Cardiovascular Disorder after Cardiotoxic Non-Hodking’s Lymphoma Treatment: A Case Report
Abstract
:1. Introduction
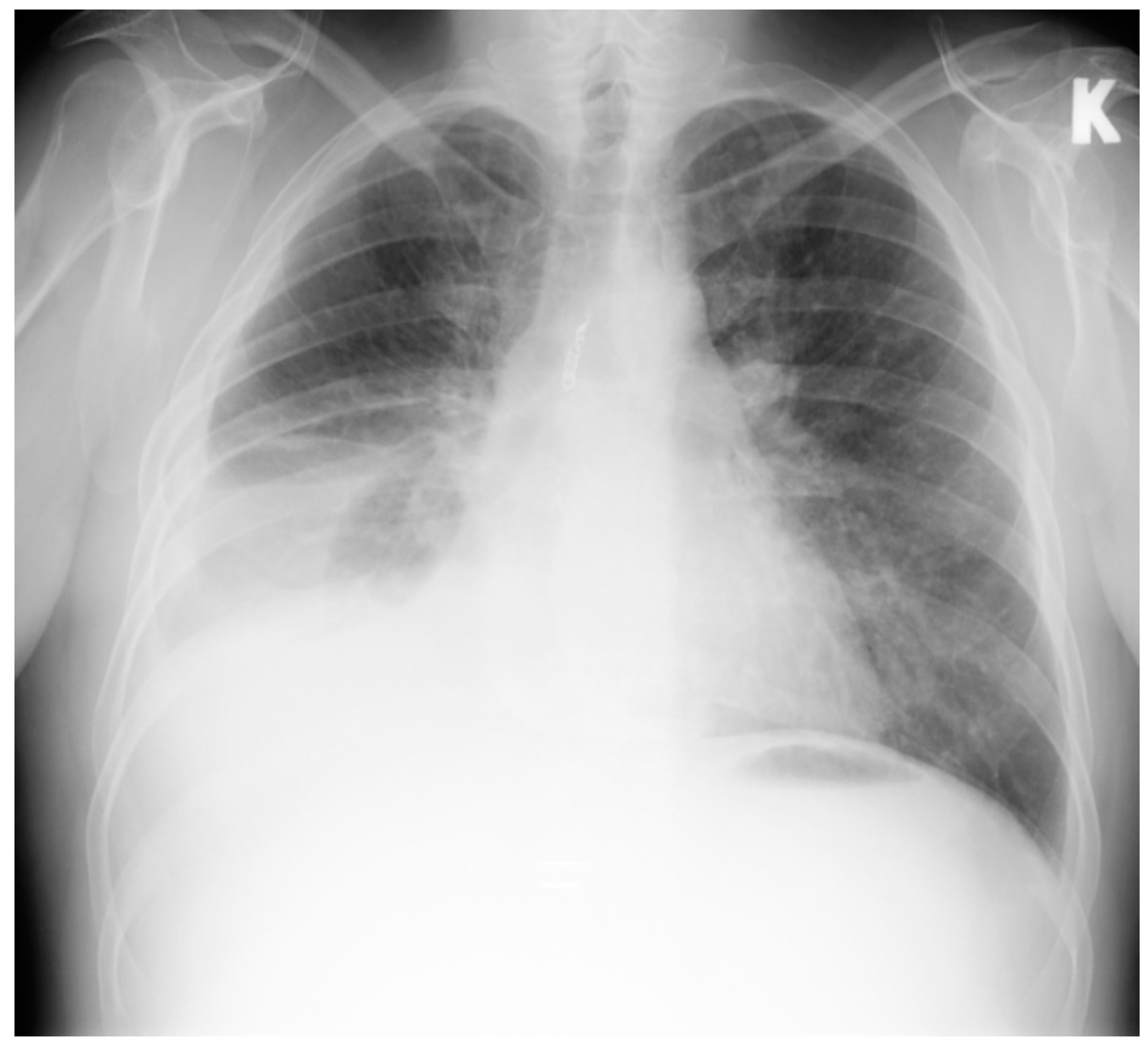
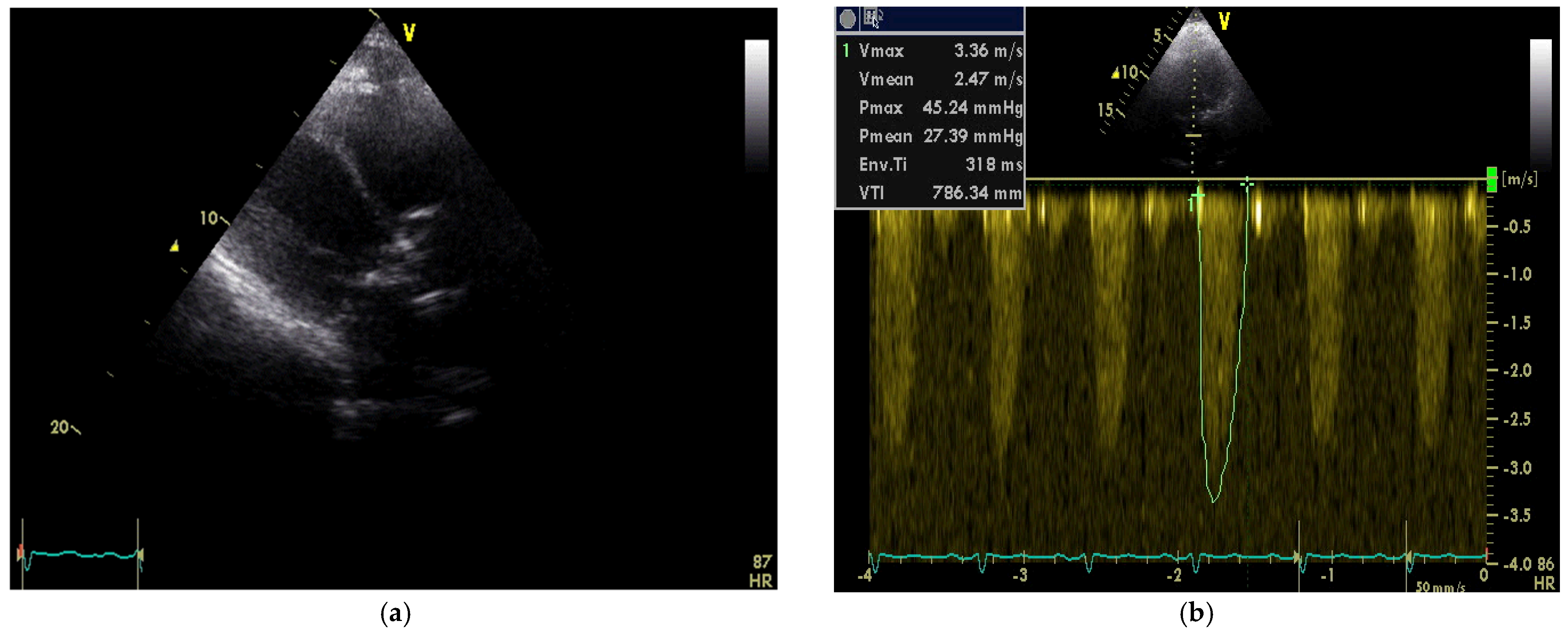
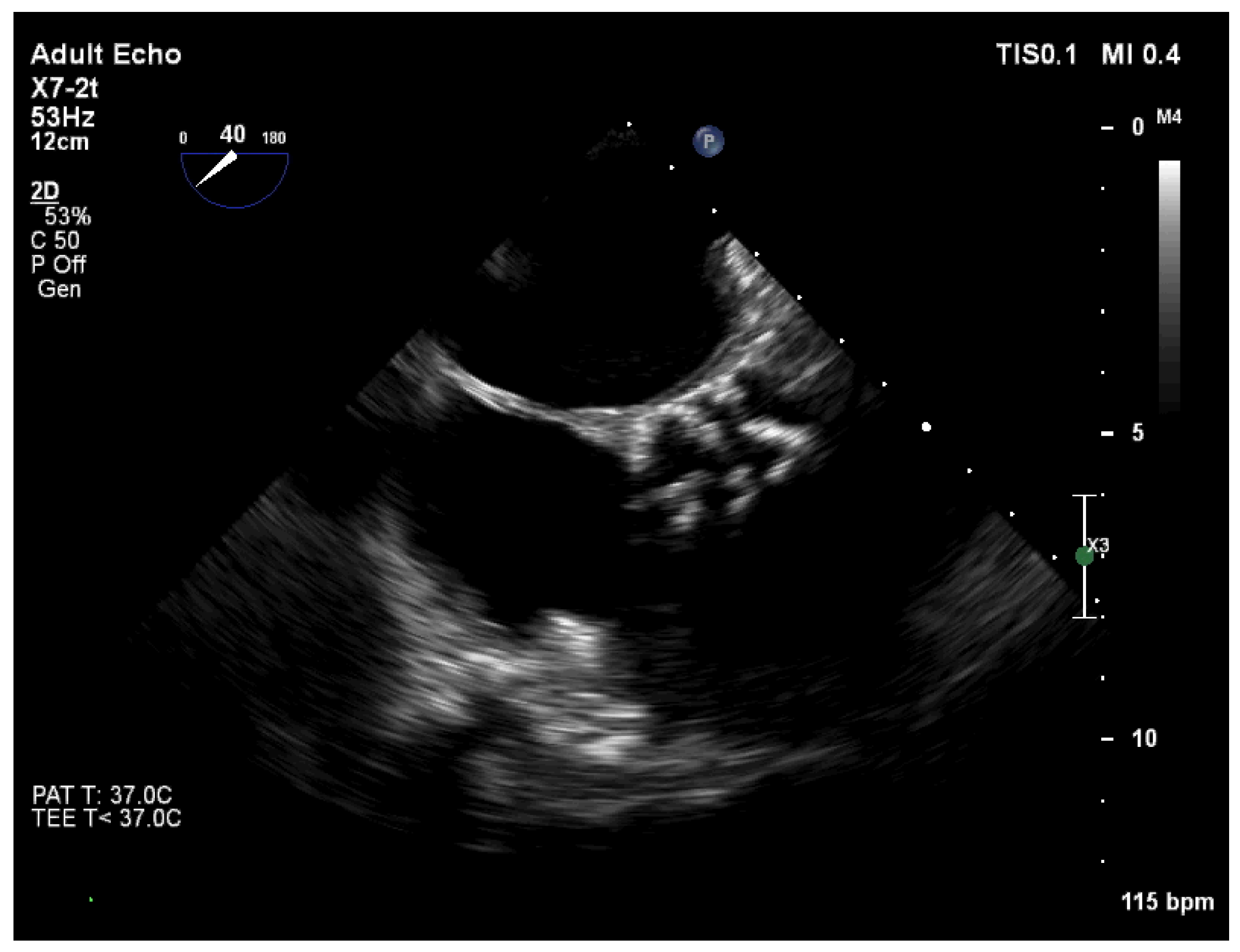
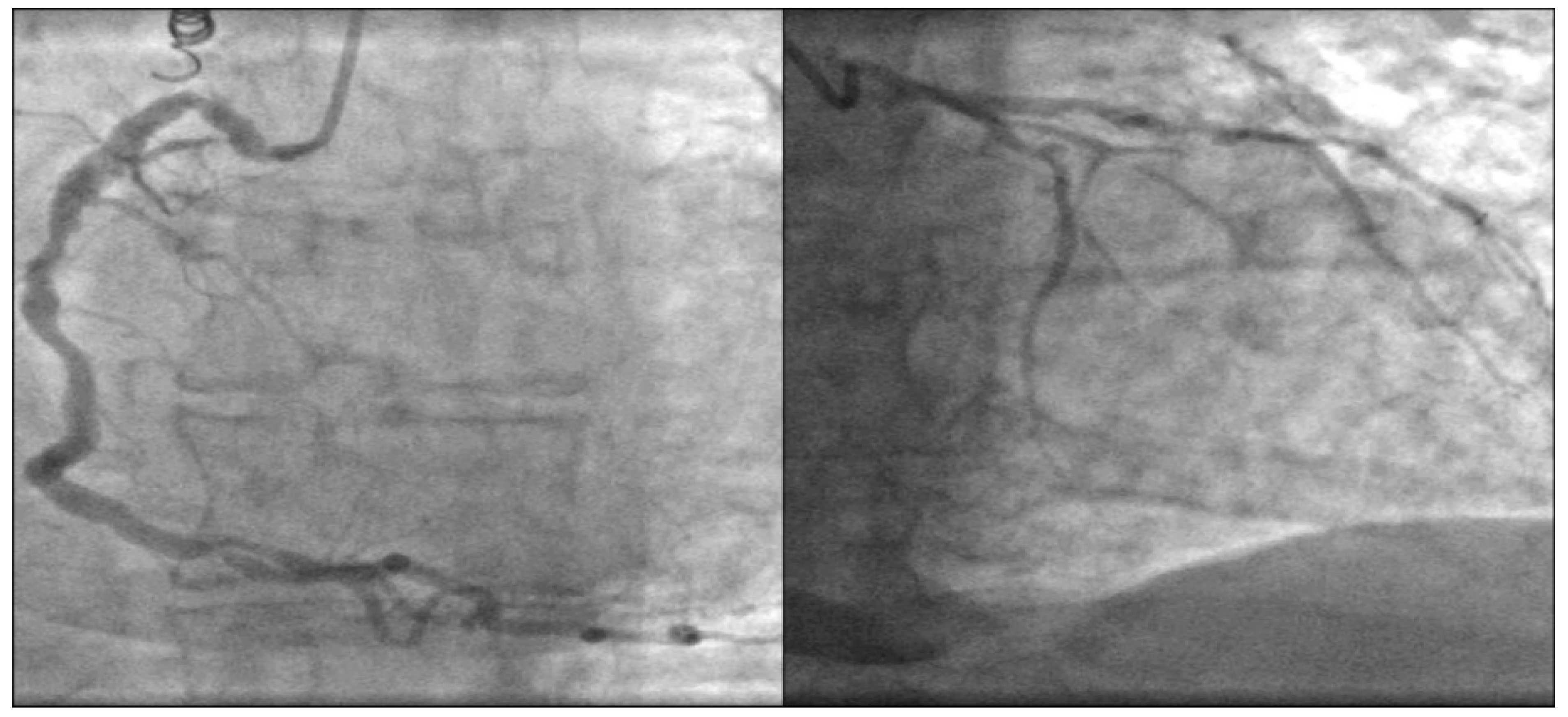
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shaik, S.; Negi, B.S.; Rajguru, J.P.; Bajirao, P.; Singh, A.; Sharma, U. Non-hodgkin lymphomas: A review. J. Family Med. Prim. Care. 2020, 9, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Salz, T.; Zabor, E.C.; Brown, P.D.N.; Dalton, S.O.; Raghunathan, N.J.; Matasar, M.J.; Steingart, R.; Vickers, A.J.; Munksgaard, P.S.; Oeffinger, K.C.; et al. Preexisting cardiovascular risk and subsequent heart failure among Non-Hodgkin lymphoma survivors. J. Clin. Oncol. 2017, 34, 3837–3843. [Google Scholar] [CrossRef] [PubMed]
