Deciphering the Role of the rs2651899, rs10166942, and rs11172113 Polymorphisms in Migraine: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Literature Search Strategy
2.3. Identification of Eligible Articles
2.4. Eligibility Criteria
2.5. Data Extraction
2.6. Statistical Analysis
2.6.1. Calculation of the Effect Size
2.6.2. Heterogeneity and Assessment of Publication Bias
3. Results
3.1. Study Selection and Study Characteristics
3.1.1. PRDM16 rs2651899
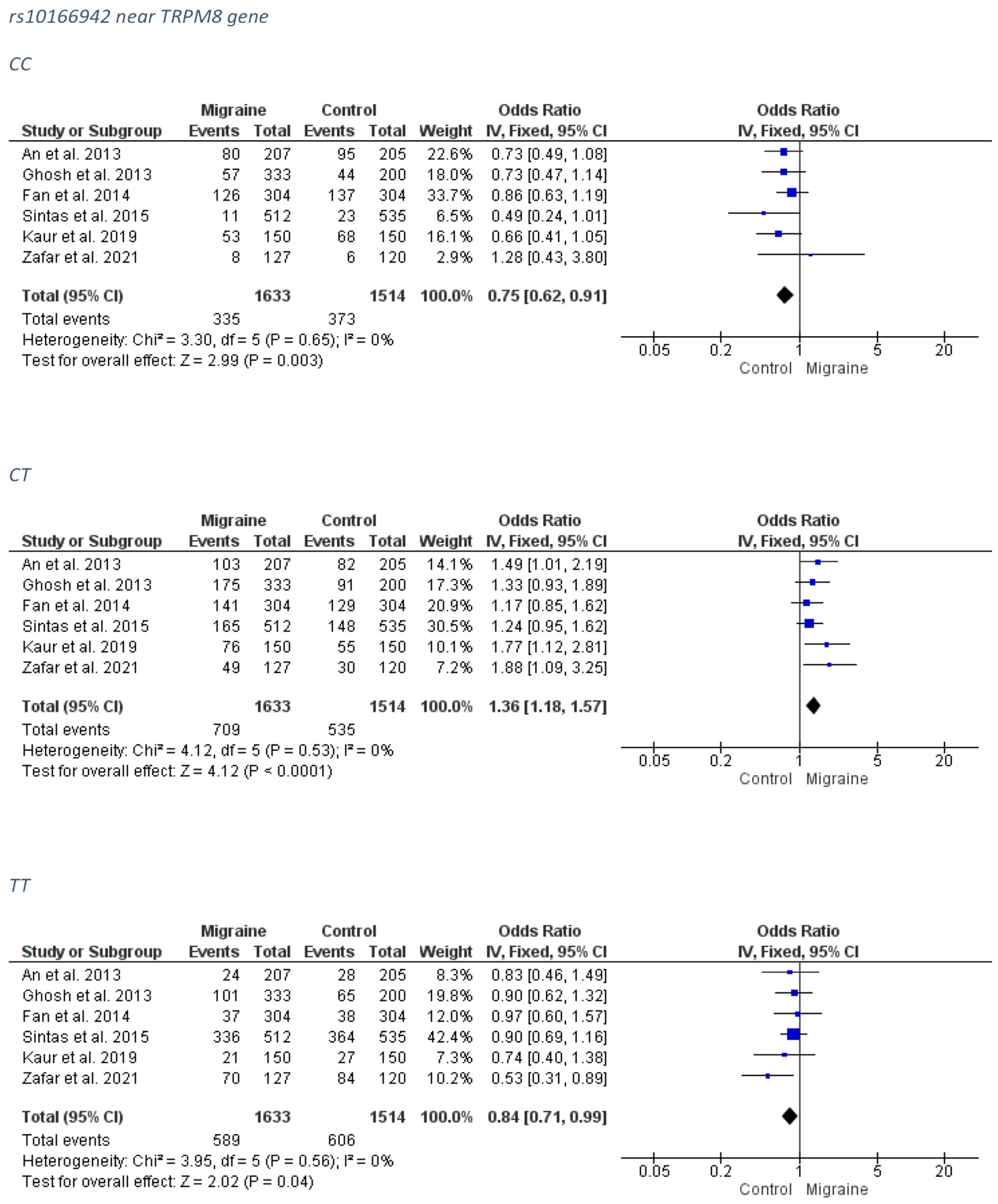
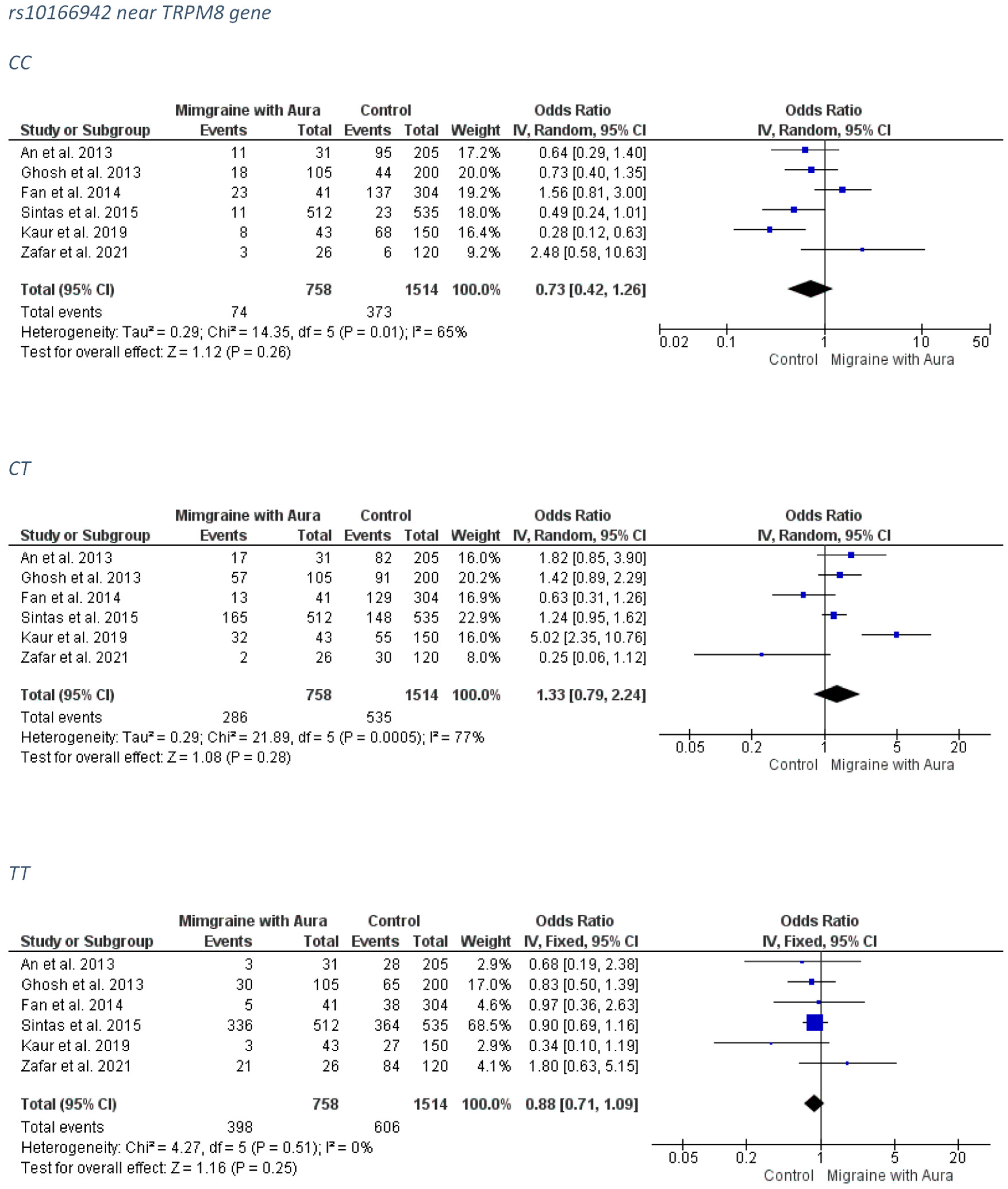
3.1.2. rs10166942 near TRPM8 Gene
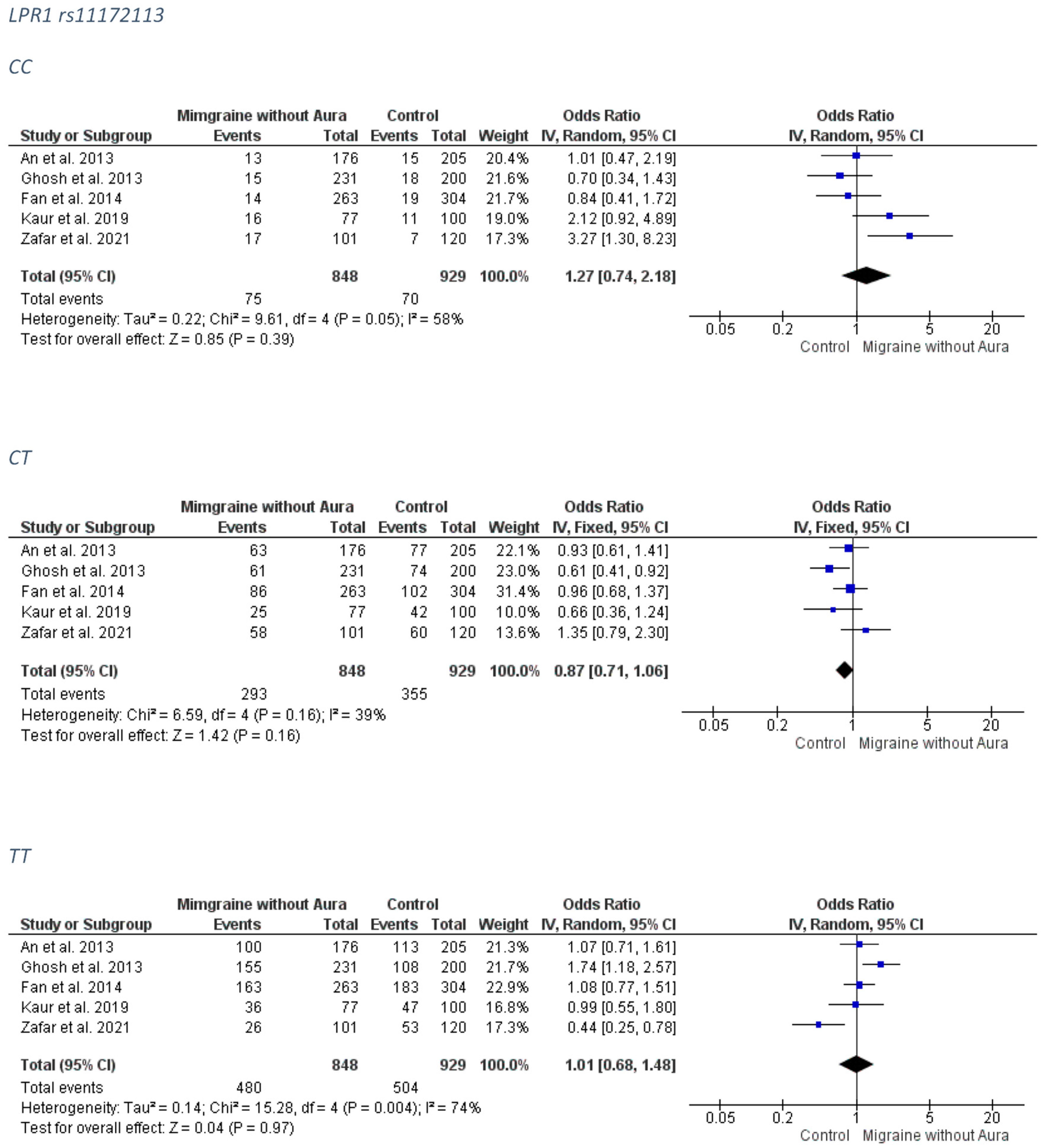
3.1.3. LPR1 rs11172113
| Cases | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (Year) [Ref] | Population or Location | Gene (Polymorphism) | HWE Test/Multiple Test Correction | Diagnosis Assessment | Mean Age ± SD/Age of Onset ± SD | n | Male/Female | Mean Age ± SD | n | Male/Female | Main Results and Comments |
| An et al. (2013) [48] | Han-Chinese | PRDM16 (rs2651899); TRPM8 (rs10166942); and LPR1 (rs11172113) | Yes (cases and controls)/- | International Classification of Headache Disorders, 2nd edition (ICHDII) | 36.0 ± 10.9 years/- | 207 | 37/170 | 35.8 ± 11.5 years | 205 | 49/156 | The rs2651899 G allele was associated with migraine and MO in allelic mode. No association for the TRPM8 rs10166942 and the LPR1 rs11172113. |
| Gosh et al. (2013) [50] | India | PRDM16 (rs2651899); TRPM8 (rs10166942); and LPR1 (rs11172113) | Yes (controls)/Yes (Benjamini–Hochberg false discovery rate (FDR) test) | International Classification of Headache Disorders, 2nd edition (ICHDII) | -/<50 years | 340 | - | matched | 200 | matched | Protective effect of the rs2651899 (T) on migraine and MO susceptibility (genotypic, dominant, allelic models). Protective effect of the LPR1 rs11172113 C allele on migraine MA and MO in various models. No association for the TRPM8 rs10166942. |
| Fan et al. (2014) [51] | Han-Chinese | PRDM16 (rs2651899); TRPM8 (rs10166942); and LPR1 (rs11172113) | Yes (controls)/Yes (Bonferroni) | International Classification of Headache Disorders, 2nd edition (ICHDII) | 40.65 ± 12.18 years/24.03 ± 11.13 years | 304 | 53/251 | matched | 304 | matched | The rs2651899 minor allele (C) was associated with migraine and MO. No association for the TRPM8 rs10166942 and the LPR1 rs11172113. |
| Sintas et al. (2015) [40] | Spanish | PRDM16 (rs2651899); TRPM8 (rs10166942); and LPR1 (rs11172113) | Yes (cases and controls)/10,000 permutations and Bonferroni’s correction | International Classification of Headache Disorders, 2nd edition (ICHDII) | -/13.5 ± 12 years | 512 | 78.13% female | matched | 535 | 78.83% female | The rs2651899 minor allele (C) was nominally associated with migraine and MA. The TRPM8 rs10166942 (T) allele nominally associated with migraine. No significance remained after multiple comparison correction. |
