Cardiometabolic Health in Adolescents and Young Adults with Congenital Adrenal Hyperplasia
Abstract
1. Introduction
2. Materials and Methods
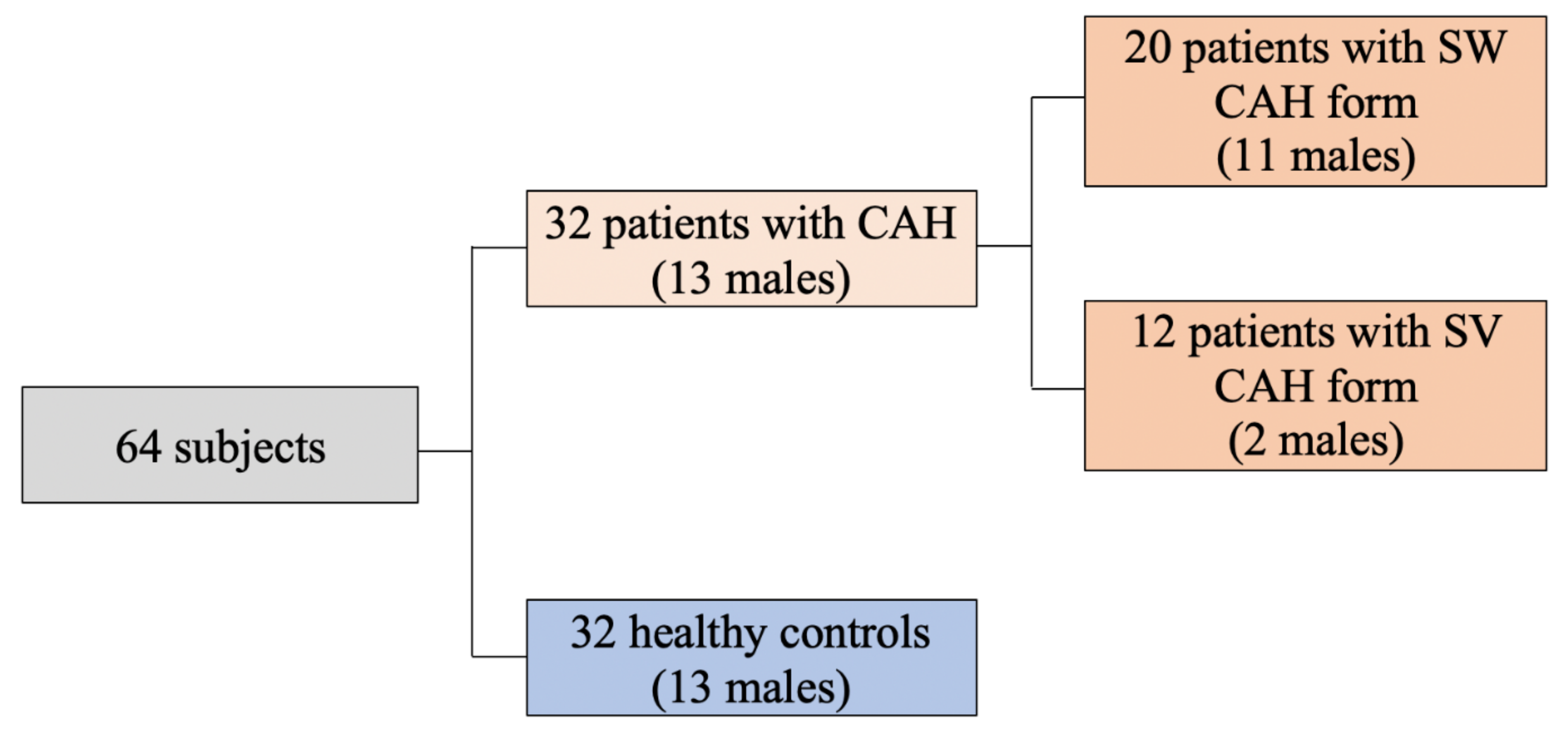
2.1. Subjects
2.2. Biochemical and Molecular Diagnosis of CAH
2.3. Therapy
2.4. Physical Examination
2.5. Laboratory Investigations
2.6. Body Composition Assessment
2.7. Biochemical and Hormonal Assays
2.8. Diagnostic Criteria for Metabolic Syndrome
2.9. Statistics
3. Results
3.1. Cardiometabolic Risk Factors
3.1.1. BMI and Adipose Tissue Distribution
3.1.2. Blood Pressure
3.1.3. Glucose Metabolism and Insulin Resistance
3.1.4. Dyslipidemia
3.2. Hormonal Profile
3.3. Relationship of HC Doses with Body Composition, Insulin Sensitivity, Blood Pressure and Lipid Profile
3.4. Metabolic Syndrome
3.5. Genotype and Phenotype Correlation
4. Discussion
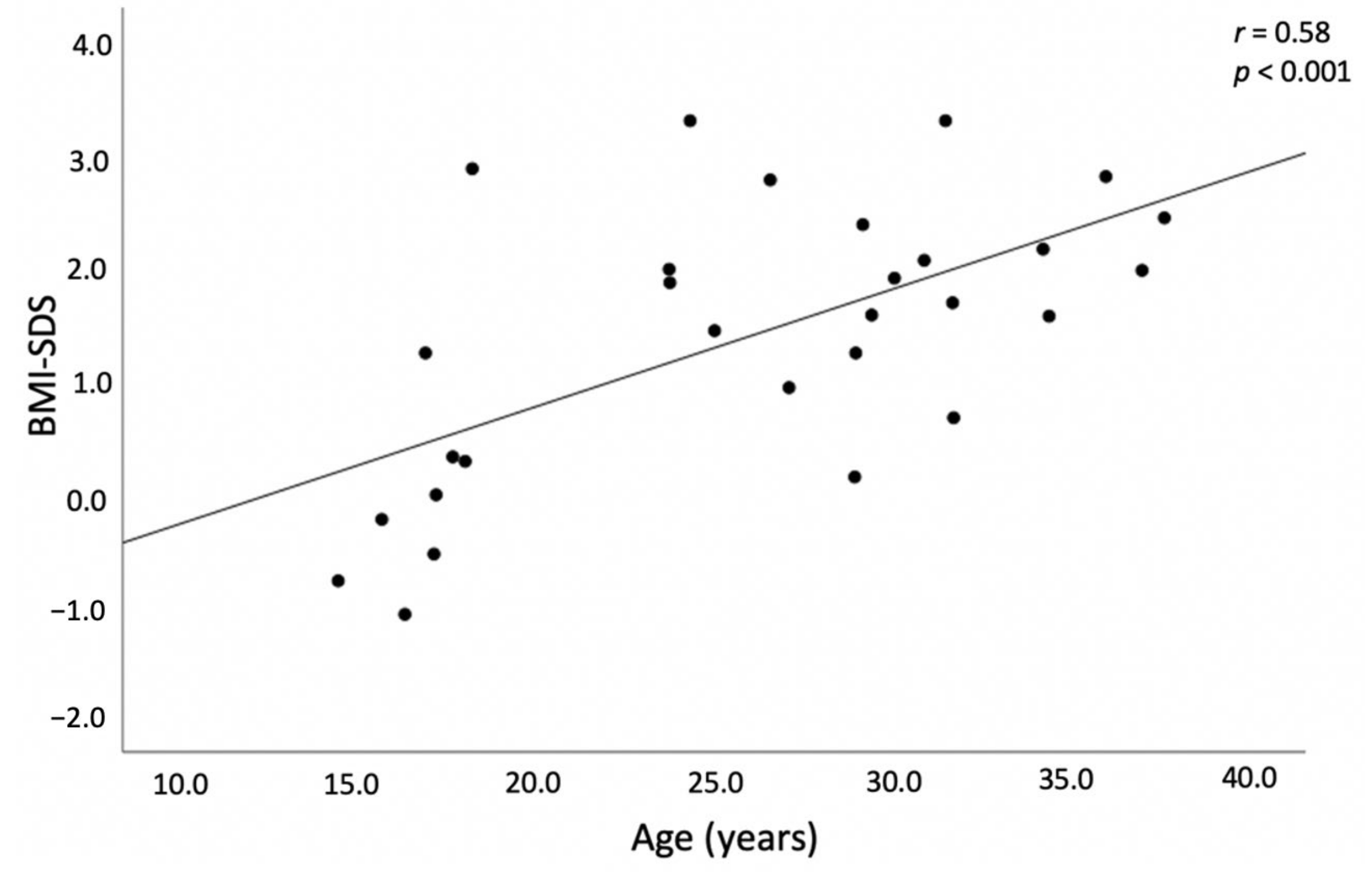
4.1. BMI and Adipose Tissue Distribution
4.2. Glucose Metabolism and Insulin Resistance
4.3. Blood Pressure
4.4. Dyslipidemia
4.5. Genotype and Phenotype Correlation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Der Claahsen-van Grinten, H.L.; Speiser, P.W.; Ahmed, S.F.; Arlt, W.; Auchus, R.J.; Falhammar, H.; Flück, C.E.; Guasti, L.; Huebner, A.; Kortmann, B.B.M.; et al. Congenital adrenal hyperplasia—Current insights in pathophysiology, diagnostics and management. Endocr. Rev. 2022, 43, 91–159. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Gong, L.; Gao, X.; Yang, N.; Zhao, J.; Yang, H.; Kong, Y. A pilot study on newborn screening for congenital adrenal hyperplasia in Beijing. J. Pediatr. Endocrinol. Metab. 2019, 32, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F. Congenital Adrenal Hyperplasia. J. Pediatr. Adolesc. Gynecol. 2017, 30, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Auchus, R.J. Novel treatment strategies in congenital adrenal hyperplasia. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 225–232. [Google Scholar] [CrossRef][Green Version]
- Tamhane, S.; Rodriguez-Gutierrez, R.; Iqbal, A.M.; Prokop, L.J.; Bancos, I.; Speiser, P.W.; Murad, M.H. Cardiovascular and Metabolic Outcomes in Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2018, 103, 4097–4103. [Google Scholar] [CrossRef]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef]