- Limat, S.; Daguindau, E.; Cahn, J.Y.; Nerich, V.; Brion, A.; Perrin, S.; Woronoff-Lemsi, M.-C.; Deconinck, E. Incidence and risk-factors of CHOP/R-CHOP-related cardiotoxicity in patients with aggressive Non-Hodgkin’s lymphoma. J. Clin. Pharm. Ther. 2014, 39, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkova, M. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2012, 7, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisberg, C.A.; Sawyer, D.B. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr. Hypertens. Rep. 2010, 12, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozdzanowska, D.; Wozniewski, M. Radiotherapy and anthracyclines—cardiovascular toxicity. Contemp. Oncol. 2015, 19, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Raghunathan, D.; Khilji, M.I.; Hassan, S.A.; Yusuf, S.W. Radiation-induced cardiovascular disease. Curr. Atheroscler. Rep. 2017, 19, 22. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Offersen, B.V.; Nielsen, H.M.; Vaage-Nilsen, M.; Yusuf, S.W. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin. Cardiol. 2017, 40, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Aonuma, K.; Sekine, I. Cardio-oncology: A multidisciplinary approach for detection, prevention and management of cardiac dysfunction in cancer patients. Jpn. J. Clin. Oncol. 2017, 1, 678–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žaliaduonytė, D.; Kleinauskienė, R.; Muckienė, G.; Zabiela, V. Cardiovascular Disorder after Cardiotoxic Non-Hodking’s Lymphoma Treatment: A Case Report. Medicina 2022, 58, 489. https://doi.org/10.3390/medicina58040489
Žaliaduonytė D, Kleinauskienė R, Muckienė G, Zabiela V. Cardiovascular Disorder after Cardiotoxic Non-Hodking’s Lymphoma Treatment: A Case Report. Medicina. 2022; 58(4):489. https://doi.org/10.3390/medicina58040489
Chicago/Turabian StyleŽaliaduonytė, Diana, Rita Kleinauskienė, Gintarė Muckienė, and Vytautas Zabiela. 2022. "Cardiovascular Disorder after Cardiotoxic Non-Hodking’s Lymphoma Treatment: A Case Report" Medicina 58, no. 4: 489. https://doi.org/10.3390/medicina58040489
APA StyleŽaliaduonytė, D., Kleinauskienė, R., Muckienė, G., & Zabiela, V. (2022). Cardiovascular Disorder after Cardiotoxic Non-Hodking’s Lymphoma Treatment: A Case Report. Medicina, 58(4), 489. https://doi.org/10.3390/medicina58040489




