Video-Assisted Thoracoscopic Surgery with Bullectomy and Partial Pleurectomy versus Chest Tube Drainage for Treatment of Secondary Spontaneous Pneumothorax—A Retrospective Single-Center Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique: VATS-Bullectomy with Partial Pleurectomy (VBPP)
2.2. Outpatient Care and Follow-Up
2.3. Statistical Analysis
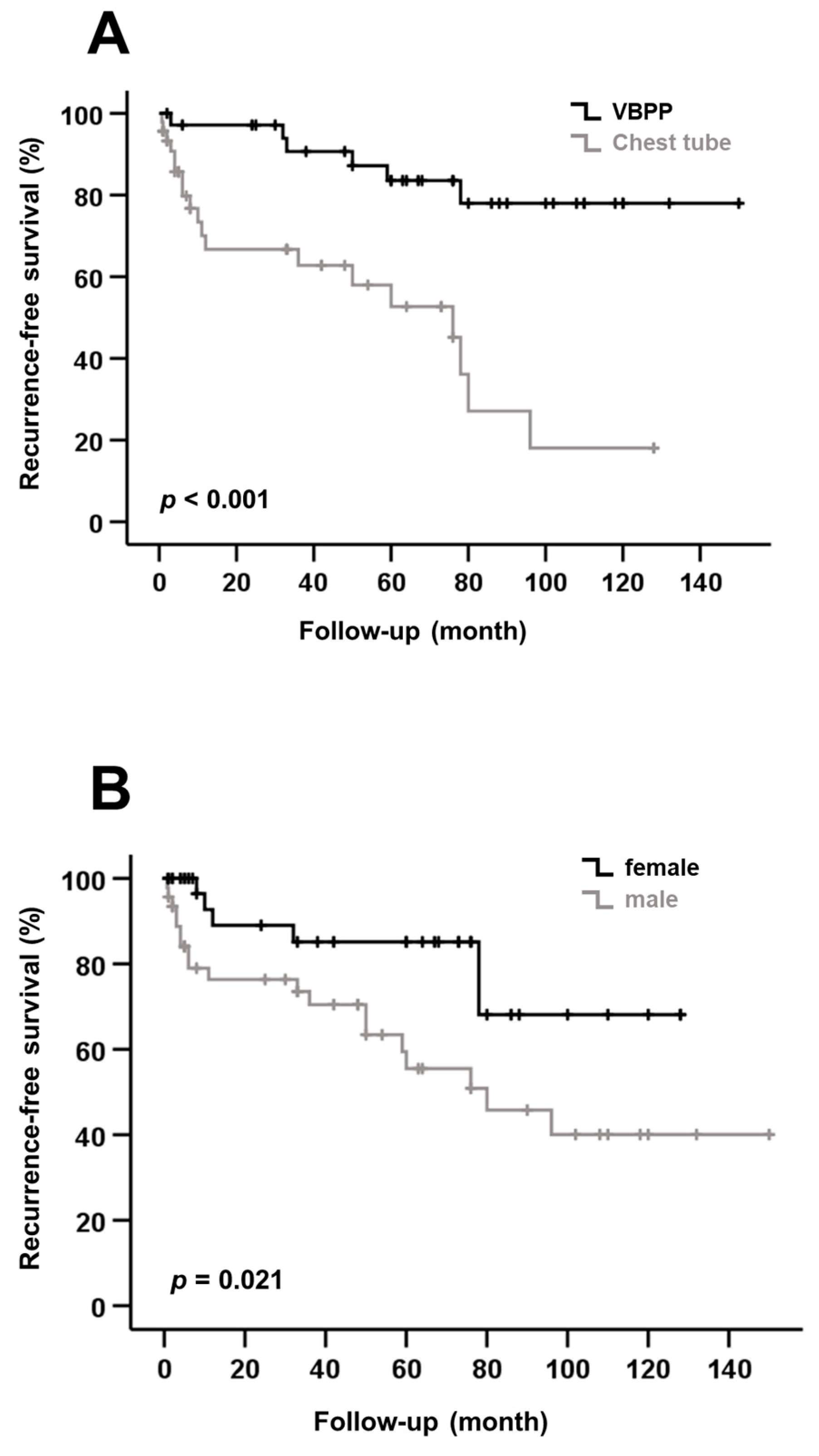
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnell, J.; Beer, M.; Eggeling, S.; Gesierich, W.; Gottlieb, J.; Herth, F.J.F.; Hofmann, H.S.; Jany, B.; Kreuter, M.; Ley-Zaporozhan, J.; et al. Management of Spontaneous Pneumothorax and Post-Interventional Pneumothorax: German S3 Guideline. Respiration 2019, 97, 370–402. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Hepper, N.G.; Offord, K.P. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am. Rev. Respir. Dis. 1979, 120, 1379–1382. [Google Scholar] [PubMed]
- Nakajima, J. Surgery for secondary spontaneous pneumothorax. Curr. Opin. Pulm. Med. 2010, 16, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Bintcliffe, O.; Maskell, N. Spontaneous pneumothorax. BMJ (Clin. Res. Ed.) 2014, 348, bg2928. [Google Scholar] [CrossRef] [PubMed]
- MacDuff, A.; Arnold, A.; Harvey, J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65 (Suppl. 2), ii18–ii31. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.M.; Bintcliffe, O.; Astoul, P.; Canalis, E.; Driesen, P.; Janssen, J.; Krasnik, M.; Maskell, N.; Van Schil, P.; Tonia, T.; et al. ERS task force statement: Diagnosis and treatment of primary spontaneous pneumothorax. Eur. Respir. J. 2015, 46, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, J.; Nagayama, K.; Hino, H.; Nitadori, J.; Anraku, M.; Murakawa, T.; Nakajima, J. Results of surgical treatment for secondary spontaneous pneumothorax according to underlying diseases. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2016, 49, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Sano, A. Multidisciplinary team approach for complicated pneumothorax. J. Thorac. Dis. 2018, 10 (Suppl. 18), S2109–S2110. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.H.; Hassaballa, A.; Ahmed, T. Is video-assisted thoracoscopic surgery talc pleurodesis superior to talc pleurodesis via tube thoracostomy in patients with secondary spontaneous pneumothorax? Interact. Cardiovasc. Thorac. Surg. 2016, 23, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Asai, K.; Urabe, N. Surgery for secondary spontaneous pneumothorax: Risk factors for recurrence and morbidity. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.D.; Lopez, A.; Mathie, A.; Wood, V.; Jackson, J.E.; Roddie, M.E. Quantification of pneumothorax size on chest radiographs using interpleural distances: Regression analysis based on volume measurements from helical CT. AJR Am. J. Roentgenol. 1995, 165, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Itoh, M.; Esaki, H.; Isobe, J.; Ueno, Y.; Inoue, R. Secondary spontaneous pneumothorax. Ann. Thorac. Surg. 1993, 55, 372–376. [Google Scholar] [CrossRef]