| An et al. (2017) [47] | Chinese | PRDM16 (rs2651899); and LPR1 (rs11172113) | Yes (controls)/Yes (Benjamini–Hochberg false discovery rate (FDR) test and Bonferroni) | International Classification of Headache Disorders (ICHD-III beta) | -/35.4 ± 10.2 years | 581 | 61/520 | 34.8 ± 8.9 years | 533 | 57/476 | The rs2651899 C allele was associated MO and migraine with family history subgroup. No association for the LPR1 rs11172113. |
| Ran et al. (2018) [49] | Swedish | PRDM16 (rs2651899); | Yes/- | International Classification of Headache Disorders, 2nd edition (ICHDII) | - | 100 | - | - | 581 | 56.3% male | No association. |
| Kaur et al. (2019) [39] | North Indian | PRDM16 (rs2651899) and TRPM8 (rs1016694) | Yes (controls)/- | International Classification of Headache disorders, 3rd edition | 35.28 ± 6.6 years/ | 150 | 40/110 | no statistical difference in terms of age as p = 0.35 | 150 | 60% females | The rs2651899 T allele was associated with migraine in genotypic, allelic, and dominant model. Association was found for the variant with the MO and the female migraineurs. The TRPM8 rs1016694 was associated with MA and in males. |
| Kaur et al. (2019) [58] | India | LPR1 (rs11172113) | Yes/- | International Classification of Headache disorders, 3rd edition | MA:35.13 ± 6.0 years/- MO: 36.40 ± 5.2 years/- | 100 | 28/72 | 34.45 ± 7.6 years | 100 | 38/62 | No association |
| Zafar et al. (2021) [41] | Pakistan | PRDM16 (rs2651899); TRPM8 (rs10166942); and LPR1 (rs11172113 | Yes (controls)/- | International Classification of Headache Disorders, 2nd edition (ICHDII) | 25.79 ± 5.19 years/- | 127 | 31/96 | 26.26 ± 5.57 years | 120 | 38/82 | The rs2651899 G allele was associated with migraine, MO, and MA. The TRPM8 rs10166942 and the LPR1 rs11172113 were associated with migraine and MO. |
3.2. Tests of Heterogeneity, Effect Size, and Publication Bias
3.2.1. PRDM16 rs2651899
Overall Migraine Group
MA Group
MO Group
3.2.2. rs10166942 near TRPM8 Gene
Overall Migraine Group
MA Group
MO Group
3.2.3. LPR1 rs11172113
Overall Migraine Group
MA Group
MO Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.-C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Agosti, R. Migraine Burden of Disease: From the Patient’s Experience to a Socio-Economic View. Headache 2018, 58 (Suppl. 1), 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, E.; Dorsey, R.; Elbaz, A.; Richard, G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Robbins, M.S. Diagnosis and Management of Headache: A Review. JAMA 2021, 325, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [CrossRef] [Green Version]
- Akerman, S.; Holland, P.R.; Hoffmann, J. Pearls and pitfalls in experimental in vivo models of migraine: Dural trigeminovascular nociception. Cephalalgia 2013, 33, 577–592. [Google Scholar] [CrossRef]
- Gasparini, C.F.; Sutherland, H.G.; Griffiths, L.R. Studies on the pathophysiology and genetic basis of migraine. Curr. Genom. 2013, 14, 300–315. [Google Scholar] [CrossRef]