- Gomes, L.G.; Mendonca, B.B.; Bachega, T.A.S.S. Long-term cardio-metabolic outcomes in patients with classical congenital adrenal hyperplasia: Is the risk real? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 155–161. [Google Scholar] [CrossRef]
- Falhammar, H.; Frisen, L.; Hirschberg, A.L.; Norrby, C.; Almqvist, C.; Nordenskjold, A.; Nordenstrom, A. Increased Cardiovascular and Metabolic Morbidity in Patients With 21-Hydroxylase Deficiency: A Swedish Population-Based National Cohort Study. J. Clin. Endocrinol. Metab. 2015, 100, 3520–3528. [Google Scholar] [CrossRef]
- Arlt, W.; Willis, D.S.; Wild, S.H.; Krone, N.; Doherty, E.J.; Hahner, S.; Han, T.S.; Carroll, P.V.; Conway, G.S.; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE); et al. Health status of adults with congenital adrenal hyperplasia: A cohort study of 203 patients. J. Clin. Endocrinol. Metab. 2010, 95, 5110–5121. [Google Scholar] [CrossRef]
- Kim, M.S.; Ryabets-Lienhard, A.; Dao-Tran, A.; Mittelman, S.D.; Gilsanz, V.; Schrager, S.M.; Geffner, M.E. Increased Abdominal Adiposity in Adolescents and Young Adults With Classical Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. J. Clin. Endocrinol. Metab. 2015, 100, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, E.M.; de Lemos-Marini, S.H.; de Mello, M.P.; Baptista, M.T.; D’Souza-Li, L.F.; Baldin, A.D.; Carvalho, W.R.; Farias, E.S.; Guerra-Junior, G. Impairment in anthropometric parameters and body composition in females with classical 21-hydroxylase deficiency. J. Pediatr. Endocrinol. Metab. 2009, 22, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Deeb, A.; Ong, K.K.; Bich, W.; Murgatroyd, P.R.; Hughes, I.A.; Acerini, C.L. Insulin sensitivity and body composition in children with classical and nonclassical congenital adrenal hyperplasia. Clin. Endocrinol. 2010, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Navardauskaite, R.; Baneviciute, K.; Songailiene, J.; Grigalioniene, K.; Cereskevicius, D.; Sukys, M.; Mockeviciene, G.; Smirnova, M.; Utkus, A.; Verkauskiene, R. Impact of Newborn Screening on Clinical Presentation of Congenital Adrenal Hyperplasia. Medicina 2021, 57, 1035. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef]