- Videm, V.; Pillgram-Larsen, J.; Ellingsen, O.; Andersen, G.; Ovrum, E. Spontaneous pneumothorax in chronic obstructive pulmonary disease: Complications, treatment and recurrences. Eur. J. Respir. Dis. 1987, 71, 365–371. [Google Scholar] [PubMed]
- Qureshi, R.; Nugent, A.; Hayat, J.; Qureshi, M.; Norton, R. Should surgical pleurectomy for spontaneous pneumothorax be always thoracoscopic? Interact. Cardiovasc. Thorac. Surg. 2008, 7, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Mithiran, H.; Leow, L.; Ong, K.; Liew, T.; Siva, D.; Liang, S.; Tam, J.K.C. Video-Assisted Thoracic Surgery (VATS) Talc Pleurodesis Versus Pleurectomy for Primary Spontaneous Pneumothorax: A Large Single-Centre Study with No Conversion. World J. Surg. 2019, 43, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Neudecker, J.; Malzahn, U.; Heuschmann, P.; Behrens, U.; Walles, T. Pulmonary wedge resection plus parietal pleurectomy (WRPP) versus parietal pleurectomy (PP) for the treatment of recurrent primary pneumothorax (WOPP trial): Study protocol for a randomized controlled trial. Trials 2015, 16, 540. [Google Scholar] [CrossRef] [PubMed]
- Shaikhrezai, K.; Thompson, A.I.; Parkin, C.; Stamenkovic, S.; Walker, W.S. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax-long-term results. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2011, 40, 120–123. [Google Scholar] [CrossRef]
- Ng, C.; Maier, H.T.; Kocher, F.; Jud, S.; Lucciarini, P.; Öfner, D.; Schmid, T.; Augustin, F. VATS Partial Pleurectomy Versus VATS Pleural Abrasion: Significant Reduction in Pneumothorax Recurrence Rates After Pleurectomy. World J. Surg. 2018, 42, 3256–3262. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, H.S.; Kim, H.S.; Shin, H.S.; Lee, J.W.; Kim, K.I.; Cho, S.W.; Lee, W.Y. Outcome of Video-assisted Thoracoscopic Surgery for Spontaneous Secondary Pneumothorax. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 225–228. [Google Scholar] [CrossRef]
- Brown, S.G.; Ball, E.L.; Macdonald, S.P.; Wright, C.; Mc, D.T.D. Spontaneous pneumothorax; a multicentre retrospective analysis of emergency treatment, complications and outcomes. Intern. Med. J. 2014, 44, 450–457. [Google Scholar] [CrossRef] [PubMed]
| Variables | VBPP (n = 36) | CT (n = 46) | p-Value |
|---|---|---|---|
| Gender | |||
| Male | 19 (52.8%) | 27 (58.7%) | |
| Female | 17 (47.2%) | 19 (41.3%) | 0.592 |
| Smoking status | |||