- Kassab, M.; Bakhtar, O.; Wack, D.; Bednarczyk, E. Resting brain glucose uptake in headache-free migraineurs. Headache 2009, 49, 90–97. [Google Scholar] [CrossRef]
- Grech, O.; Mollan, S.P.; Wakerley, B.R.; Fulton, D.; Lavery, G.G.; Sinclair, A.J. The Role of Metabolism in Migraine Pathophysiology and Susceptibility. Life 2021, 11, 415. [Google Scholar] [CrossRef]
- Hoffmann, U.; Sukhotinsky, I.; Eikermann-Haerter, K.; Ayata, C. Glucose modulation of spreading depression susceptibility. J. Cereb. Blood Flow Metab. 2013, 33, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, G.; Suzuki, S.; Morishita, N.; Takeshita, M.; Kanou, K.; Takamatsu, T.; Suzuki, S.; Morichi, S.; Watanabe, Y.; Ishida, Y.; et al. Role of Neuroinflammation and Blood-Brain Barrier Permutability on Migraine. Int. J. Mol. Sci. 2021, 22, 8929. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Chojnacki, C.; Chojnacki, J.; Blasiak, J. Is an “Epigenetic Diet” for Migraines Justified? The Case of Folate and DNA Methylation. Nutrients 2019, 11, 2763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koute, V.; Michalopoulou, A.; Siokas, V.; Aloizou, A.M.; Rikos, D.; Bogdanos, D.P.; Kontopoulos, E.; Grivea, I.N.; Syrogiannopoulos, G.A.; Papadimitriou, A.; et al. Val66Met polymorphism is associated with decreased likelihood for pediatric headache and migraine. Neurol. Res. 2021, 43, 715–723. [Google Scholar] [CrossRef]
- Amin, F.M.; Aristeidou, S.; Baraldi, C.; Czapinska-Ciepiela, E.K.; Ariadni, D.D.; Di Lenola, D.; Fenech, C.; Kampouris, K.; Karagiorgis, G.; Braschinsky, M.; et al. The association between migraine and physical exercise. J. Headache Pain 2018, 19, 83. [Google Scholar] [CrossRef]
- López-Mesonero, L.; Márquez, S.; Parra, P.; Gámez-Leyva, G.; Muñoz, P.; Pascual, J. Smoking as a precipitating factor for migraine: A survey in medical students. J. Headache Pain 2009, 10, 101–103. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Liang, X.; Wang, S.; Mu, X. Association between Body Mass Index and Migraine: A Survey of Adult Population in China. Behav. Neurol. 2018, 2018, 6585734. [Google Scholar] [CrossRef] [Green Version]
- Stewart, W.F.; Roy, J.; Lipton, R.B. Migraine prevalence, socioeconomic status, and social causation. Neurology 2013, 81, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Liampas, I.; Siokas, V.; Brotis, A.; Dardiotis, E. Vitamin D serum levels in patients with migraine: A meta-analysis. Rev. Neurol. (Paris) 2020, 176, 560–570. [Google Scholar] [CrossRef]
- Liampas, I.N.; Siokas, V.; Aloizou, A.M.; Tsouris, Z.; Dastamani, M.; Aslanidou, P.; Brotis, A.; Dardiotis, E. Pyridoxine, folate and cobalamin for migraine: A systematic review. Acta Neurol. Scand. 2020, 142, 108–120. [Google Scholar] [CrossRef]