- Krone, N.; Braun, A.; Roscher, A.A.; Knorr, D.; Schwarz, H.P. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J. Clin. Endocrinol. Metab. 2000, 85, 1059–1065. [Google Scholar] [CrossRef]
- Concolino, P.; Costella, A. Congenital Adrenal Hyperplasia (CAH) due to 21-Hydroxylase Deficiency: A Comprehensive Focus on 233 Pathogenic Variants of CYP21A2 Gene. Mol. Diagn. Ther. 2018, 22, 261–280. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef]
- Tutkuvienė, J. Vaikų Augimo ir Brendimo Vertinimas; Meralas: Vilnius, Lithuania, 1995; pp. 2–20. [Google Scholar]
- WHO Expert Consultation. Waist Circumference and Waist-Hip Ratio; Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008; pp. 8–11. [Google Scholar]
- Katzmarzyk, P.T.; Shen, W.; Baxter-Jones, A.; Bell, J.D.; Butte, N.F.; Demerath, E.W.; Gilsanz, V.; Goran, M.I.; Hirschler, V.; Hu, H.H.; et al. Adiposity in children and adolescents: Correlates and clinical consequences of fat stored in specific body depots. Pediatr. Obes. 2012, 7, 42–61. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics 1996, 98, 649–658. [Google Scholar]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia; Report of a WHO/IDF Consultation; WHO/IDF: Geneva, Switzerland, 2016; Available online: https://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ (accessed on 10 January 2022).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rodden, A.M.; Diaz, V.A.; Mainous, A.G.; Koopman, R.J.; Geesey, M.E. Insulin resistance in adolescents. J. Pediatr. 2007, 151, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; American Heart Association; National Heart, Lung, and Blood Institute; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.E.A.M.; Soliman, A.T.; Ramadan, M.A.; Elawwa, A.; Abugabal, A.M.S.; Emam, M.H.A.; De Sanctis, V. Long-term prednisone versus hydrocortisone treatment in children with classic Congenital Adrenal Hyperplasia (CAH) and a brief review of the literature. Acta Biomed. 2019, 90, 360–369. [Google Scholar] [CrossRef]
- Volkl, T.M.; Simm, D.; Beier, C.; Dorr, H.G. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 2006, 117, 98–105. [Google Scholar] [CrossRef]
