NPS-1034 Induce Cell Death with Suppression of TNFR1/NF-κB Signaling in Testicular Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay
2.3. Cell Cycle Analysis
2.4. Hoechst 33342 Staining
2.5. The Annexin-V/FITC Assay
2.6. The Human Apoptosis Array for Proteome Profiling
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
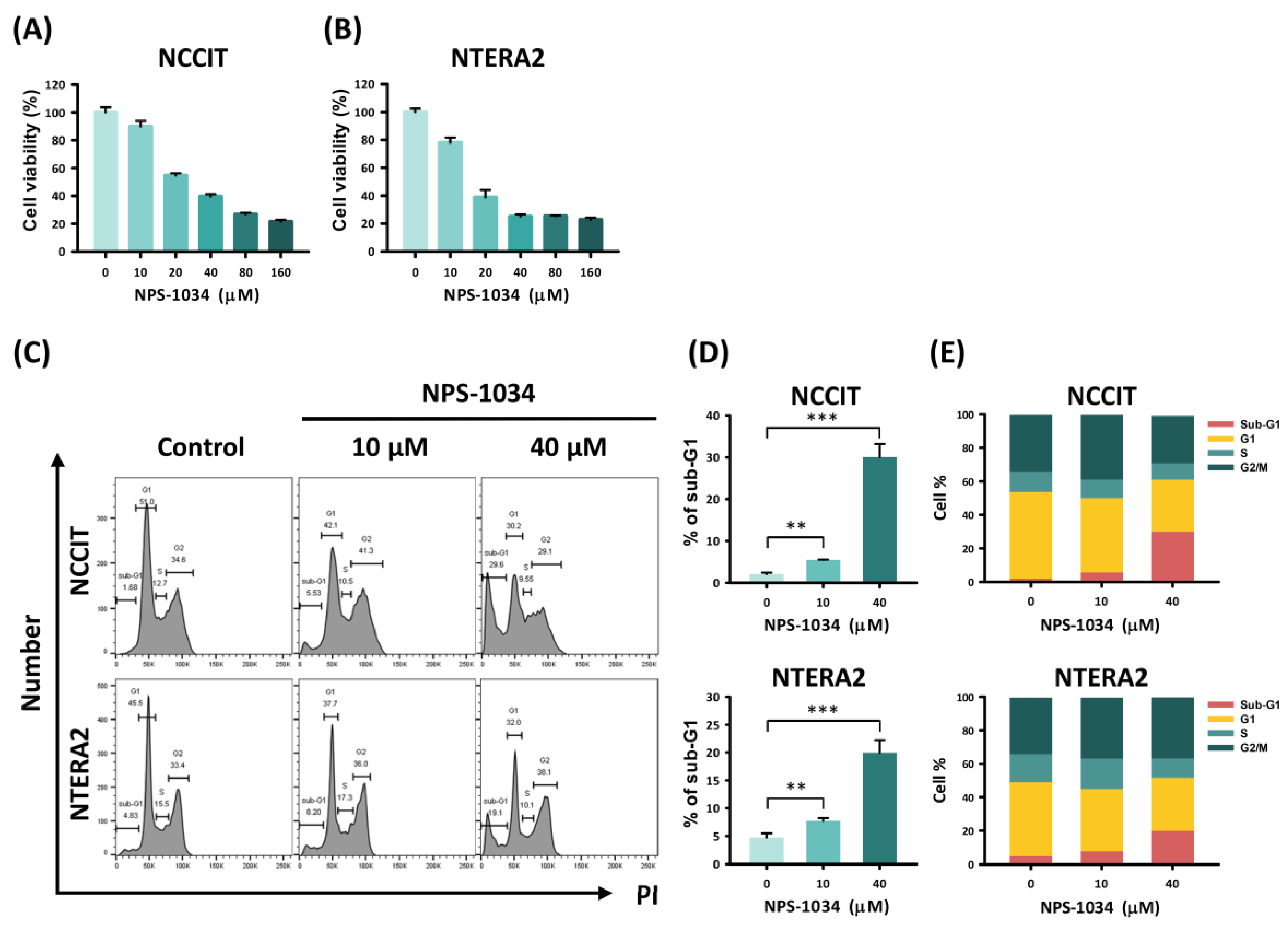
3.1. NPS-1034 Inhibits the Cell Progression of NCCIT and NTERA2 Cell Lines In Vitro
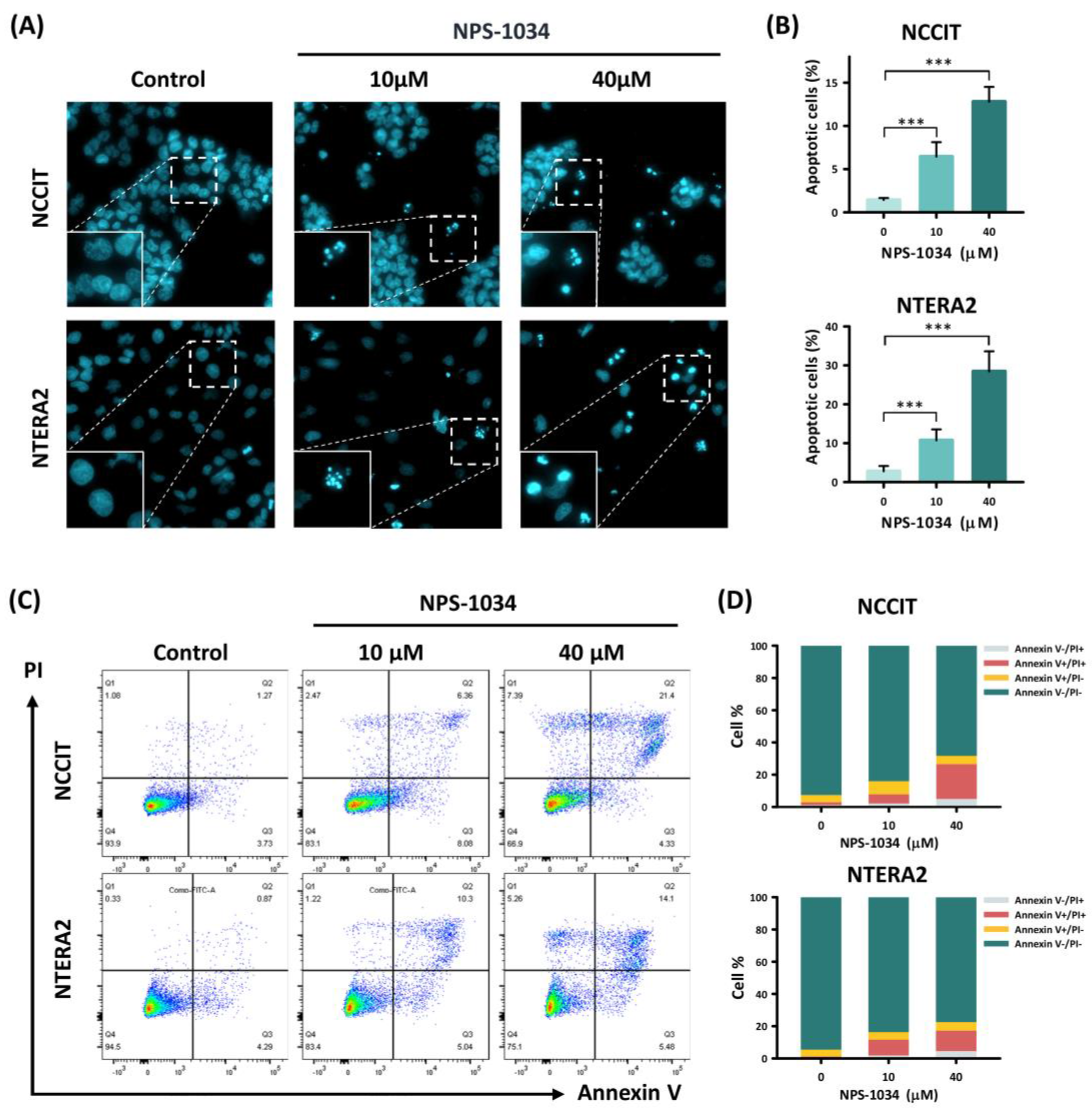
3.2. NPS-1034 Enhances Apoptosis in NCCIT and NTERA2 Cell Lines
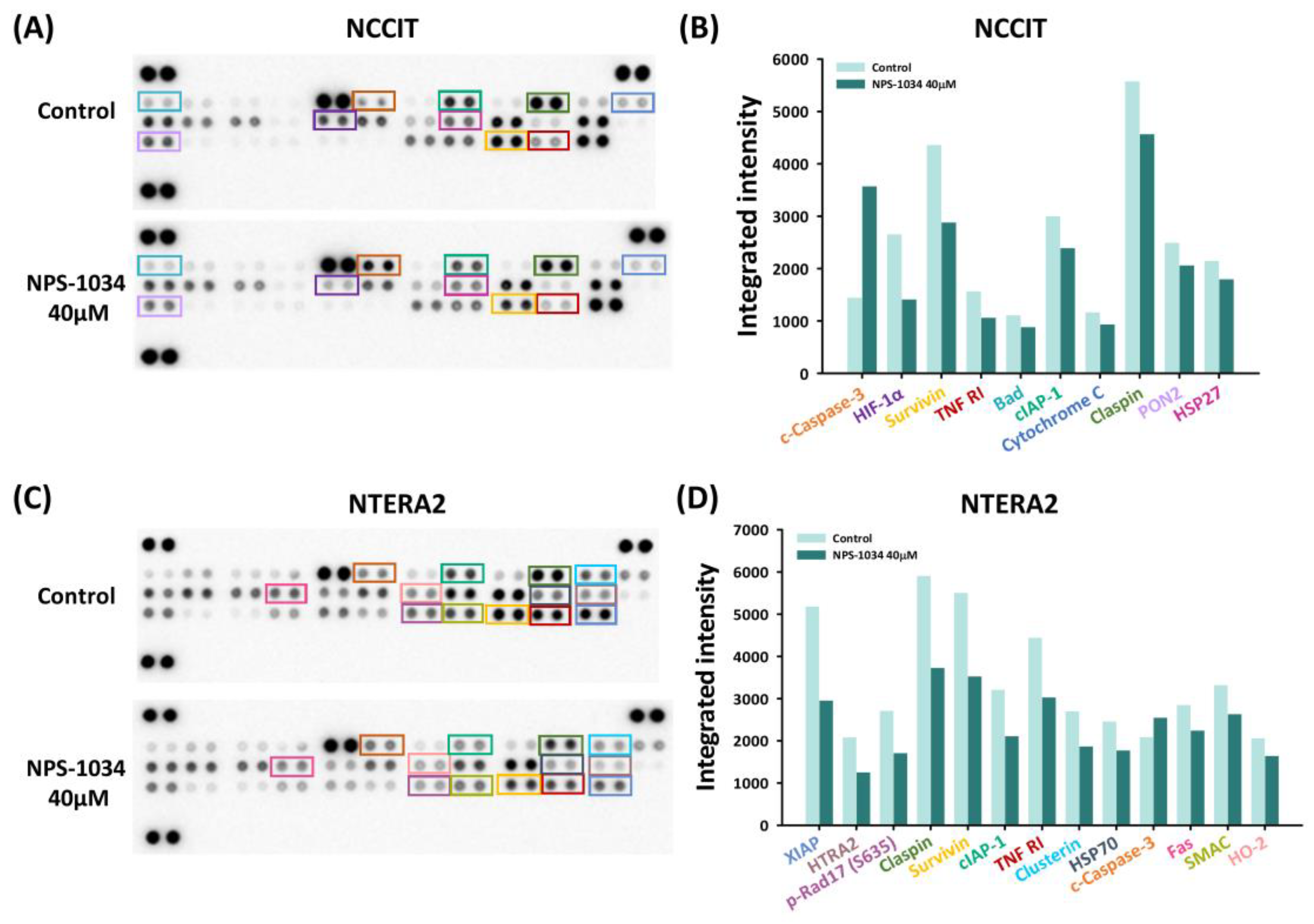
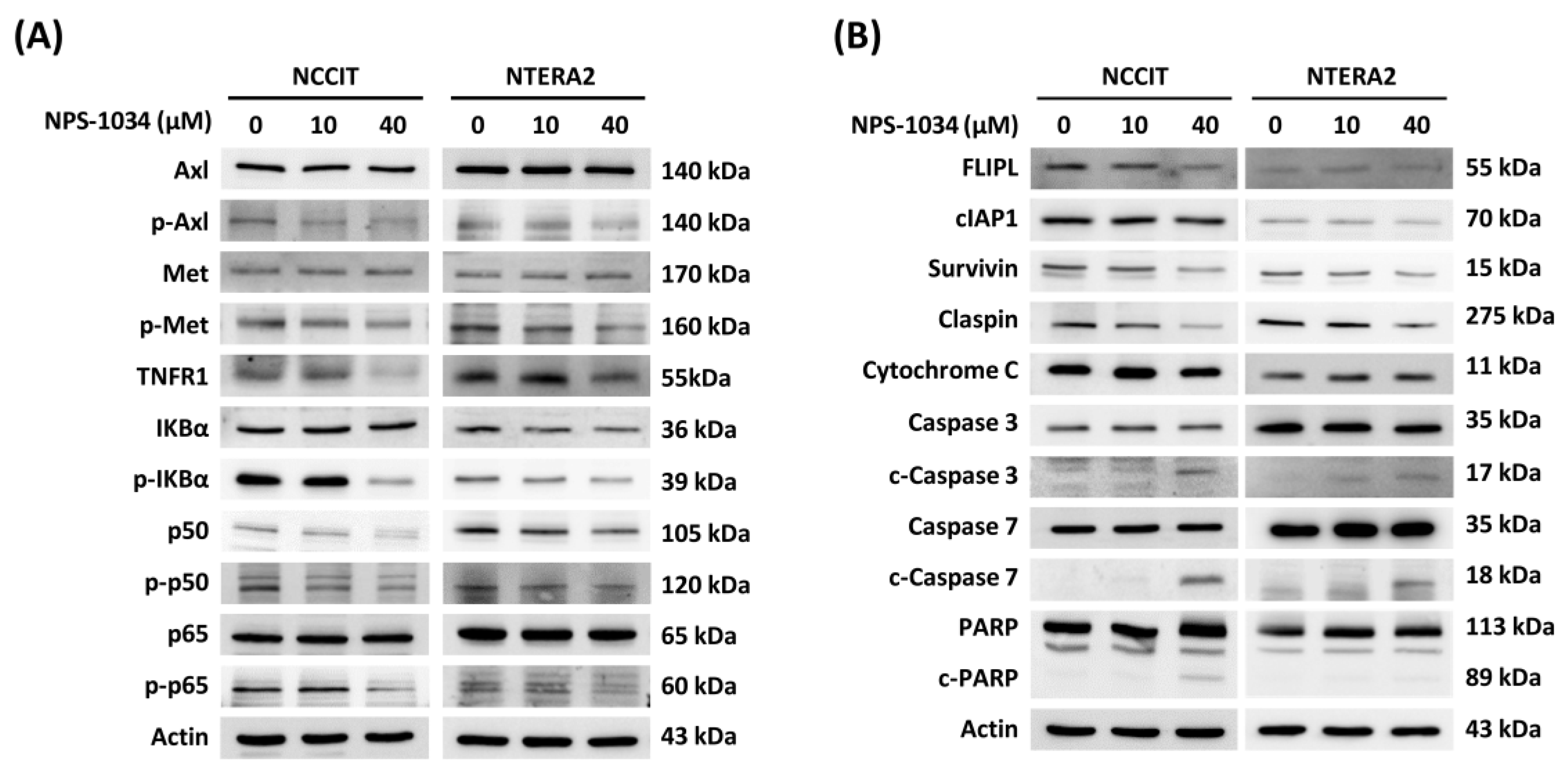
3.3. Identification of Proteins Related to NPS-1034-Facilitated Apoptotic Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldman, D.R.; Bosl, G.J.; Sheinfeld, J.; Motzer, R.J. Medical treatment of advanced testicular cancer. JAMA 2008, 299, 672–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajpert-De Meyts, E. Developmental model for the pathogenesis of testicular carcinoma in situ: Genetic and environmental aspects. Hum. Reproduct. Update 2006, 12, 303–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Cornet, C.; Lortet-Tieulent, J.; Forman, D.; Béranger, R.; Flechon, A.; Fervers, B.; Schüz, J.; Bray, F. