Analysis of the Common Femoral Artery and Vein: Anatomical Morphology, Vessel Relationship, and Factors Affecting Vessel Size
Abstract
:1. Introduction
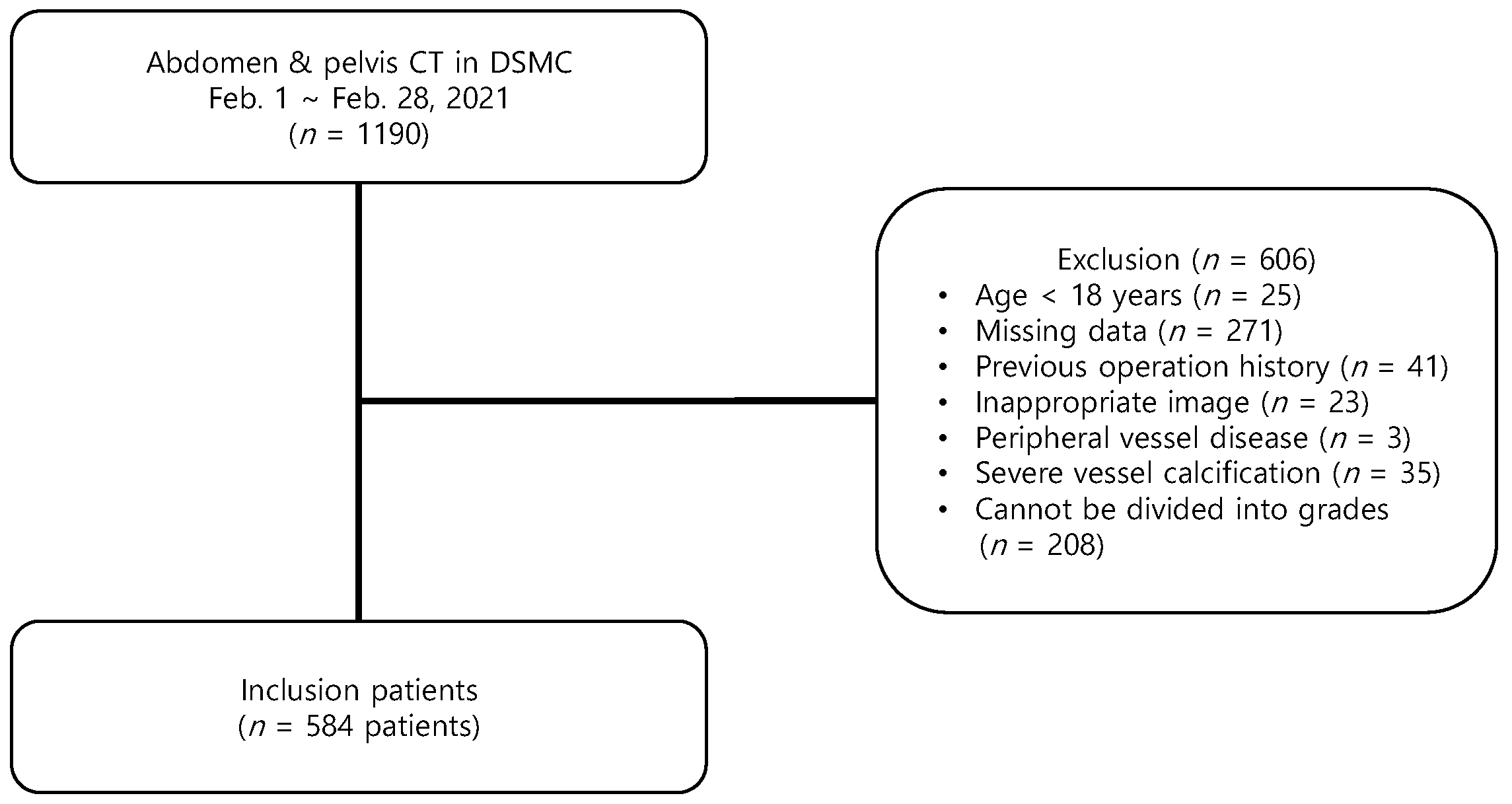
2. Materials and Methods
2.1. Study Design and Search Strategy
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leibowitz, A.; Oren-Grinberg, A.; Matyal, R. Ultrasound Guidance for Central Venous Access: Current Evidence and Clinical Recommendations. J. Intensive Care Med. 2020, 35, 303–321. [Google Scholar] [CrossRef]
- Manning, J.E.; Moore, E.E.; Morrison, J.J.; Lyon, R.F.; DuBose, J.J.; Ross, J.D. Femoral vascular access for endovascular resuscitation. J. Trauma Acute Care. Surg. 2021, 91, e104–e113. [Google Scholar] [CrossRef]
- Parienti, J.J.; Mongardon, N.; Mégarbane, B.; Mira, J.P.; Kalfon, P.; Gros, A.; Marqué, S.; Thuong, M.; Pottier, V.; Ramakers, M.; et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N. Engl. J. Med. 2015, 373, 1220–1229. [Google Scholar] [CrossRef] [Green Version]
- Frenckner, B.; Broman, M.; Broomé, M. Position of draining venous cannula in extracorporeal membrane oxygenation for respiratory and respiratory/circulatory support in adult patients. Crit. Care 2018, 22, 163. [Google Scholar] [CrossRef] [Green Version]
- Brass, P.; Hellmich, M.; Kolodziej, L.; Schick, G.; Smith, A.F. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst. Rev. 2015, 1, Cd011447. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.; Scott, C.; Bodenham, A. Ultrasonography of the femoral vessels in the groin: Implications for vascular access. Anaesthesia 2000, 55, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, G.; Sawhney, N.; Whisenant, B.; Tsimikas, S.; Turi, Z.G. Common femoral artery anatomy is influenced by demographics and comorbidity: Implications for cardiac and peripheral invasive studies. Catheter. Cardiovasc. Interv. 2001, 53, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Keiler, J.; Seidel, R.; Wree, A. The femoral vein diameter and its correlation with sex, age and body mass index—An anatomical parameter with clinical relevance. Phlebology 2019, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Swift, H.; Bordoni, B. Anatomy, bony pelvis and lower limb, femoral artery. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lorbeer, R.; Grotz, A.; Dörr, M.; Völzke, H.; Lieb, W.; Kühn, J.P.; Mensel, B. Reference values of vessel diameters, stenosis prevalence, and arterial variations of the lower limb arteries in a male population sample using contrast-enhanced MR angiography. PLoS ONE 2018, 13, e0197559. [Google Scholar] [CrossRef]