- Bachelot, A.; Golmard, J.L.; Dulon, J.; Dahmoune, N.; Leban, M.; Bouvattier, C.; Cabrol, S.; Leger, J.; Polak, M.; Touraine, P. Determining clinical and biological indicators for health outcomes in adult patients with childhood onset of congenital adrenal hyperplasia. Eur. J. Endocrinol. 2015, 173, 175–184. [Google Scholar] [CrossRef]
- Moreira, R.P.; Villares, S.M.; Madureira, G.; Mendonca, B.B.; Bachega, T.A. Obesity and familial predisposition are significant determining factors of an adverse metabolic profile in young patients with congenital adrenal hyperplasia. Horm. Res. Paediatr. 2013, 80, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ariyawatkul, K.; Tepmongkol, S.; Aroonparkmongkol, S.; Sahakitrungruang, T. Cardio-metabolic risk factors in youth with classical 21-hydroxylase deficiency. Eur. J. Pediatr. 2017, 176, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Torky, A.; Sinaii, N.; Jha, S.; Desai, J.; El-Maouche, D.; Mallappa, A.; Merke, D.P. Cardiovascular Disease Risk Factors and Metabolic Morbidity in a Longitudinal Study of Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2021, 106, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.P.; Gomes, L.G.; Mendonca, B.B.; Bachega, T.A. Impact of glucocorticoid receptor gene polymorphisms on the metabolic profile of adult patients with the classical form of 21-hydroxylase deficiency. PLoS ONE 2012, 7, e44893. [Google Scholar] [CrossRef]
- Santen, R.J.; Jewell, C.M.; Yue, W.; Heitjan, D.F.; Raff, H.; Katen, K.S.; Cidlowski, J.A. Glucocorticoid Receptor Mutations and Hypersensitivity to Endogenous and Exogenous Glucocorticoids. J. Clin. Endocrinol. Metab. 2018, 103, 3630–3639. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.S.; Cho, A.R.; Lee, Y.J.; Kwon, Y.J. Relationship between muscle mass index and LDL cholesterol target levels: Analysis of two studies of the Korean population. Atherosclerosis 2021, 325, 1–7. [Google Scholar] [CrossRef]
- Sartorato, P.; Zulian, E.; Benedini, S.; Mariniello, B.; Schiavi, F.; Bilora, F.; Pozzan, G.; Greggio, N.; Pagnan, A.; Mantero, F.; et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2007, 92, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Bonfig, W.; Roehl, F.W.; Riedl, S.; Dorr, H.G.; Bettendorf, M.; Bramswig, J.; Schonau, E.; Riepe, F.; Hauffa, B.; Aquape CAH Study Group; et al. Blood Pressure in a Large Cohort of Children and Adolescents With Classic Adrenal Hyperplasia (CAH) Due to 21-Hydroxylase Deficiency. Am. J. Hypertens. 2016, 29, 266–272. [Google Scholar] [CrossRef]
