Prenat. Diagn. 2020, 40, 1085–1091. [Google Scholar] [CrossRef]
- Tobias, D.K.; Chavarro, J.E.; Williams, M.A.; Buck Louis, G.M.; Hu, F.B.; Rich-Edwards, J.; Missmer, S.A.; Zhang, C. History of Infertility and Risk of Gestational Diabetes Mellitus: A Prospective Analysis of 40,773 Pregnancies. Am. J. Epidemiol 2013, 178, 1219–1225. [Google Scholar] [CrossRef]
- Holst, S.; Kjær, S.K.; Jørgensen, M.E.; Damm, P.; Jensen, A. Fertility Problems and Risk of Gestational Diabetes Mellitus: A Nationwide Cohort Study. Fertil. Steril. 2016, 106, 427.e1–434.e1. [Google Scholar] [CrossRef][Green Version]
- Mohammadi, M.; Khedmati Morasae, E.; Maroufizadeh, S.; Almasi-Hashiani, A.; Navid, B.; Amini, P.; Omani-Samani, R.; Alizadeh, A. Assisted Reproductive Technology and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Middle East Fertil. Soc. J. 2020, 25, 6. [Google Scholar] [CrossRef]
- Grady, R.; Alavi, N.; Vale, R.; Khandwala, M.; McDonald, S.D. Elective Single Embryo Transfer and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Fertil. Steril. 2012, 97, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gong, T.-T.; Jiang, Y.-T.; Zhang, S.; Zhao, Y.-H.; Wu, Q.-J. Global, Regional, and National Prevalence and Disability-Adjusted Life-Years for Infertility in 195 Countries and Territories, 1990–2017: Results from a Global Burden of Disease Study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef] [PubMed]
- Enquobahrie, D.A.; Williams, M.A.; Qiu, C.; Meller, M.; Sorensen, T.K. Global Placental Gene Expression in Gestational Diabetes Mellitus. Am. J. Obs. Gynecol. 2009, 200, 206.e1–206.e13. [Google Scholar] [CrossRef] [PubMed]
- Dłuski, D.F.; Wolińska, E.; Skrzypczak, M. Epigenetic Changes in Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 7649. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Lynch, K.F.; Shaat, N.; Håkansson, R.; Ivarsson, S.A.; Berntorp, K.; Agardh, C.D.; Lernmark, Å. DiPiS Study Group Gestational Diabetes Mellitus Is Associated with TCF7L2 Gene Polymorphisms Independent of HLA-DQB1*0602 Genotypes and Islet Cell Autoantibodies. Diabetes Med. 2011, 28, 1018–1027. [Google Scholar] [CrossRef]
