Temporal Changes in Incidence Rates of the Most Common Gynecological Cancers in the Female Population in Central Serbia
Abstract
1. Background and Objectives
2. Material and Methods
Statistical Analysis
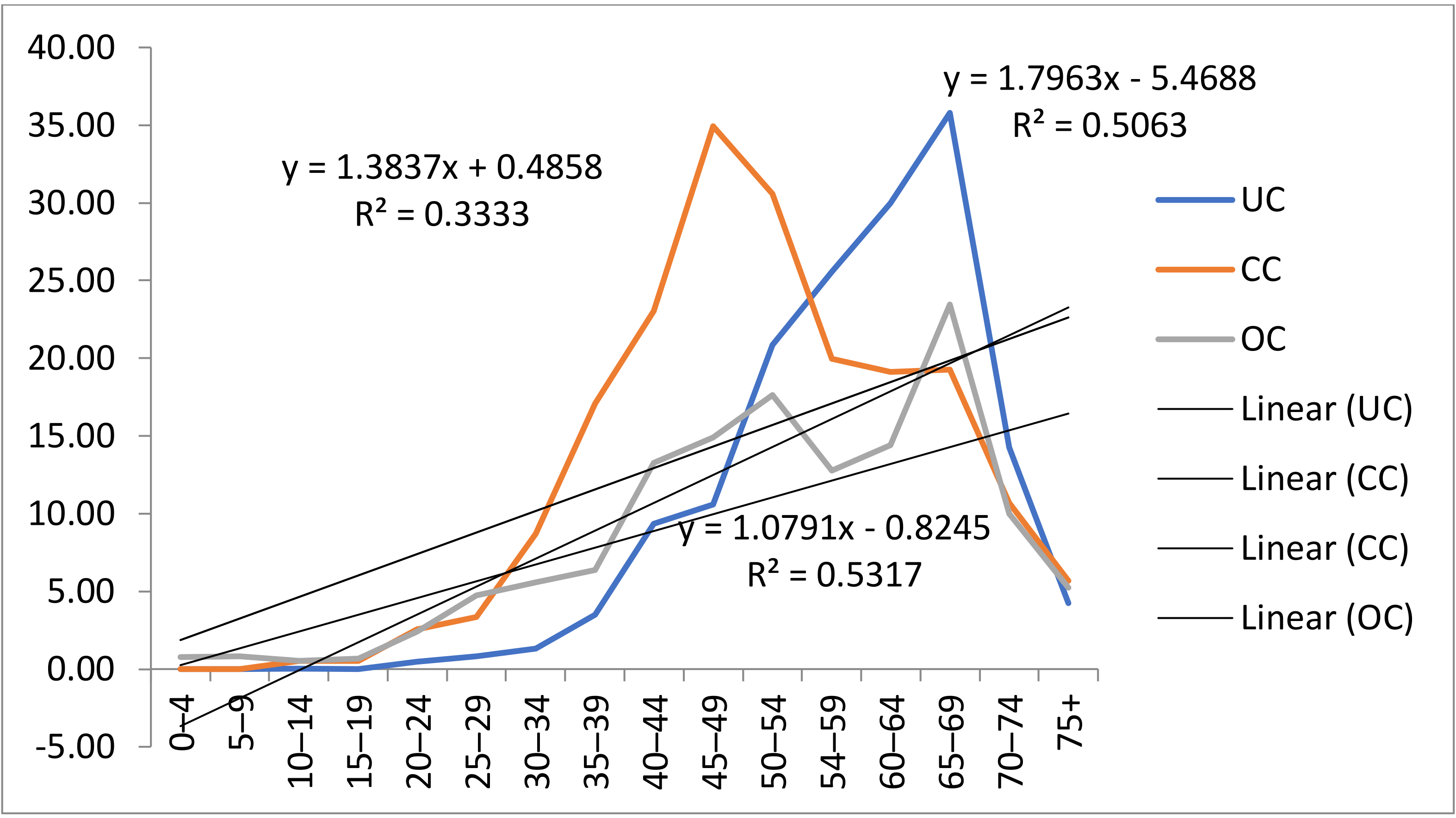
3. Results
4. Discussion
Prevention and Age Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLOBOCAN | Global Cancer Observatory |
| CC | cervical cancer |
| UC | uterine cancer |
| OC | ovarian cancer |
| ASR | age-standardized rate |
| APC | annual percentage change |
| CI | confidence interval |
| CBHT | compounded bioidentical hormone therapy |
| HT | hormone therapy |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Int. Agency Res. Cancer 2020. Available online: www.Gco.iarc.fr/today (accessed on 25 November 2020). [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, L.R. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Risk Factors for Cervical Cancer. Available online: https://www.cancer.org/cancer/cervical-cancer/causes-risks-prevention/risk-factors.html (accessed on 15 December 2019).