- Kurzyńska, A.; Skalniak, A.; Franson, K.; Bistika, V.; Hubalewska-Dydejczyk, A.; Przybylik-Mazurek, E. Molecular analysis and genotype-phenotype correlations in patients with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency from southern Poland-experience of a clinical center. Hormones 2022, 1–9. [Google Scholar] [CrossRef]
- De Carvalho, D.F.; Miranda, M.C.; Gomes, L.G.; Madureira, G.; Marcondes, J.A.; Billerbeck, A.E.; Rodrigues, A.S.; Presti, P.F.; Kuperman, H.; Damiani, D.; et al. Molecular C.CYP21A2 diagnosis in 480 Brazilian patients with congenital adrenal hyperplasia before newborn screening introduction. Eur. J. Endocrinol. 2016, 175, 107–116. [Google Scholar] [CrossRef]
- Narasimhan, M.L.; Khattab, A. Genetics of congenital adrenal hyperplasia and genotype-phenotype correlation. Fertil. Steril. 2019, 111, 24–29. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Ye, J.; Han, L.; Qiu, W.; Zhang, H.; Liang, L.; Gong, Z.; Wang, L.; Gu, X. 21-hydroxylase deficiency-induced congenital adrenal hyperplasia in 230 Chinese patients: Genotypephenotype correlation and identification of nine novel mutations. Steroids 2016, 108, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Milacic, I.; Barac, M.; Milenkovic, T.; Ugrin, M.; Klaassen, K.; Skakic, A.; Jesic, M.; Joksic, I.; Mitrovic, K.; Todorovic, S.; et al. Molecular genetic study of congenital adrenal hyperplasia in Serbia: Novel p.Leu129Pro and p.Ser165Pro CYP21A2 gene mutations. J. Endocrinol. Investig. 2015, 38, 1199–1210. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, L.; Lu, Z. Molecular diagnosis of Chinese patients with 21-hydroxylase deficiency and analysis of genotype–phenotype correlations. J. Int. Med. Res. 2017, 45, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Neocleous, V.; Fanis, P.; Toumba, M.; Stylianou, C.; Picolos, M.; Andreou, E.; Kyriakou, A.; Iasonides, M.; Nicolaou, S.; Kyriakides, T.C.; et al. The spectrum of genetic defects in congenital adrenal hyperplasia in the population of Cyprus: A retrospective analysis. Horm. Metab. Res. 2019, 51, 586–594. [Google Scholar] [CrossRef] [PubMed]