- Včelák, J.; Vejražková, D.; Vaňková, M.; Lukášová, P.; Bradnová, O.; Hálková, T.; Bešťák, J.; Andělová, K.; Kvasničková, H.; Hoskovcová, P.; et al. T2D Risk Haplotypes of the TCF7L2 Gene in the Czech Population Sample: The Association with Free Fatty Acids Composition. Physiol. Res. 2012, 61, 229–240. [Google Scholar] [CrossRef]
- Cho, Y.M.; Kim, T.H.; Lim, S.; Choi, S.H.; Shin, H.D.; Lee, H.K.; Park, K.S.; Jang, H.C. Type 2 Diabetes-Associated Genetic Variants Discovered in the Recent Genome-Wide Association Studies Are Related to Gestational Diabetes Mellitus in the Korean Population. Diabetologia 2009, 52, 253–261. [Google Scholar] [CrossRef]
- Lui, J.C.; Finkielstain, G.P.; Barnes, K.M.; Baron, J. An Imprinted Gene Network That Controls Mammalian Somatic Growth Is Down-Regulated during Postnatal Growth Deceleration in Multiple Organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R189–R196. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational Diabetes Mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhou, H.; Ma, J.; Lu, Y.; Pan, B. Prevalence of Diabetes Mellitus and Hypertension during Pregnancy in Eastern China after the Implementation of Universal Two-Child Policy. Int. J. Diabetes Dev. Ctries. 2020, 41, 221–227. [Google Scholar] [CrossRef]
- Hunt, K.J.; Schuller, K.L. The Increasing Prevalence of Diabetes in Pregnancy. Obs. Gynecol. Clin. N. Am. 2007, 34, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Visser, G.H.A. Obesity, Gestational Diabetes and Pregnancy Outcome. Semin. Fetal Neonatal Med. 2009, 14, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the Ovarian Follicular Antrum and Follicular Fluid. Biol. Reprod. 2010, 82, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Baskaran, S.; Agarwal, A. Proteomics of Reproduction: Prospects and Perspectives. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 92, pp. 217–243. ISBN 978-0-12-817477-7. [Google Scholar]