| Smokers (current and past) | 30 (83.3%) | 31 (67.4%) | |
| Non-smokers | 6 (16.7%) | 15 (32.6%) | 0.101 |
| Pneumothorax size (cm) | |||
| Collins < 4 cm | 5 (13.9%) | 4 (8.7%) | |
| Collins ≥ 4 cm | 30 (83.3%) | 41 (89.1%) | |
| Missing | 1 (2.8%) | 1 (2.2%) | 0.449 |
| Age (yrs.) | |||
| Median (Range) | 65 (41–87) | 60.5 (41–92) | 0.144 |
| Height (m) | |||
| Mean (SD) | 1.73 (0.08) | 1.72 (0.08) | 0.498 |
| Weight (kg) | |||
| Mean (SD) | 65.8 (10.51) | 64.7 (11.39) | 0.655 |
| BMI (kg/m2) | |||
| Median (Mean) | 21.5 (22) | 20.8 (21.8) | 0.483 |
| ECOG status | |||
| Grade 0–1 | 27 (75%) | 23 (50%) | |
| Grade 2–3 | 8 (22.2%) | 12 (26.1%) | |
| Grade 4 | 1 (2.8%) | 11 (23.9%) | 0.238 |
| Charlson Comorbidity Index (score) | |||
| 1–2 | 20 (55.6%) | 24 (52.2%) | |
| 3–4 | 14 (38.9%) | 12 (23.1%) | |
| 5 | 2 (5.5%) | 10 (24.7%) | 0.302 |
| Length of hospital stay (LOS) (days) | |||
| Mean (SD) | 14.1 (8.99) | 9.3 (4.82) | 0.006 * |
| Days until operation | |||
| Mean (SD) | 7.1 (2.65) | / | / |
| Complications | |||
| Hemothorax | 5 (13.9%) | 1 (2.8%) | 0.043 * |
| Acute pneumonia | 11 (30.6%) | 5 (10.9%) | 0.026 * |
| Clavien-Dindo grade | |||
| Grade I | 0 (0%) | 0 (0%) | |
| Grade II | 14 (87.5%) | 6 (100%) | |
| Grade IIIa | 2 (12.5%) | 0 (0%) | 0.375 |
| In-hospital mortality | 0 (0%) | 0 (0%) | 1.00 |
| COPD stage | |||
| No COPD | 7 (19.4%) | 11(23.9%) | |
| COPD Gold I | 5 (13.9%) | 2 (4.3%) | |
| COPD Gold II | 6 (16.7%) | 3 (6.5%) | |
| COPD Gold III | 13 (36.1%) | 21 (45.7%) | |
| COPD Gold IV | 5 (13.9%) | 9 (19.6%) | 0.282 |
| Cause of SSP | Total (N) | ||
| COPD | 29 (80.6%) | 35 (76.1%) | 64 |
| Tuberculosis | 6 (16.7%) | 5 (10.9%) | 11 |
| Lung cancer | 1 (2.7%) | 6 (13%) | 7 |
| Variable | Recurrence n (%) | p-Value |
|---|---|---|
| Gender | ||
| Male | 19 (41.3) | |
| Female | 6 (16.7) | 0.016 * |
| Age | ||
| ≤median (63 years) | 14 (31.8) | |
| >median (63 years) | 11 (28.9) | 0.778 |
| BMI | ||
| ≤median (21.1 kg/m2) | 13 (31.0) | |
| >median (21.1 kg/m2) | 12 (30.0) | 0.925 |
| Smoking status | ||
| Smokers (past and current) | 8 (38.1) | |
| Non-smokers | 17 (27.3) | 0.380 |
| Treatment | ||
| VBPP | 6 (16.7) | |
| CT | 19 (41.3) | 0.016 * |
| Pneumothorax size | ||
| Collins < 4 cm | 3 (33.3) | |
| Collins ≥ 4 cm | 20 (28.2) | 0.747 |
| Side of recurrence at presentation | ||
| Ipsilateral | 12 (60) | |
| Contralateral | 8 (40) | 0.211 |
| COPD stage | ||
| No COPD | 4 (22.2) | |
| COPD Gold I-IV | 21 (32.8) | 0.389 |
| Other causes of SSP | ||
| Tuberculosis | 4 (22.2) | |
| Lung cancer | 0 (77.8) | 0.024 * |
| Risk Factor | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Gender | |||
| Female vs. Male | 2.803 | 1.118–7.030 | 0.021 * |
| Age | |||