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur. J. Cancer 2014, 50, 831–839. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent global trends in testicular cancer incidence and mortality. Medicine 2018, 97, e12390. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Trabert, B. Adolescent and adult risk factors for testicular cancer. Nat. Rev. Urol. 2012, 9, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H.J. Testicular cancer. Nat. Rev. Dis. Primers 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Karimi, N.; Wu, X.N.; Chen, S.R.; Liu, Y.X. Testicular germ cell tumor: A comprehensive review. Cell. Mol. Life Sci. CMLS 2019, 76, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- Coursey Moreno, C.; Small, W.C.; Camacho, J.C.; Master, V.; Kokabi, N.; Lewis, M.; Hartman, M.; Mittal, P.K. Testicular tumors: What radiologists need to know—Differential diagnosis, staging, and management. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2015, 35, 400–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijnsdijk, E.A.M.; Supit, S.J.; Looijenga, L.H.J.; de Koning, H.J. Screening for cancers with a good prognosis: The case of testicular germ cell cancer. Cancer Med. 2021, 10, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.K.; Choi, Y.J.; Kim, S.Y.; Kim, T.W.; Choi, E.K.; Yoon, S.J.; Park, B.M.; Park, E.; Bae, J.H.; Choi, C.M.; et al. MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation. Cancer Res. 2014, 74, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Peruzzi, B.; Bottaro, D.P. Targeting the c-Met signaling pathway in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 3657–3660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Organ, S.L.; Tsao, M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011, 3, S7–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Siemann, D.W. Gas6/Axl Signaling Pathway in the Tumor Immune Microenvironment. Cancers 2020, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Okura, N.; Nishioka, N.; Yamada, T.; Taniguchi, H.; Tanimura, K.; Katayama, Y.; Yoshimura, A.; Watanabe, S.; Kikuchi, T.; Shiotsu, S.; et al. ONO-7475: A Novel AXL Inhibitor, Suppresses the Adaptive Resistance to Initial EGFR-TKI Treatment in EGFR-Mutated Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Hong, S.W.; Moon, J.H.; Kim, J.S.; Jung, K.A.; Kim, S.M.; Lee, D.H.; Kim, I.; Yoon, S.J.; Lee, C.G.; et