- Sandgren, T.; Sonesson, B.; Ahlgren, R.; Länne, T. The diameter of the common femoral artery in healthy human: Influence of sex, age, and body size. J. Vasc. Surg. 1999, 29, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Baum, P.A.; Matsumoto, A.H.; Teitelbaum, G.P.; Zuurbier, R.A.; Barth, K.H. Anatomic relationship between the common femoral artery and vein: CT evaluation and clinical significance. Radiology 1989, 173, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Bhatt, D.; Kapadia, S.; Lee, D.; Yen, M.; Whitlow, P.L. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2006, 67, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Kontopantelis, E.; Kinnaird, T.; Potts, J.; Rashid, M.; Shoaib, A.; Nolan, J.; Bagur, R.; de Belder, M.A.; Ludman, P.; et al. Retroperitoneal Hemorrhage after Percutaneous Coronary Intervention: Incidence, Determinants, and Outcomes as Recorded by the British Cardiovascular Intervention Society. Circ. Cardiovasc. Interv. 2018, 11, e005866. [Google Scholar] [CrossRef] [PubMed]
- Uhl, J.F.; Gillot, C.; Chahim, M. Anatomical variations of the femoral vein. J. Vasc. Surg. 2010, 52, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Crişan, S. Ultrasound examination of the femoral and popliteal arteries. Med. Ultrason. 2012, 14, 74–77. [Google Scholar]
- Rajebi, H.; Rajebi, M.R. Optimizing Common Femoral Artery Access. Technol. Vasc. Interv. Radiol. 2015, 18, 76–81. [Google Scholar] [CrossRef]
- Maecken, T.; Grau, T. Ultrasound imaging in vascular access. Crit. Care Med. 2007, 35, S178–S185. [Google Scholar] [CrossRef]
- Jeon, J.C.; Choi, W.I.; Lee, J.H.; Lee, S.H. Anatomical Morphology Analysis of Internal Jugular Veins and Factors Affecting Internal Jugular Vein Size. Medicina 2020, 56, 135. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, J.D.; Talbot, S.; Burkart, J.A. Cross-sectional internal diameters of human cervical and femoral blood vessels: Relationship to subject’s sex, age, body size. Anat. Rec. 1990, 226, 115–124. [Google Scholar] [CrossRef]
- Greenwald, S.E. Ageing of the conduit arteries. J. Pathol. 2007, 211, 157–172. [Google Scholar] [CrossRef]