- New, M.I.; Abraham, M.; Gonzalez, B.; Dumic, M.; Razzaghy-Azar, M.; Chitayat, D.; Sun, L.; Zaidi, M.; Wilson, R.C.; Yuen, T. Genotype-phenotype correlation in 1507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc. Natl. Acad. Sci. USA 2013, 110, 2611–2616. [Google Scholar] [CrossRef]

| Enzyme Activity | Phenotype | CYP21A2 Pathogenic Variant | Grouping of Mutations According to 21OH Activity |
|---|---|---|---|
| 0% | Severe (classic) | Whole-gene deletion Large-gene conversion p.Gly111ValfsTer21 p.[Ile237Asn;Val238Glu;Met240Lys] p.Leu308PhefsTer6 p.Gln319Ter p.Arg357Trp | Null |
| <1% | c.293-13A > G c.293C > G | A | |
| 2–11% | p.Ile173Asn | B | |
| ~20–50% | Mild (non-classic) | p.Pro31Leu p.Val282Leu p.Pro454Ser | C |
| Variables | SW/M (n = 11) | SV/M (n = 2) | SW/F (n = 9) | SV/F (n = 9) | SW (n = 20) | SV (n = 12) | All CAH (n = 32) | Controls (n = 32) |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 26.6 (16.9–29.2) | 30.2 (28.9–30.45) | 24.3 (20.8–28.4) | 30.9 (20.9–36.5) | 25.0 (17.1–29.2) c | 30.9 (23.7–36.0) c | 26.9 (17.9–31.6) | 28.6 (17.1–31.3) |
| Median cumulative HC dose (mg/m2/day) | 16.6 (14.2–17.7) | 13.5 (13.3–13.5) | 16.8 (13.6–20.7) | 14.4 (12.3–16.1) | 16.6 (14.1–18.5) c | 13.9 (12.6–15.2) c | 15.4 (13.2–17.7) | |
| Median cumulative MC dose (μg/day) * | 100 (87.5–125) | 100 (50–100) | 100 (50–125) | |||||
| BMI-SDS ** | 0.31 (−0.4–1.87) | 2.28 (1.24–2.5) | 1.84 (1.14–3.01) b | 1.8 (1.7–2.63) b | 0.93 (−0.03–2.17) | 1.98 (1.57–2.82) | 1.63 (0.3–2.4) | 0.41 (−0.68–1.19) |
| WHR | 0.79 (0.76–0.9) | 1.0 (0.9–1.1) | 0.86 (0.81–0.95) b | 0.84 (0.79–0.91) b | 0.85 (0.78–0.9) | 0.88 (0.8–0.94) | 0.85 (0.78–0.91) d | 0.79 (0.76–0.84) d |
| WHtR | 0.45 (0.41–0.52) | 0.65 (0.55–0.7) | 0.54 (0.47–0.61) b | 0.58 (0.53–0.59) b | 0.48 (0.41–0.55) c | 0.58 (0.54–0.6) c | 0.52 (0.44–0.58) d | 0.44 (0.4–0.47) d |
| Systolic BP (mmHg) | 129 (117–138) | 127 (121–129) | 130 (124–135) b | 120 (113–125) b | 130 (120–138) c | 121 (120–126) c | 125 (120–132) | 122 (112–131) |
| Diastolic BP (mmHg) | 82 (72–90) | 80 (77–82) | 78 (68–92) | 80 (74–80) | 82 (72–92) | 80 (77–80) | 80 (72–86) | 80 (70–83.7) |