al. NPS-1034: A novel MET inhibitor, inhibits the activated MET receptor and its constitutively active mutants. Investig. New Drugs 2014, 32, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Facchini, G.; Rossetti, S.; Cavaliere, C.; D′Aniello, C.; Di Franco, R.; Iovane, G.; Grimaldi, G.; Piscitelli, R.; Muto, P.; Botti, G.; et al. Exploring the molecular aspects associated with testicular germ cell tumors: A review. Oncotarget 2018, 9, 1365–1379. [Google Scholar] [CrossRef] [Green Version]
- Lobo, J.; Jerónimo, C.; Henrique, R. Cisplatin Resistance in Testicular Germ Cell Tumors: Current Challenges from Various Perspectives. Cancers 2020, 12, 1601. [Google Scholar] [CrossRef]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-related heart disease: Current knowledge and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 656–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerring, A.W.; Fosså, S.D.; Haugnes, H.S.; Nome, R.; Stokke, T.M.; Haugaa, K.H.; Kiserud, C.E.; Edvardsen, T.; Sarvari, S.I. The cardiac impact of cisplatin-based chemotherapy in survivors of testicular cancer: A 30-year follow-up. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Ge, Y.; Ma, P.; Ma, Q.; Ma, J.; Wang, H.; Xue, S.; Han, D. Immunoexpression of Tyro 3 family receptors--Tyro 3, Axl, and Mer--and their ligand Gas6 in postnatal developing mouse testis. J. Histochem. Cytochem. Off. J. Histoche. Soc. 2005, 53, 1355–1364. [Google Scholar] [CrossRef]
- Scheri, K.C.; Leonetti, E.; Laino, L.; Gigantino, V.; Gesualdi, L.; Grammatico, P.; Bizzari, M.; Franco, R.; Oosterhuis, J.W.; Stoop, H.; et al. c-MET receptor as potential biomarker and target molecule for malignant testicular germ cell tumors. Oncotarget 2018, 9, 31842–31860. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chang, Y.C.; Wu, M.Y.; Yu, C.Y.; Chen, S.L.; Sung, W.W. Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer. Cancers 2021, 13, 3933. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Yamada, T.; Wang, R.; Tanimura, K.; Adachi, Y.; Nishiyama, A.; Tanimoto, A.; Takeuchi, S.; Araujo, L.H.; Boroni, M.; et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat. Commun. 2019, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Luo, Y.; He, Y.; Zhang, H.; Zhang, R.; Li, X.; Dobrucki, W.L.; Sinusas, A.J.; Sessa, W.C.; Min, W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am. J. Pathol. 