- Liampas, I.; Siokas, V.; Mentis, A.A.; Aloizou, A.M.; Dastamani, M.; Tsouris, Z.; Aslanidou, P.; Brotis, A.; Dardiotis, E. Serum Homocysteine, Pyridoxine, Folate, and Vitamin B12 Levels in Migraine: Systematic Review and Meta-Analysis. Headache 2020, 60, 1508–1534. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in genetics of migraine. J. Headache Pain 2019, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.; Terwindt, G.M.; van den Maagdenberg, A.M.J.M. Genetics of migraine aura: An update. J. Headache Pain 2020, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Mikol, D.D.; Picard, H.; Klatt, J.; Wang, A.; Peng, C.; Stefansson, K. Migraine Polygenic Risk Score Is Associated with Severity of Migraine—Analysis of Genotypic Data from Four Placebo-controlled Trials of Erenumab (1214). Neurology 2020, 94, 1214. [Google Scholar]
- May, A.; Schulte, L.H. Chronic migraine: Risk factors, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 455–464. [Google Scholar] [CrossRef]
- Di Stefano, V.; Rispoli, M.G.; Pellegrino, N.; Graziosi, A.; Rotondo, E.; Napoli, C.; Pietrobon, D.; Brighina, F.; Parisi, P. Diagnostic and therapeutic aspects of hemiplegic migraine. J. Neurol. Neurosurg. Psychiatry 2020, 91, 764–771. [Google Scholar] [CrossRef]
- Bron, C.; Sutherland, H.G.; Griffiths, L.R. Exploring the Hereditary Nature of Migraine. Neuropsychiatr. Dis. Treat. 2021, 17, 1183–1194. [Google Scholar] [CrossRef]
- van den Maagdenberg, A.; Nyholt, D.R.; Anttila, V. Novel hypotheses emerging from GWAS in migraine? J. Headache Pain 2019, 20, 5. [Google Scholar] [CrossRef] [Green Version]
- Anttila, V.; Winsvold, B.S.; Gormley, P.; Kurth, T.; Bettella, F.; McMahon, G.; Kallela, M.; Malik, R.; de Vries, B.; Terwindt, G.; et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat. Genet. 2013, 45, 912–917. [Google Scholar] [CrossRef]
- Gormley, P.; Anttila, V.; Winsvold, B.S.; Palta, P.; Esko, T.; Pers, T.H.; Farh, K.H.; Cuenca-Leon, E.; Muona, M.; Furlotte, N.A.; et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 2016, 48, 856–866. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.; Kumar, P. Relation Between Methylenetetrahydrofolate Reductase Polymorphisms (C677T and A1298C) and Migraine Susceptibility. Indian J. Clin. Biochem. 2022, 37, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Terrazzino, S.; Cargnin, S.; Viana, M.; Sances, G.; Tassorelli, C. Brain-Derived Neurotrophic Factor Val66Met Gene Polymorphism Impacts on Migraine Susceptibility: A Meta-analysis of Case-Control Studies. Front. Neurol. 2017, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Papasavva, M.; Vikelis, M.; Katsarou, M.S.; Siokas, V.; Dermitzakis, E.; Papademetriou, C.; Karakostis, K.; Lazopoulos, G.; Dardiotis, E.; Drakoulis, N. Evidence That HFE H63D Variant Is a Potential Disease Modifier in Cluster Headache. J. Mol. Neurosci. 2021, 72, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Papasavva, M.; Vikelis, M.; Siokas, V.; Katsarou, M.S.; Dermitzakis, E.; Raptis, A.; Dardiotis, E.; Drakoulis, N. VDR Gene Polymorphisms and Cluster Headache Susceptibility: Case-Control Study in a Southeastern European Caucasian Population. J. Mol. Neurosci. 2021, 72, 382–392. [Google Scholar] [CrossRef]