- Scalici, E.; Traver, S.; Molinari, N.; Mullet, T.; Monforte, M.; Vintejoux, E.; Hamamah, S. Cell-Free DNA in Human Follicular Fluid as a Biomarker of Embryo Quality. Hum. Reprod. 2014, 29, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Ramachandran, V.; Hayat, S.; Dargham, S.R.; Cunningham, T.K.; Benurwar, M.; Sathyapalan, T.; Najafi-Shoushtari, S.H.; Atkin, S.L. Expression of MicroRNA in Follicular Fluid in Women with and without PCOS. Sci Rep. 2019, 9, 16306. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Girish Kumar, V.; Manjunatha, B.M.; Ramesh, H.S.; Gupta, P.S.P. Follicular Fluid Concentrations of Glucose, Lactate and Pyruvate in Buffalo and Sheep, and Their Effects on Cultured Oocytes, Granulosa and Cumulus Cells. Theriogenology 2008, 69, 186–196. [Google Scholar] [CrossRef]
- Identification of New Biomarkers of Human Endometrial Receptivity in the Natural Cycle-Human Reproduction-Oxford Academic. Available online: https://academic.oup.com/humrep/article/24/1/198/691688 (accessed on 7 November 2021).
- Vrhovac Madunić, I.; Karin-Kujundžić, V.; Madunić, J.; Šola, I.M.; Šerman, L. Endometrial Glucose Transporters in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 2381. [Google Scholar] [CrossRef]
- von Wolff, M.; Ursel, S.; Hahn, U.; Steldinger, R.; Strowitzki, T. Glucose Transporter Proteins (GLUT) in Human Endometrium: Expression, Regulation, and Function throughout the Menstrual Cycle and in Early Pregnancy. J. Clin. Endocrinol. Metab. 2003, 88, 3885–3892. [Google Scholar] [CrossRef]
- Su, R.; Wang, C.; Feng, H.; Lin, L.; Liu, X.; Wei, Y.; Yang, H. Alteration in Expression and Methylation of IGF2/H19 in Placenta and Umbilical Cord Blood Are Associated with Macrosomia Exposed to Intrauterine Hyperglycemia. PLoS ONE 2016, 11, e0148399. [Google Scholar] [CrossRef]
- White, V.; Jawerbaum, A.; Mazzucco, M.B.; Gauster, M.; Desoye, G.; Hiden, U. IGF2 Stimulates Fetal Growth in a Sex- and Organ-Dependent Manner. Pediatr. Res. 2018, 83, 183–189. [Google Scholar] [CrossRef]
- Smith, F.M.; Holt, L.J.; Garfield, A.S.; Charalambous, M.; Koumanov, F.; Perry, M.; Bazzani, R.; Sheardown, S.A.; Hegarty, B.D.; Lyons, R.J.; et al. Mice with a Disruption of the Imprinted Grb10 Gene Exhibit Altered Body Composition, Glucose Homeostasis, and Insulin Signaling during Postnatal Life. Mol. Cell. Biol. 2007, 27, 5871–5886. [Google Scholar] [CrossRef] [PubMed]