- Green, D.J.; Spence, A.; Rowley, N.; Thijssen, D.H.; Naylor, L.H. Vascular adaptation in athletes: Is there an ‘athlete’s artery’? Exp. Physiol. 2012, 97, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Arfvidsson, B.; Eklof, B.; Balfour, J. Iliofemoral venous pressure correlates with intraabdominal pressure in morbidly obese patients. Vasc. Endovasc. Surg. 2005, 39, 505–509. [Google Scholar] [CrossRef]
- Farnsworth, R.H.; Lackmann, M.; Achen, M.G.; Stacker, S.A. Vascular remodeling in cancer. Oncogene 2014, 33, 3496–3505. [Google Scholar] [CrossRef] [Green Version]
| Patient Characteristics | Total (n = 584) | Men (n = 340) | Women (n = 244) | p Value |
|---|---|---|---|---|
| Age, years | 65 (56–73) | 66 (59–73) | 64 (50–72) | 0.016 |
| Height (cm) | 163.0 (156.9–169.0) | 168.0 (163.0–172.0) | 156.0 (150.6–160.1) | <0.001 |
| Weight (kg) | 60.7 (53.9–69.6) | 65.0 (56.9–73.1) | 55.5 (50.9–62.8) | <0.001 |
| Body mass index (kg/m2) | 23.3 (20.9–25.7) | 23.3 (20.9–25.6) | 23.1 (20.9–25.9) | 0.859 |
| Previous illness | ||||
| Hypertension | 210 (36.0) | 125 (36.8) | 85 (34.8) | 0.348 |
| Diabetes | 151 (25.9) | 101 (29.7) | 50 (20.5) | 0.008 |
| Dyslipidemia | 54 (9.2) | 31 (9.1) | 23 (9.4) | 0.505 |
| Chronic kidney disease | 22 (3.8) | 12 (3.5) | 10 (4.1) | 0.442 |
| Cerebrovascular accident | 34 (5.8) | 22 (6.5) | 12 (4.9) | 0.273 |
| Coronary artery disease | 60 (10.3) | 46 (13.5) | 14 (5.7) | 0.001 |
| Pulmonary thromboembolism | 12 (2.1) | 4 (1.2) | 8 (3.3) | 0.072 |
| Malignancy | 367 (62.8) | 225 (66.2) | 142 (58.2) | 0.030 |
| History of Chemotherapy | 231 (39.6) | 132 (38.8) | 99 (40.6) | 0.366 |
| History of Radiotherapy | 64 (11.0) | 43 (12.6) | 21 (8.6) | 0.079 |
| Location | Total (n = 1168) | Right (n = 584) | Left (n = 584) | p Value |
|---|---|---|---|---|
| Femoral artery size (mm2) | ||||
| Proximal | 61.9 (49.8–74.8) | 62.3 (50.4–75.0) | 61.6 (49.6–74.1) | 0.306 |
| Middle | 66.9 (55.0–81.4) | 67.0 (55.1–82.7) | 66.9 (54.8–81.1) | 0.592 |
| Distal | 73.1 (58.7–88.7) | 73.7 (59.8–90.4) | 72.0 (58.2–87.5) | 0.151 |
| p value | <0.001 | <0.001 | <0.001 | |
| Femoral vein size (mm2) | ||||
| Proximal | 97.5 (74.5–122.2) | 97.1 (75.1–122.9) | 97.9 (74.3–122.1) | 1.000 |
| Middle | 111.0 (81.6–147.2) | 109.9 (81.8–147.6) | 113.5 (80.8–147.9) | 0.944 |
| Distal | 126.6 (91.1–169.2) | 123.8 (90.4–164.2) | 128.8 (91.8–173.7) | 0.219 |
| p value | <0.001 | <0.001 | <0.001 | |