- Pilleron, S.; Cabasag, C.J.; Ferlay, J.; Bray, F.; Luciani, S.; Almonte, M.; Piñeros, M. Cervical cancer burden in Latin America and the Caribbean: Where are we? Int. J. Cancer 2020, 147, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.E.L.; Zielonke, N.; Gini, A.; Anttila, A.; Segnan, N.; Voko, Z.; Ivanuš, U.; McKee, M.; de Koning, H.J.; de Kok, I.M.; et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 207–223. [Google Scholar] [CrossRef]
- Castanon, A.; Sasieni, P. Is the recent increase in cervical cancer in women aged 20–24 years in England a cause for concern? Prev. Med. 2018, 107, 21–28. [Google Scholar] [CrossRef]
- Utada, M.; Chernyavskiy, P.; Lee, W.J.; Franceschi, S.; Sauvaget, C.; Berrington de Gonzalez, A.; Withrow, D.R. Increasing risk of uterine cervical cancer among young Japanese women: Comparison of incidence trends in Japan, South Korea and Japanese-Americans between 1985 and 2012. Int. J. Cancer 2019, 144, 2144–2152. [Google Scholar] [CrossRef]
- Mezei, A.K.; Armstrong, H.L.; Pedersen, H.N.; Campos, N.G.; Mitchell, S.M.; Sekikubo, M.; Byamugisha, J.K.; Kim, J.J.; Bryan, S.; Ogilvie, G.S.; et al. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: A systematic review. Int. J. Cancer 2017, 141, 437–446. [Google Scholar] [CrossRef]
- Lemp, J.M.; De Neve, J.W.; Bussmann, H.; Chen, S.; Manne-Goehler, J.; Theilmann, M.; Marcus, M.E.; Ebert, C.; Probst, C.; Tsabedze-Sibanyoni, L.; et al. Lifetime prevalence of cervical cancer screening in 55 low- and middle-income countries. JAMA 2020, 324, 1532–1542. [Google Scholar] [CrossRef]
- Jovanovic, V.; Jovanovic, A.M.; Kocic, S.; Vasiljevic, M.; Krasic, V. Knowledge about cervical cancer, Pap test, and barriers to women’s participation in screening in Belgrade, Serbia. Eur. J. Gynaecol. Oncol. 2017, 38, 69–75. [Google Scholar] [PubMed]
- Aimagambetova, G.; Chee, K.; Chee Kai Chan, K.C. Cervical cancer screening and prevention in Kazakhstan and Central Asia. J. Med. Screen. 2020, 28, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Jedy-Agba, E.; Joko, W.Y.; Liu, B.; Buziba, N.G.; Borok, M.; Korir, A.; Masamba, L.; Manraj, S.S.; Finesse, A.; Wabinga, H.; et al. Trends in cervical cancer incidence in sub-Saharan Africa. Br. J Cancer 2020, 123, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mistry, M.; Parkin, D.M.; Ahmad, A.S.; Sasieni, P. Cancer incidence in the United Kingdom: Projections to the year 2030. Br. J. Cancer 2011, 105, 1759–1803. [Google Scholar] [CrossRef] [PubMed]
- Brüggmann, D.; Ouassou, K.; Klingelhöfer, D.; Bohlmann, M.K.; Jaque, J.; Groneberg, D.A. Endometrial cancer: Mapping the global landscape of research. J. Transl. Med. 2020, 18, 386. [Google Scholar] [CrossRef] [PubMed]
- Constantine, G.D.; Kessler, G.B.A.; Graham, S.; Goldsteine, S.R. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J. Womens Health 2019, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, R.; Sinha, D.N.; Mehrotra, R. Relationship between type of smokeless tobacco and risk of cancer: A systematic review. Indian J. Med. Res. 2018, 148, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, G.; Li, M.; Guo, P.; Xiao, Y.; Ji, H.; Hao, Y. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer 2019, 19, 984. [Google Scholar] [CrossRef]
- Everatt, R.; Intaite, B. Trends in Mortality Rates of Corpus Uteri and Ovarian Cancer in Lithuania, 1987–2016. Medicina 2020, 56, 347. [Google Scholar] [CrossRef]
- Ministry of Health of Republic of Serbia. National Guide for Diagnosis and Treatment of Cervical Cancer; Ministry of Health of Republic of Serbia: Belgrade, Serbia, 2013.