| Sodium (mmol/L) | 138 (136–141) | 136 (136–136) | 139 (135.3–139) | 137 (133.2–138.8) | 138 (136–139) | 136 (133.5–138.5) | 138 (135.2–139) | 139 (137–140) |
| Potassium (mmol/L) | 3.8 (3.6–4.0) | 3.9 (3.9–3.94) | 3.8 (3.6–4.0) | 3.9 (3.6–3.9) | 3.8 (3.6–4.0) | 3.7 (3.6–3.9) | 3.8 (3.6–4.0) | 4.0 (3.8–4.2) |
| Total-C (mmol/L) | 3.84 (3.27–4.25) | 4.5 (4.4–4.6) | 4.63 (3.66–5.17) | 4.42 (4.09–5.47) | 3.98 (3.39–4.51) c | 4.46 (4.14–5.43) c | 4.2 (3.7–4.7) | 4.5 (3.8–5.4) |
| HDL-C (mmol/L) | 1.12 (0.98–1.38) a | 1.79 (1.57–1.85) a | 1.54 (1.38–1.54) | 1.52 (1.36–1.71) | 1.21 (1.01–1.58) c | 1.53 (1.39–1.74) c | 1.44 (1.12–1.64) | 1.47 (1.27–156) |
| LDL-C (mmol/L) | 2.18 (1.86–2.54) | 2.64 (2.4–2.7) | 2.62 (2.07–3.18) | 2.75 (2.2–3.81) | 2.27 (1.88–2.62) | 2.72 (2.25–3.59) | 2.49 (1.89–2.92) | 2.69 (2.2–2.7) |
| TG (mmol/L) | 1.01 (0.62–1.19) | 0.8 (0.68–0.91) | 1.36 (0.57–1.66) | 1.24 (0.87–1.67) | 1.07 (0.61–1.37 | 1.15 (0.78–1.65) | 1.27 (0.72–1.66) d | 0.82 (0.57–1.01) d |
| Fasting insulin (mU/L) | 9.1 (3.5–12.3) | 10.9 (9.8–12.3) | 12.5 (10.1–14.0) b | 16.2 (8.6–23.0) b | 10.2 (5.1–12.9) | 15.4 (9.2–22.3) | 11.2 (7.8–20.3) d | 6.4 (4.0–10.7) d |
| Fasting glycemia (mmol/L) | 4.92 (4.55–5.1) a | 5.09 (4.8–5.2) a | 4.53 (4.39–4.55) | 4.93 (4.49–5.04) | 4.74 (4.48–5.05) | 4.97 (4.6–5.07) | 4.9 (4.5–5.06) d | 5.16 (4.8–5.44) d |
| Glycemia after OGTT (mmol/L) | 5.68 (5.5–8.0) a | 4.9 (4.7–5.7) a | 5.54 (4.78–5.87) b | 6.35 (6.14–7.27) b | 5.6 (5.4–6.32) c | 6.3 (6.1–7.2) c | 5.95 (5.53–7.08) | 5.35 (4.92–5.89) |
| HOMA-IR ** | 2.22 (1.82–5.1) | 2.6 (2.4–3.8) | 2.52 (1.95–2.72) | 3.4 (1.25–4.56) | 2.26 (1.95–4.78) | 3.4 (1.25–4.55) | 2.5 (1.9–4.8) d | 1.51 (0.92–2.35) d |
| T (nmol/L) ** | 19.1 (8.3–23.9) | 9.7 (7.5–10.2) | 3.78 (1.54–5.46) | 1.74 (0.52–4.7) | 14.4 (5.1–22.5) c | 1.87 (0.84–6.48) c | 7.08 (2.7–19.3) | 1.99 (1.39–14.9) |
| 17OHP (nmol/L) ** | 124.2 (20.3–420) | 61.5 (4.1–70.6) | 434 (258–472) | 229 (20.7–410) | 161 (35.2–520) | 119 (20.6–329) | 156 (20–410) | |
| Renin (ng/L) ** | 13.7 (4.0–29.4) | 9.2 (4.5–10.2) | 28.3 (7.5–57.8) | 14.9 (9.1–21.8) | 13.7 (5.6–31.4) | 14.6 (9.0–19.8) | 14.6 96.8–29.1) | |
| TFM (kg) *** | 16.2 (13.9–18.9) a | 32.4 (19.7–35.7) a | 29.4 (18.5–32.4) b | 25.2 (24.6–37.4) b | 17.5 (14.6–23.5) c | 25.2 (23.5–37.4) c | 22.5 (16.2–27.1) | 19.3 (16.8–24.7) |