- Chasman, D.I.; Schürks, M.; Anttila, V.; de Vries, B.; Schminke, U.; Launer, L.J.; Terwindt, G.M.; van den Maagdenberg, A.M.J.M.; Fendrich, K.; Völzke, H.; et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 2011, 43, 695–698. [Google Scholar] [CrossRef] [Green Version]
- Freilinger, T.; Anttila, V.; de Vries, B.; Malik, R.; Kallela, M.; Terwindt, G.M.; Pozo-Rosich, P.; Winsvold, B.; Nyholt, D.R.; van Oosterhout, W.P.; et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat. Genet. 2012, 44, 777–782. [Google Scholar] [CrossRef]
- Lee, H.H.; Chen, C.C.; Ong, J.R.; Lin, Y.F.; Lee, F.P.; Hu, C.J.; Wang, Y.H. Association of rs2651899 Polymorphism in the Positive Regulatory Domain 16 and Common Migraine Subtypes: A Meta-Analysis. Headache 2020, 60, 71–80. [Google Scholar] [CrossRef]
- Fu, X.; Yang, J.; Wu, X.; Lin, Q.; Zeng, Y.; Xia, Q.; Cao, L.; Huang, B.; Huang, G. Association between PRDM16, MEF2D, TRPM8, LRP1 gene polymorphisms and migraine susceptibility in the She ethnic population in China. Clin. Invest. Med. 2019, 42, E21–E30. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Ali, A.; Ahmad, U.; Pandey, A.K.; Singh, B. rs2651899 variant is associated with risk for migraine without aura from North Indian population. Mol. Biol. Rep. 2019, 46, 1247–1255. [Google Scholar] [CrossRef]
- Sintas, C.; Fernández-Morales, J.; Vila-Pueyo, M.; Narberhaus, B.; Arenas, C.; Pozo-Rosich, P.; Macaya, A.; Cormand, B. Replication study of previous migraine genome-wide association study findings in a Spanish sample of migraine with aura. Cephalalgia 2015, 35, 776–782. [Google Scholar] [CrossRef]
- Zafar, R.; Saleem, T.; Sheikh, N.; Maqbool, H.; Mukhtar, M.; Abbasi, M.H. PRDM16, LRP1 and TRPM8 genetic polymorphisms are risk factor for Pakistani migraine patients. Saudi J. Biol. Sci. 2021, 28, 5793–5799. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [PubMed]
- Ran, C.; Graae, L.; Magnusson, P.K.; Pedersen, N.L.; Olson, L.; Belin, A.C. A replication study of GWAS findings in migraine identifies association in a Swedish case-control sample. BMC Med. Genet. 2014, 15, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esserlind, A.L.; Christensen, A.F.; Le, H.; Kirchmann, M.; Hauge, A.W.; Toyserkani, N.M.; Hansen, T.; Grarup, N.; Werge, T.; Steinberg, S.; et al. Replication and meta-analysis of common variants identifies a genome-wide significant locus in migraine. Eur. J. Neurol. 2013, 20, 765–772. [Google Scholar] [CrossRef] [PubMed]
- An, X.K.; Fang, J.; Yu, Z.Z.; Lin, Q.; Lu, C.X.; Qu, H.L.; Ma, Q.L. Multilocus analysis reveals three candidate genes for Chinese migraine susceptibility. Clin. Genet. 2017, 92, 143–149. [Google Scholar] [CrossRef]
- An, X.K.; Ma, Q.L.; Lin, Q.; Zhang, X.R.; Lu, C.X.; Qu, H.L. PRDM16 rs2651899 variant is a risk factor for Chinese common migraine patients. Headache 2013, 53, 1595–1601. [Google Scholar] [CrossRef]