- Ilic, M.; Ilic, I. Gynaecological Cancer Mortality in Serbia, 1991–2010: A Joinpoint Regression Analysis. Asian Pac. J. Cancer Prev. 2015, 16, 157–162. [Google Scholar] [CrossRef][Green Version]
- Institute for Public Health of Serbia Dr. Milan Jovanovic “Batut”. Malignant Tumors in Republic of Serbia 2018; Institute for Public Health of Serbia Dr. Milan Jovanovic “Batut”: Belgrade, Serbia, 2020. [Google Scholar]
- Sagy, M. Cancer Mortality for Selected Sites in 24 Countries (1950–1957); Tohoku University School of Public Health: Sendai, Japan, 1960. [Google Scholar]
- World Health Organization. International Classification of Diseases and Related Health Problems, 10th Revision. Available online: www.who.int/classifications/icd/en/ (accessed on 25 June 2021).
- National Programme. Official Gazette Number 54. Available online: http://www.slglasnik.com (accessed on 8 December 2021).
- The 2019 Serbian National Health Survey; Institute of Public Health of Serbia: Serbia, Belgrade, 2021.
- Mihajlović, J.; Pechlivanoglou, P.; Miladinov-Mikov, M.; Živković, S.S.; Postma, M.J. Cancer incidence and mortality in Serbia 1999–2009. BMC Cancer 2013, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K. Current Status of Gynecologic Cancer in Japan. J. Gynecol. Oncol. 2009, 20, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tang, H.; Chen, T. Epidemiology of gynecologic cancers in China. J. Gynecol. Oncol. 2018, 29, e7. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases. Kazakhstan. Summary Report. 2019. Available online: http://www.hpvcentre.net/statistics/reports/ (accessed on 17 June 2019).
- Aitken, C.A.; van Agt, H.M.; Siebers, A.S.; van Kemenade, F.J.; Niesters, H.G.M.; Melchers, W.J.G.; Vedder, J.E.; Schuurman, R.; van den Brule, A.J.; van der Linden, H.C.; et al. Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: A population-based cohort study. BMC Med. 2019, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Rancic, N.K.; Golubovic, M.; Ilic, M.; Ignjatovic, A.; Zivadinovic, R.; Djenic, S.; Momčilović, S.D.; Kocić, B.N.; Milošević, Z.G.; Otašević, S.A. Knowledge about Cervical Cancer and Awareness of Human Papillomavirus (HPV) and HPV Vaccine among Female Students from Serbia. Medicina 2020, 56, 406. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, V. The Impact of Knolwedge and Attitudes of Women toward Cervical Cancer and Pap Test on Their Participation in Screening. Ph.D. Thesis, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia, 2015. [Google Scholar]
- Kesic, V.; Markovic, M.; Matejic, B.; Topic, L. Awareness of cervical cancer screening among women in Serbia. Gynecol. Oncol. 2005, 99, S222–S225. [Google Scholar] [CrossRef]
- Ministry of Health of RS. The Rulebook on the Program of Mandatory and Recommended Immunization against Certain Infectious Diseases. Official Gazette of RS; Ministry of Health of RS: Belgrade, Serbia, 2020.