| TFM (Z-score) *** | 0.4 (0.2–0.5) | 1.25 (0.1–1.3) | 0.8 (−0.2–0.82) b | 0.3 (0.2–1.1) b | 0.45 (0.12–0.8) | 0.3 (0.15–1.2) | 0.4 (0.2–0.8) d | 0.05 (−0.5–0.27) d |
| VAT (grams) *** | 274 (252–359) a | 750 (360–800) a | 434 (197–450) b | 543 (247–566) b | 283 (249–387) | 543 (266–662) | 348 (252–501) | 293 (201–358) |
| SAT (kg) *** | 16.0 (13.7–18.5) a | 31.7 (19.3–32.6) a | 28.9 (18.3–30.5) b | 25.0 (24.3–36.6) b | 17.3 (14.4–23.1) c | 25.0 (23.3–36.8) c | 22.3 (16.0–26.6) | 18.9 (16.6–18.9) |
| Lean body mass (kg) *** | 43.3 (40.0–52.6) | 56.5 (54.1–58.5) | 41.1 (37.1–43.2) | 41.0 (39.6–43.3) | 43.3 (39.7–52.8) | 43.1 (39.7–51.5) | 43.2 (39.7–52.6) | 44.3 (36.6–55.6) |
| Variable | Null + Null and Null + A | Other Combinations of Mutations | p Value |
|---|---|---|---|
| BMI-SDS | 0.48 (−0.08–1.6) | 2.1 (1.6–2.8) | p = 0.004 |
| Total-C | 3.89 (3.23–4.2) | 4.42 (4.1–5.3) | p = 0.046 |
| LDL-C | 2.24 (1.88–2.49) | 2.75 (2.36–3.23) | p = 0.004 |
| TFM (kg) | 18.5 (14.9–25.0) | 25.2 (22.5–37.4) | p = 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navardauskaite, R.; Semeniene, K.; Sukys, M.; Pridotkaite, A.; Vanckaviciene, A.; Zilaitiene, B.; Verkauskiene, R. Cardiometabolic Health in Adolescents and Young Adults with Congenital Adrenal Hyperplasia. Medicina 2022, 58, 500. https://doi.org/10.3390/medicina58040500
Navardauskaite R, Semeniene K, Sukys M, Pridotkaite A, Vanckaviciene A, Zilaitiene B, Verkauskiene R. Cardiometabolic Health in Adolescents and Young Adults with Congenital Adrenal Hyperplasia. Medicina. 2022; 58(4):500. https://doi.org/10.3390/medicina58040500
Chicago/Turabian StyleNavardauskaite, Ruta, Kristina Semeniene, Marius Sukys, Agne Pridotkaite, Aurika Vanckaviciene, Birute Zilaitiene, and Rasa Verkauskiene. 2022. "Cardiometabolic Health in Adolescents and Young Adults with Congenital Adrenal Hyperplasia" Medicina 58, no. 4: 500. https://doi.org/10.3390/medicina58040500
APA StyleNavardauskaite, R., Semeniene, K., Sukys, M., Pridotkaite, A., Vanckaviciene, A., Zilaitiene, B., & Verkauskiene, R. (2022). Cardiometabolic Health in Adolescents and Young Adults with Congenital Adrenal Hyperplasia. Medicina, 58(4), 500. https://doi.org/10.3390/medicina58040500