- Ran, C.; Fourier, C.; Zinnegger, M.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E.; Belin, A.C. Implications for the migraine SNP rs1835740 in a Swedish cluster headache population. J. Headache Pain 2018, 19, 100. [Google Scholar] [CrossRef]
- Ghosh, J.; Pradhan, S.; Mittal, B. Multilocus analysis of hormonal, neurotransmitter, inflammatory pathways and genome-wide associated variants in migraine susceptibility. Eur. J. Neurol. 2014, 21, 1011–1020. [Google Scholar] [CrossRef]
- Fan, X.; Wang, J.; Fan, W.; Chen, L.; Gui, B.; Tan, G.; Zhou, J. Replication of migraine GWAS susceptibility loci in Chinese Han population. Headache 2014, 54, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Fuh, J.L.; Chung, M.Y.; Lin, Y.C.; Liao, Y.C.; Wang, Y.F.; Hsu, C.L.; Yang, U.C.; Lin, M.W.; Chiou, J.J.; et al. Genome-wide association study identifies novel susceptibility loci for migraine in Han Chinese resided in Taiwan. Cephalalgia 2018, 38, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.H.; Chen, S.P.; Fann, C.S.; Wang, S.J.; Wang, Y.F. TRPM8 genetic variant is associated with chronic migraine and allodynia. J. Headache Pain 2019, 20, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasman, D.I.; Anttila, V.; Buring, J.E.; Ridker, P.M.; Schürks, M.; Kurth, T. Selectivity in genetic association with sub-classified migraine in women. PLoS Genet. 2014, 10, e1004366. [Google Scholar] [CrossRef] [Green Version]
- Meng, W.; Adams, M.J.; Hebert, H.L.; Deary, I.J.; McIntosh, A.M.; Smith, B.H. A Genome-Wide Association Study Finds Genetic Associations with Broadly-Defined Headache in UK Biobank (N = 223,773). EBioMedicine 2018, 28, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Daghals, I.; Sargurupremraj, M.; Danning, R.; Gormley, P.; Malik, R.; Amouyel, P.; Metso, T.; Pezzini, A.; Kurth, T.; Debette, S.; et al. Migraine, Stroke, and Cervical Arterial Dissection: Shared Genetics for a Triad of Brain Disorders With Vascular Involvement. Neurol. Genet. 2022, 8, e653. [Google Scholar] [CrossRef]
- Shoba, U.S.; Srinivasan, G.; Gundlapally, J.; Kuppamuthu, K. Association of Single Nucleotide Polymorphism rs11172113 of LRP1 Gene with Migraine in South Indian Population–A Study. Helix-Sci. Explor. Peer Rev. Bimon. Int. J. 2020, 10, 7–11. [Google Scholar]
- Kaur, S.; Ali, A.; Siahbalaei, Y.; Ahmad, U.; Pandey, A.K.; Singh, B. Could rs4379368 be a genetic marker for North Indian migraine patients with aura?: Preliminary evidence by a replication study. Neurosci. Lett. 2019, 712, 134482. [Google Scholar] [CrossRef]
- Casamassimi, A.; Rienzo, M.; Di Zazzo, E.; Sorrentino, A.; Fiore, D.; Proto, M.C.; Moncharmont, B.; Gazzerro, P.; Bifulco, M.; Abbondanza, C. Multifaceted Role of PRDM Proteins in Human Cancer. Int. J. Mol. Sci. 2020, 21, 2648. [Google Scholar] [CrossRef] [Green Version]