| ≤63 years vs. >63 years | 0.811 | 0.368–1.790 | 0.602 |
| BMI | |||
| ≤21.1 kg/m2 vs. >21.1 kg/m2 | 1.073 | 0.489–2.355 | 0.861 |
| Smoking status | |||
| Non-Smokers vs. Smokers | 0.690 | 0.297–1.601 | 0.382 |
| Treatment | |||
| CT vs. VBPP | 0.196 | 0.077–0.498 | <0.001 * |
| Pneumothorax size (cm) | |||
| Collins < 4 vs. Collins ≥4 | 1.075 | 0.318–3.630 | 0.907 |
| COPD stage | |||
| COPD vs. no COPD | 1.051 | 0.358–3.082 | 0.927 |
| Risk Factor | B | SE | Wald | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female vs. Male | 0.931 | 0.494 | 3.544 | 2.536 | 0.962–6.684 | 0.060 |
| Age | ||||||
| ≤63 years vs. >63 years | 0.005 | 0.018 | 0.065 | 1.005 | 0.969–1.041 | 0.799 |
| BMI | ||||||
| ≤21.1 kg/m2 vs. >21.1 kg/m2 | 0.237 | 0.487 | 0.237 | 1.268 | 0.488–3.294 | 0.627 |
| Smoking status | ||||||
| Non-smokers vs. Smokers | −0.090 | 0.500 | 0.032 | 0.914 | 0.343–2.437 | 0.857 |
| Treatment | ||||||
| CT vs. VBPP | −1.893 | 0.548 | 11.938 | 0.151 | 0.051–0.441 | 0.001 * |
| Pneumothorax size (cm) | ||||||
| Collins < 4 vs. Collins ≥ 4 | −0.433 | 0.738 | 0.345 | 0.648 | 0.153–2.755 | 0.557 |
| COPD | ||||||
| no COPD vs. COPD | 0.610 | 0.685 | 0.793 | 1.840 | 0.481–7.041 | 0.373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fung, S.; Kivilis, M.; Krieg, A.; Schauer, A.; Rehders, A.; Dizdar, L.; Knoefel, W.-T. Video-Assisted Thoracoscopic Surgery with Bullectomy and Partial Pleurectomy versus Chest Tube Drainage for Treatment of Secondary Spontaneous Pneumothorax—A Retrospective Single-Center Analysis. Medicina 2022, 58, 354. https://doi.org/10.3390/medicina58030354
Fung S, Kivilis M, Krieg A, Schauer A, Rehders A, Dizdar L, Knoefel W-T. Video-Assisted Thoracoscopic Surgery with Bullectomy and Partial Pleurectomy versus Chest Tube Drainage for Treatment of Secondary Spontaneous Pneumothorax—A Retrospective Single-Center Analysis. Medicina. 2022; 58(3):354. https://doi.org/10.3390/medicina58030354
Chicago/Turabian StyleFung, Stephen, Marius Kivilis, Andreas Krieg, Anja Schauer, Alexander Rehders, Levent Dizdar, and Wolfram-Trudo Knoefel. 2022. "Video-Assisted Thoracoscopic Surgery with Bullectomy and Partial Pleurectomy versus Chest Tube Drainage for Treatment of Secondary Spontaneous Pneumothorax—A Retrospective Single-Center Analysis" Medicina 58, no. 3: 354. https://doi.org/10.3390/medicina58030354
APA StyleFung, S., Kivilis, M., Krieg, A., Schauer, A., Rehders, A., Dizdar, L., & Knoefel, W.-T. (2022). Video-Assisted Thoracoscopic Surgery with Bullectomy and Partial Pleurectomy versus Chest Tube Drainage for Treatment of Secondary Spontaneous Pneumothorax—A Retrospective Single-Center Analysis. Medicina, 58(3), 354. https://doi.org/10.3390/medicina58030354