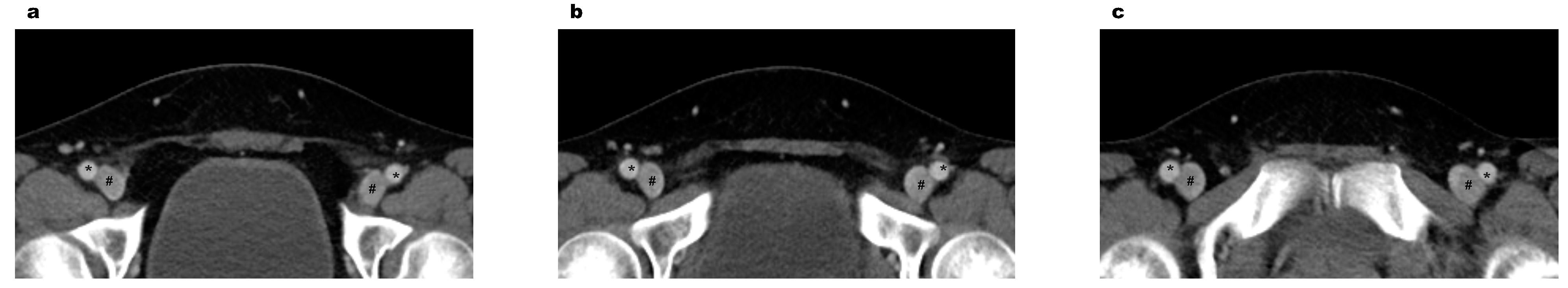
| Morphology types between veins and arteries | ||||
| Proximal | 0.206 | |||
| Type I | 1060 (90.8) | 534 (91.4) | 526 (90.1) | |
| Type II | 102 (8.7) | 48 (8.2) | 54 (9.2) | |
| Type III | 6 (0.5) | 2 (0.3) | 4 (0.7) | |
| Middle | <0.001 | |||
| Type I | 924 (79.1) | 491 (84.1) | 433 (74.1) | |
| Type II | 224 (19.2) | 84 (14.4) | 140 (24.0) | |
| Type III | 20 (1.7) | 9 (1.5) | 11 (1.9) | |
| Distal | 0.011 | |||
| Type I | 680 (58.2) | 359 (61.5) | 321 (55.0) | |
| Type II | 419 (35.9) | 195 (33.4) | 224 (38.4) | |
| Type III | 69 (5.9) | 30 (5.1) | 39 (6.7) | |
| p value | <0.001 | <0.001 | <0.001 | |
| Variables | Crude OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Common femoral artery | ||||
| Age | 1.038 | 1.026–1.051 | 1.045 | 1.030–1.060 |
| Men | 4.324 | 3.036–6.157 | 4.956 | 3.340–7.354 |
| Body mass index | 1.105 | 1.057–1.155 | 1.176 | 1.116–1.240 |
| Malignancy | 2.246 | 1.592–3.168 | 2.136 | 1.414–3.227 |
| Common femoral vein | ||||
| Age | 1.022 | 1.010–1.034 | 1.032 | 1.018–1.047 |
| Men | 4.380 | 3.075–6.239 | 5.385 | 3.625–7.999 |
| Body mass index | 1.171 | 1.115–1.229 | 1.231 | 1.166–1.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-h.; Yu, D.u.; Kim, T.k.; Jeon, J.-c.; Jin, S.c.; Choi, W.i.; Lee, J.h. Analysis of the Common Femoral Artery and Vein: Anatomical Morphology, Vessel Relationship, and Factors Affecting Vessel Size. Medicina 2022, 58, 325. https://doi.org/10.3390/medicina58020325
Lee S-h, Yu Du, Kim Tk, Jeon J-c, Jin Sc, Choi Wi, Lee Jh. Analysis of the Common Femoral Artery and Vein: Anatomical Morphology, Vessel Relationship, and Factors Affecting Vessel Size. Medicina. 2022; 58(2):325. https://doi.org/10.3390/medicina58020325
Chicago/Turabian StyleLee, Sang-hun, Dong uk Yu, Tae kwon Kim, Jae-cheon Jeon, Sang chan Jin, Woo ik Choi, and Jae ho Lee. 2022. "Analysis of the Common Femoral Artery and Vein: Anatomical Morphology, Vessel Relationship, and Factors Affecting Vessel Size" Medicina 58, no. 2: 325. https://doi.org/10.3390/medicina58020325
APA StyleLee, S.-h., Yu, D. u., Kim, T. k., Jeon, J.-c., Jin, S. c., Choi, W. i., & Lee, J. h. (2022). Analysis of the Common Femoral Artery and Vein: Anatomical Morphology, Vessel Relationship, and Factors Affecting Vessel Size. Medicina, 58(2), 325. https://doi.org/10.3390/medicina58020325