- González-Renteria, S.M.; Sosa-Macías, M.; Rodríguez-Moran, M.; Chairez-Hernández, I.; Lares-Aseff, I.A.; Guerrero-Romero, F.; Galaviz-Hernández, C. Association of the Intronic Polymorphism Rs12540874 A>G of the GRB10 Gene with High Birth Weight. Early Hum. Dev. 2014, 90, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Ravikumar, G.; Dwarkanath, P.; Meraaj, H.; Thomas, A.; Crasta, J.; Thomas, T.; Kurpad, A.V.; Sridhar, T.S. Placental Expression of the Insulin Receptor Binding Protein GRB10: Relation to Human Fetoplacental Growth and Fetal Gender. Placenta 2015, 36, 1225–1230. [Google Scholar] [CrossRef]
- Kamiya, M.; Judson, H.; Okazaki, Y.; Kusakabe, M.; Muramatsu, M.; Takada, S.; Takagi, N.; Arima, T.; Wake, N.; Kamimura, K.; et al. The Cell Cycle Control Gene ZAC/PLAGL1 Is Imprinted—A Strong Candidate Gene for Transient Neonatal Diabetes. Hum. Mol. Genet. 2000, 9, 453–460. [Google Scholar] [CrossRef]
- Wurst, U.; Ebert, T.; Kralisch, S.; Stumvoll, M.; Fasshauer, M. Serum Levels of the Adipokine Pref-1 in Gestational Diabetes Mellitus. Cytokine 2014, 71, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kleiblova, P.; Dostalova, I.; Bartlova, M.; Lacinova, Z.; Ticha, I.; Krejci, V.; Springer, D.; Kleibl, Z.; Haluzik, M. Expression of Adipokines and Estrogen Receptors in Adipose Tissue and Placenta of Patients with Gestational Diabetes Mellitus. Mol. Cell Endocrinol. 2010, 314, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Knabl, J.; Hiden, U.; Hüttenbrenner, R.; Riedel, C.; Hutter, S.; Kirn, V.; Günthner-Biller, M.; Desoye, G.; Kainer, F.; Jeschke, U. GDM Alters Expression of Placental Estrogen Receptor α in a Cell Type and Gender-Specific Manner. Reprod. Sci. 2015, 22, 1488–1495. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Louden, E.D.; Chi, M.M.-Y.; Frolova, A.I.; Riley, J.K.; Moley, K.H. Preimplantation Exposure of Mouse Embryos to Palmitic Acid Results in Fetal Growth Restriction Followed by Catch-Up Growth in the Offspring. Biol. Reprod. 2011, 85, 678–683. [Google Scholar] [CrossRef]
- Tavakol, S.; Azedi, F.; Hoveizi, E.; Ai, J.; Joghataei, M.T. Human Endometrial Stem Cell Isolation from Endometrium and Menstrual Blood. Bio-Protocol 2018, 8, e2693. [Google Scholar] [CrossRef]