- Di Zazzo, E.; De Rosa, C.; Abbondanza, C.; Moncharmont, B. PRDM Proteins: Molecular Mechanisms in Signal Transduction and Transcriptional Regulation. Biology 2013, 2, 107–141. [Google Scholar] [CrossRef] [Green Version]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristoffersen, E.S.; Børte, S.; Hagen, K.; Zwart, J.A.; Winsvold, B.S. Migraine, obesity and body fat distribution—A population-based study. J. Headache Pain 2020, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Shimada, I.S.; Acar, M.; Burgess, R.J.; Zhao, Z.; Morrison, S.J. Prdm16 is required for the maintenance of neural stem cells in the postnatal forebrain and their differentiation into ependymal cells. Genes Dev. 2017, 31, 1134–1146. [Google Scholar] [CrossRef]
- Geyik, S.; Altunısık, E.; Neyal, A.M.; Taysi, S. Oxidative stress and DNA damage in patients with migraine. J. Headache Pain 2016, 17, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, E.C.; Putananickal, N.; Orsini, A.-L.; Vogt, D.R.; Sandor, P.S.; Schoenen, J.; Fischer, D. Mitochondrial function and oxidative stress markers in higher-frequency episodic migraine. Sci. Rep. 2021, 11, 4543. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Baca, S.M.; Akerman, S. Neurovascular mechanisms of migraine and cluster headache. J. Cereb. Blood Flow Metab. 2019, 39, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Dussor, G.; Cao, Y.Q. TRPM8 and Migraine. Headache 2016, 56, 1406–1417. [Google Scholar] [CrossRef] [Green Version]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front. Immunol. 2020, 11, 2742. [Google Scholar] [CrossRef]
- Burgos-Vega, C.C.; Ahn, D.D.; Bischoff, C.; Wang, W.; Horne, D.; Wang, J.; Gavva, N.; Dussor, G. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia 2016, 36, 185–193. [Google Scholar] [CrossRef]
- Prince, P.B.; Rapoport, A.M.; Sheftell, F.D.; Tepper, S.J.; Bigal, M.E. The effect of weather on headache. Headache 2004, 44, 596–602. [Google Scholar] [CrossRef]
- González-Muñiz, R.; Bonache, M.A.; Martín-Escura, C.; Gómez-Monterrey, I. Recent Progress in TRPM8 Modulation: An Update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, W.M.; Daniels, R.L.; Palkar, R.; McCoy, D.D.; McKemy, D.D. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS ONE 2011, 6, e25894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyer, A.D.; Lehto, S.G. Development of TRPM8 Antagonists to Treat Chronic Pain and Migraine. Pharmaceuticals 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Sandrock, R.; Arnold, G.E.; Davis, M.; Lamas, E.; Lindvay, C.; Li, C.-M.; Smith, B.; Backonja, M.; Gabriel, K.; et al. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci. Rep. 2019, 9, 19655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Actis Dato, V.; Chiabrando, G.A. The Role of Low-Density Lipoprotein Receptor-Related Protein 1 in Lipid Metabolism, Glucose Homeostasis and Inflammation. Int. J. Mol. Sci. 2018, 19, 1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, M.D.; Odink, J.; Bos, K.D.; Malessy, M.J.; Bruyn, G.W. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology 1990, 40, 1582–1586. [Google Scholar] [CrossRef]