2006, 169, 1886–1898. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-T.; Wang, S.-C.; Chen, B.-S.; Chang, Y.-C.; Yu, C.-Y.; Sung, W.-W.; Song, T.-Y. NPS-1034 Induce Cell Death with Suppression of TNFR1/NF-κB Signaling in Testicular Cancer. Medicina 2022, 58, 355. https://doi.org/10.3390/medicina58030355
Chen J-T, Wang S-C, Chen B-S, Chang Y-C, Yu C-Y, Sung W-W, Song T-Y. NPS-1034 Induce Cell Death with Suppression of TNFR1/NF-κB Signaling in Testicular Cancer. Medicina. 2022; 58(3):355. https://doi.org/10.3390/medicina58030355
Chicago/Turabian StyleChen, Jian-Ting, Shao-Chuan Wang, Brian-Shiian Chen, Ya-Chuan Chang, Chia-Ying Yu, Wen-Wei Sung, and Tuzz-Ying Song. 2022. "NPS-1034 Induce Cell Death with Suppression of TNFR1/NF-κB Signaling in Testicular Cancer" Medicina 58, no. 3: 355. https://doi.org/10.3390/medicina58030355
APA StyleChen, J.-T., Wang, S.-C., Chen, B.-S., Chang, Y.-C., Yu, C.-Y., Sung, W.-W., & Song, T.-Y. (2022). NPS-1034 Induce Cell Death with Suppression of TNFR1/NF-κB Signaling in Testicular Cancer. Medicina, 58(3), 355. https://doi.org/10.3390/medicina58030355