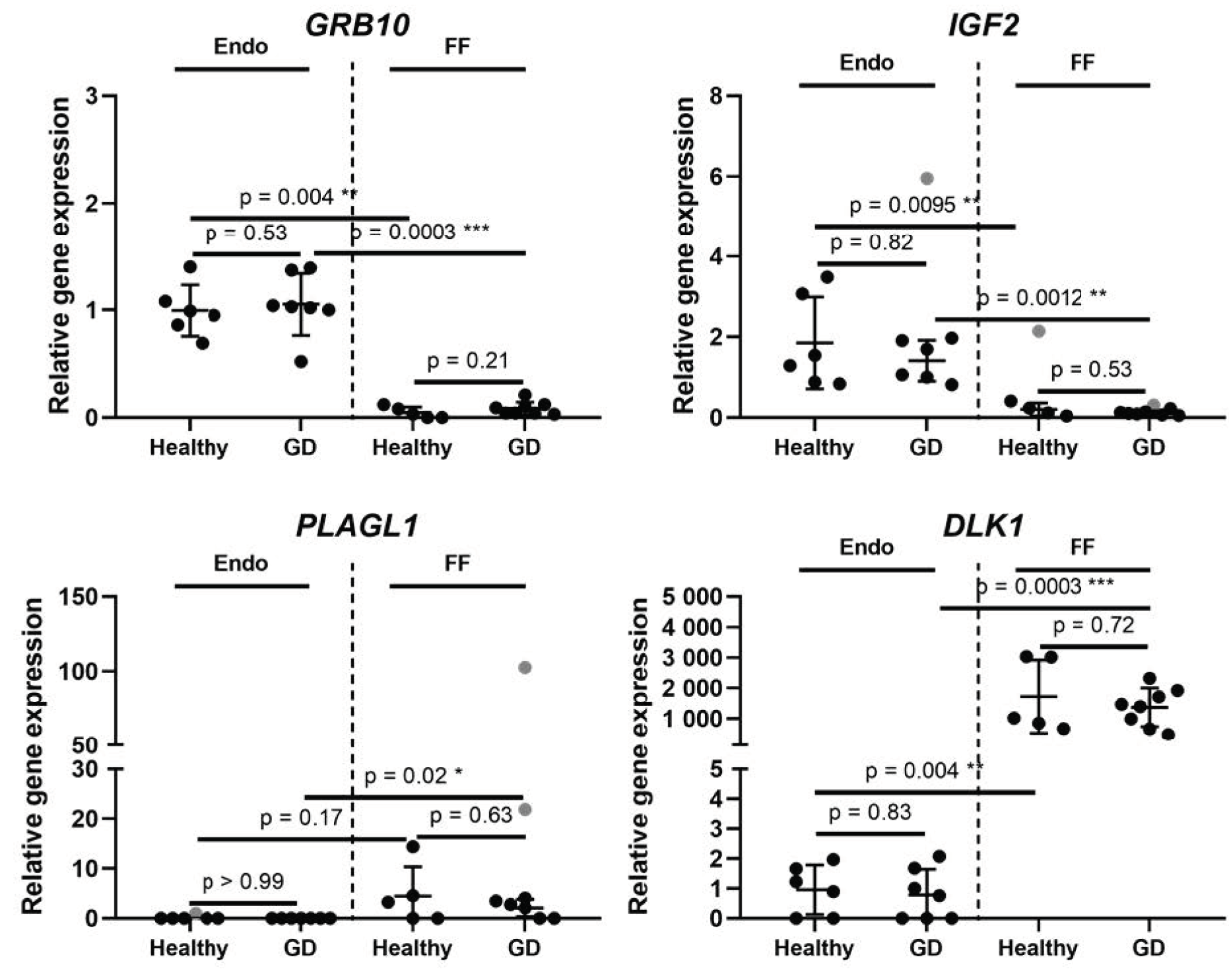
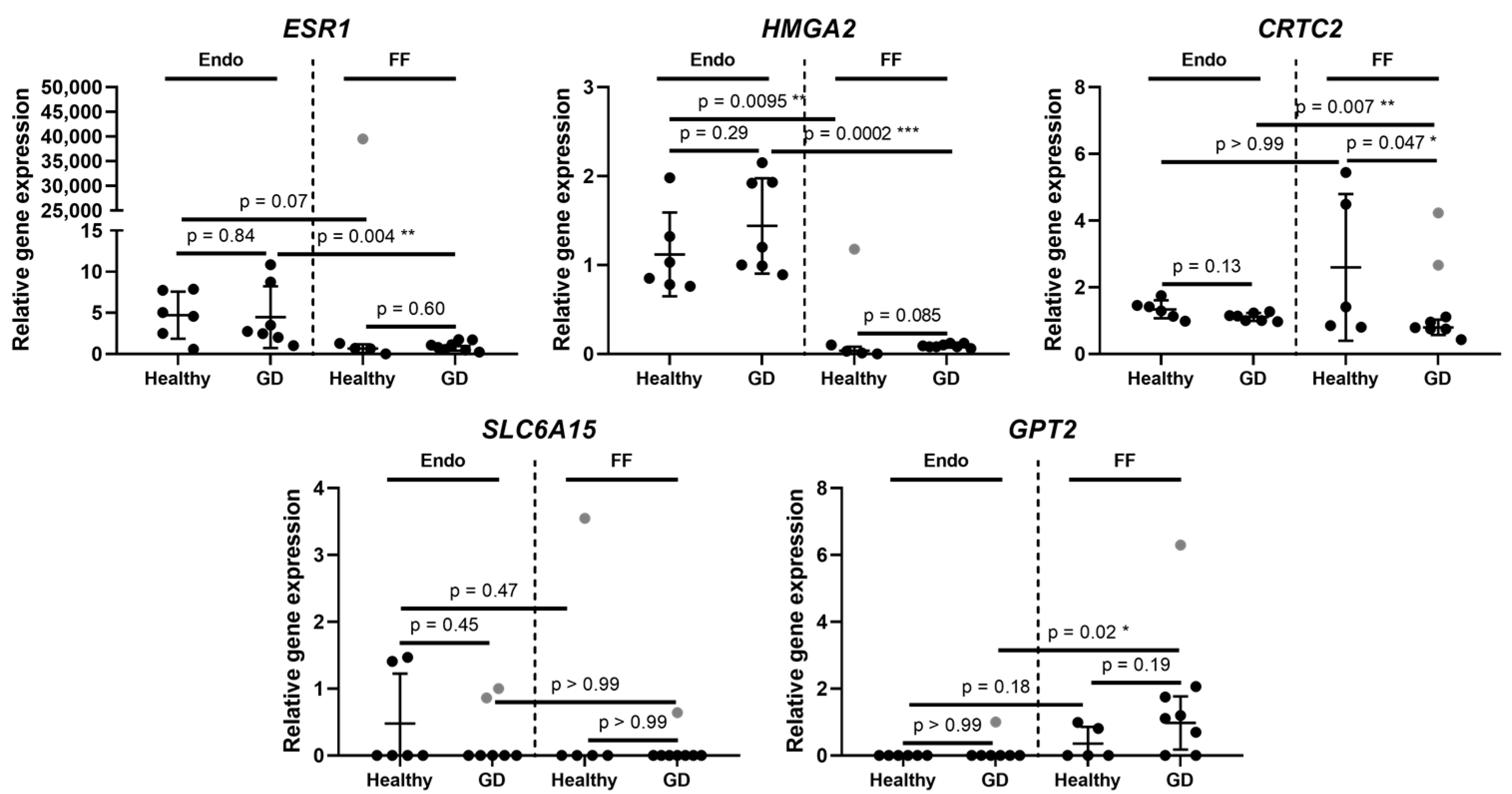
| Gene Symbol | Gene Name | Gene Ontology (Molecular Function) | Location | Endometrium | Follicular Fluid | ||
|---|---|---|---|---|---|---|---|
| Fold | p * | Fold | p * | ||||
| CRTC2 | CREB regulated transcription coactivator 2 | Transcription of genes targeted by the cAMP response element-binding protein | 1q21.3 | 0.83 | 0.13 | 0.31 | 0.047 |
| DLK1 | Delta like non-canonical Notch ligand 1 | Regulation of cell growth and differentiation | 14q32.2 | 0.82 | 0.83 | 0.79 | 0.72 |
| ESR1 | Estrogen receptor 1 | Transcription factor | 6q25.1-q25.2 | 0.95 | 0.84 | 1.47 | 0.60 |
| GPT2 | Glutamic--pyruvic transaminase 2 | Catalysation of the reversible transamination. Play roles in gluconeogenesis and amino acid metabolism | 16q11.2 | 0 | >0.99 | 2.71 | 0.19 |
| GRB10 | Growth factor receptor bound protein 10 | Interaction with insulin receptors and insulin-like growth-factor receptors | 7p12.1 | 1.06 | 0.53 | 1.82 | 0.21 |
| HMGA2 | High mobility group AT-hook 2 | Component of the enhancesome. Transcriptional regulating factor, enhancing or suppressing the transcriptional activity depending on the number and spacing of the AT-rich binding sites in DNA | 12q14.3 | 1.29 | 0.29 | 2.61 | 0.085 |
| IGF2 | Insulin like growth factor 2 | Growth factor, involved in development and growth | 11p15.5 | 0.76 | 0.82 | 0.57 | 0.53 |
| PLAGL1 | PLAG1 like zinc finger 1 | Suppressor of cell growth. Overexpression of this gene during fetal development is thought to be the causal factor for transient neonatal diabetes mellitus | 6q24.2 | 0 | >0.99 | 0.47 | 0.63 |
| SLC6A15 | Solute carrier family 6 member 15 | Transport of neutral amino acids | 12q21.31 | 0.45 | 0.45 | 0 | >0.99 |
| Characteristics | GDM Cases (n = 15) | Controls (n = 12) |
|---|---|---|
| Maternal age, in years | 33.8 ± 3.6 | 31.7± 2.9 |
| BMI before pregnancy | 23.4 ± 4.1 | 22.1 ± 3.8 |
| GDM insulin treatment, % | 13.3 | - |
| Gestational age at delivery, wks. | 37 ± 2.7 | 38 ± 4.2 |
| Delivery mode, % Vaginal Cesarean | 80.0 20.0 | 83.3 16.7 |
| Preterm rupture of membranes, % | 33.3 | 41.6 |