- Harder, A.V.E.; Vijfhuizen, L.S.; Henneman, P.; Willems van Dijk, K.; van Duijn, C.M.; Terwindt, G.M.; van den Maagdenberg, A. Metabolic profile changes in serum of migraine patients detected using (1)H-NMR spectroscopy. J. Headache Pain 2021, 22, 142. [Google Scholar] [CrossRef]
- Haigh, S.; Karanovic, O.; Wilkinson, F.; Wilkins, A. Cortical hyperexcitability in migraine and aversion to patterns. Cephalalgia Int. J. Headache 2012, 32, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Salazar, A.; Berrocal, L.; Failde, I. Prevalence of Migraine in General Spanish Population; Factors Related and Use of Health Resources. Int. J. Environ. Res. Public Health 2021, 18, 1145. [Google Scholar] [CrossRef]
- Fuh, J.L.; Wang, S.J.; Lu, S.R.; Liao, Y.C.; Chen, S.P.; Yang, C.Y. Headache disability among adolescents: A student population-based study. Headache 2010, 50, 210–218. [Google Scholar] [CrossRef]
- Yucel, A.; Thach, A.; Kumar, S.; Loden, C.; Bensink, M.; Goldfarb, N. Estimating the economic burden of migraine on US employers. Am. J. Manag. Care 2020, 26, e403–e408. [Google Scholar] [CrossRef] [PubMed]
- Abu-Arafeh, I.; Razak, S.; Sivaraman, B.; Graham, C. Prevalence of headache and migraine in children and adolescents: A systematic review of population-based studies. Dev. Med. Child. Neurol. 2010, 52, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Wöber-Bingöl, C. Epidemiology of migraine and headache in children and adolescents. Curr. Pain Headache Rep. 2013, 17, 341. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siokas, V.; Liampas, I.; Aloizou, A.-M.; Papasavva, M.; Bakirtzis, C.; Lavdas, E.; Liakos, P.; Drakoulis, N.; Bogdanos, D.P.; Dardiotis, E. Deciphering the Role of the rs2651899, rs10166942, and rs11172113 Polymorphisms in Migraine: A Meta-Analysis. Medicina 2022, 58, 491. https://doi.org/10.3390/medicina58040491
Siokas V, Liampas I, Aloizou A-M, Papasavva M, Bakirtzis C, Lavdas E, Liakos P, Drakoulis N, Bogdanos DP, Dardiotis E. Deciphering the Role of the rs2651899, rs10166942, and rs11172113 Polymorphisms in Migraine: A Meta-Analysis. Medicina. 2022; 58(4):491. https://doi.org/10.3390/medicina58040491
Chicago/Turabian StyleSiokas, Vasileios, Ioannis Liampas, Athina-Maria Aloizou, Maria Papasavva, Christos Bakirtzis, Eleftherios Lavdas, Panagiotis Liakos, Nikolaos Drakoulis, Dimitrios P. Bogdanos, and Efthimios Dardiotis. 2022. "Deciphering the Role of the rs2651899, rs10166942, and rs11172113 Polymorphisms in Migraine: A Meta-Analysis" Medicina 58, no. 4: 491. https://doi.org/10.3390/medicina58040491
APA StyleSiokas, V., Liampas, I., Aloizou, A.-M., Papasavva, M., Bakirtzis, C., Lavdas, E., Liakos, P., Drakoulis, N., Bogdanos, D. P., & Dardiotis, E. (2022). Deciphering the Role of the rs2651899, rs10166942, and rs11172113 Polymorphisms in Migraine: A Meta-Analysis. Medicina, 58(4), 491. https://doi.org/10.3390/medicina58040491