| Nulliparous, % | 86.6 | 33.3 |
| Pregestational obesity, % | 13.3 | 16.6 |
| Macrosomia, % | 20.0 | 25.0 |
| Gene Name | Primer Sequence (5′-3′), F—Forward, R—Reverse | Product Size (bp) |
|---|---|---|
| GAPDH | F: GTGAACCATGAGAAGTATGACAAC R: CATGAGTCCTTCCACGATACC | 123 |
| IGF2 | F: TCGTTGAGGAGTGCTGTTTCC R: ACACGTCCCTCTCGGACTTG | 87 |
| GRB10 | F: GCAAACAGGACGCGTGATAGAG R: GTGAATCACTGTACTTAGGG | 148 |
| CRTC2 | F: CTCTGCCCAATGTTAACCAGAT R: GAGTGCTCCGAGATGAATCC | 87 |
| HMGA2 | F: CCCAAAGGCAGCAAAAACAA R: GCCTCTTGGCCGTTTTTCTC | 81 |
| ESR1 | F: TGGGAATGAAAGGTGGGATAC R: AGGTTGGCTCTCATGTC | 124 |
| DLK1 | F: TCCTCAACAAGTGCGAGACC R: CTGTGGGAACGCTGCTTAGA | 191 |
| SLC6A15 | F: TGCTAATAGCTAGTGTTGTGAATATGG R: GGATAGCTCAGAAATTCTTCAGATG | 94 |
| GPT2 | F: GAACATAAGCGAATGAAGAAAGAAG R: CCAGCAGGTTTGGGTAGGT | 294 |
| PLAGL1 | F: GGCGGGTAGGTAGGAAAGG R: GATGTTTATGAATCAGGCAGG | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaigauskaitė, B.; Baušytė, R.; Valatkaitė, E.; Skliutė, G.; Kazėnaitė, E.; Ramašauskaitė, D.; Navakauskienė, R. Prognostic Gene Predictors of Gestational Diabetes in Endometrium and Follicular Fluid of Women after Infertility. Medicina 2022, 58, 498. https://doi.org/10.3390/medicina58040498
Vaigauskaitė B, Baušytė R, Valatkaitė E, Skliutė G, Kazėnaitė E, Ramašauskaitė D, Navakauskienė R. Prognostic Gene Predictors of Gestational Diabetes in Endometrium and Follicular Fluid of Women after Infertility. Medicina. 2022; 58(4):498. https://doi.org/10.3390/medicina58040498
Chicago/Turabian StyleVaigauskaitė, Brigita, Raminta Baušytė, Elvina Valatkaitė, Giedrė Skliutė, Edita Kazėnaitė, Diana Ramašauskaitė, and Rūta Navakauskienė. 2022. "Prognostic Gene Predictors of Gestational Diabetes in Endometrium and Follicular Fluid of Women after Infertility" Medicina 58, no. 4: 498. https://doi.org/10.3390/medicina58040498
APA StyleVaigauskaitė, B., Baušytė, R., Valatkaitė, E., Skliutė, G., Kazėnaitė, E., Ramašauskaitė, D., & Navakauskienė, R. (2022). Prognostic Gene Predictors of Gestational Diabetes in Endometrium and Follicular Fluid of Women after Infertility. Medicina, 58(4), 498. https://doi.org/10.3390/medicina58040498