- Gazibara, T.; Filipović, A.; Kesić, V.; Kisić-Tepavčević, D.; Pekmezović, T. Risk factors for epithelial ovarian cancer in the female population of Belgrade, Serbia: A case-control study. Vojn. Pregl. 2013, 70, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ruterbusch, J.J.; Cote, M.L. Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1511–1527. [Google Scholar] [CrossRef]
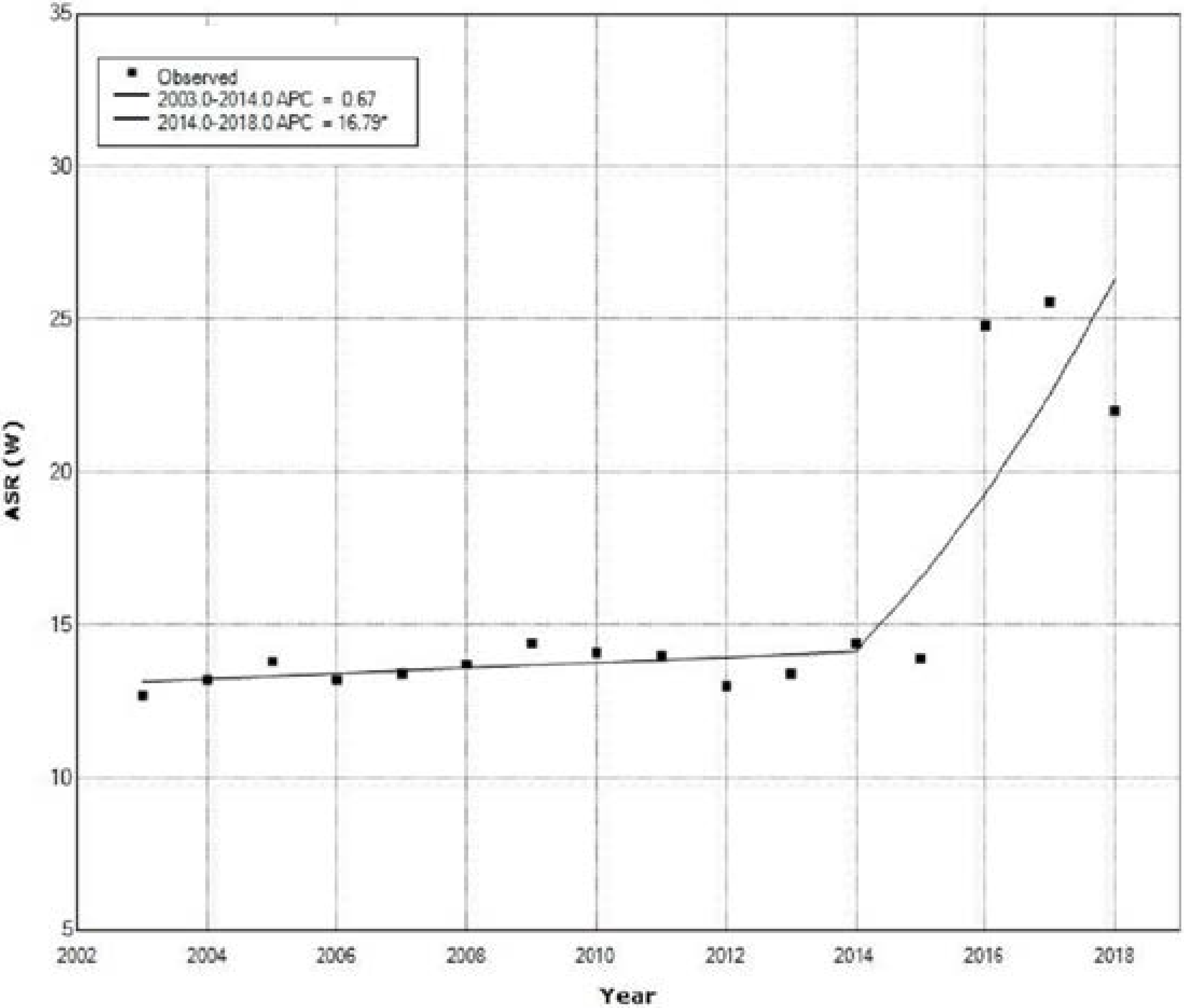
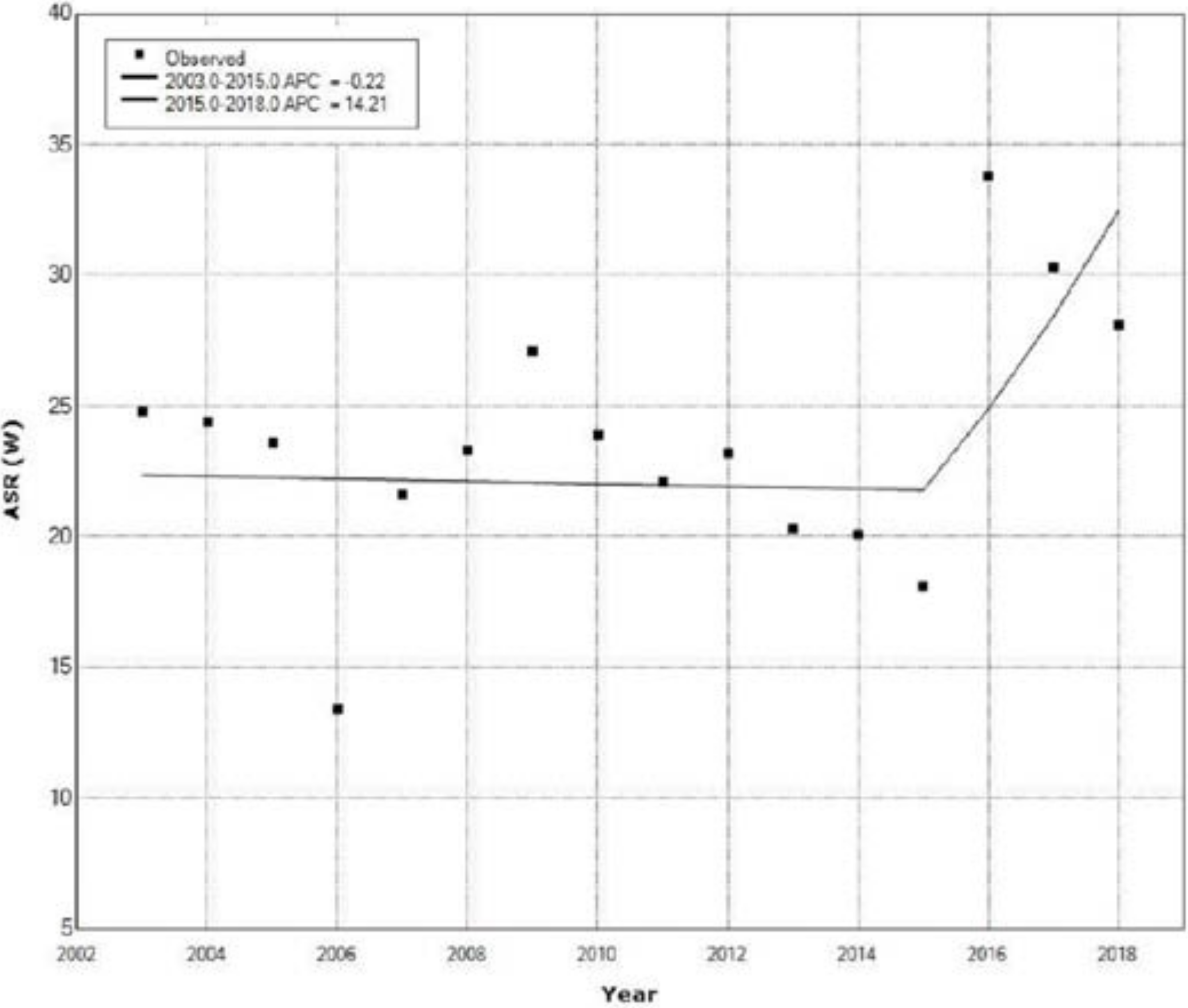
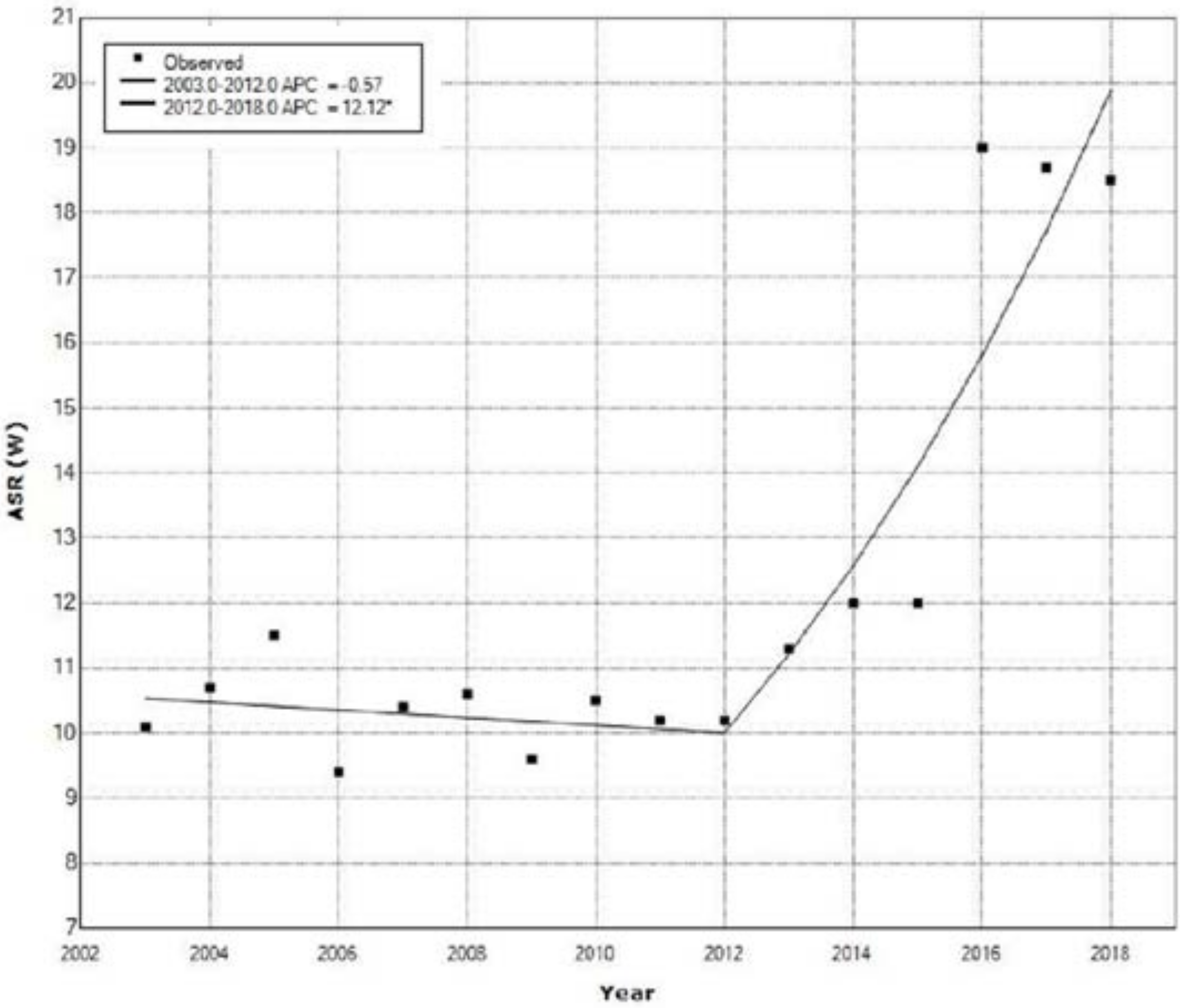
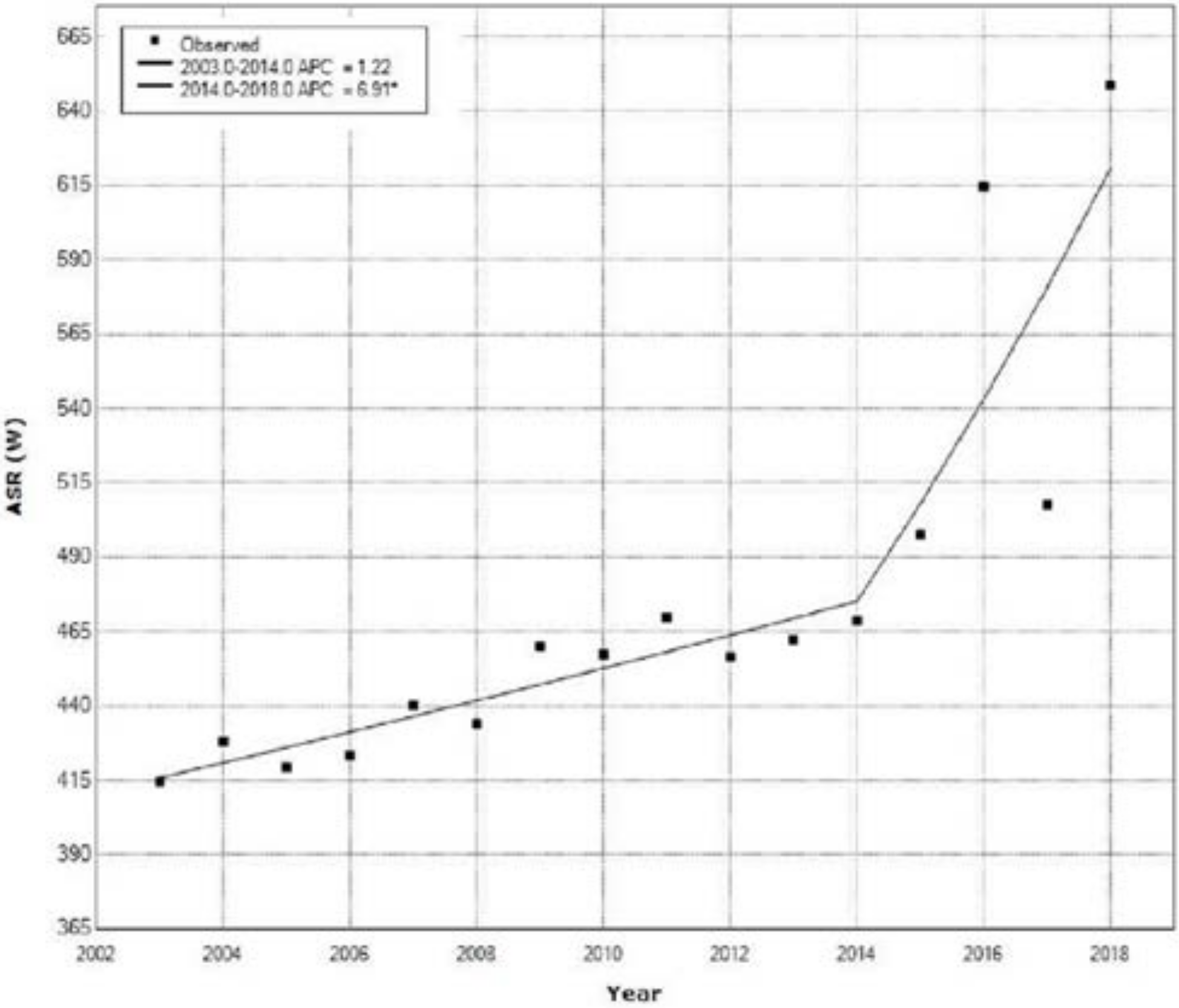
| Year | Ovarian Cancer | Cervical Cancer | Uterine Cancer | All Cancers | ||||
|---|---|---|---|---|---|---|---|---|
| Number of New Cases | ASR * (W) | Number of New Cases | ASR (W) | Number of New Cases | ASR (W) | Number of New Cases | ASR (W) | |
| 2003 | 459 | 10.1 | 1004 | 24.8 | 633 | 12.7 | 11,594 | 414.5 |
| 2004 | 472 | 10.7 | 967 | 24.4 | 630 | 13.2 | 11,954 | 428 |
| 2005 | 500 | 11.5 | 948 | 23.6 | 702 | 13.8 | 11,690 | 419.4 |
| 2006 | 428 | 9.4 | 1053 | 13.4 | 646 | 13.2 | 12,835 | 423.3 |
| 2007 | 484 | 10.4 | 889 | 21.6 | 659 | 13.4 | 12,187 | 440.2 |
| 2008 | 458 | 10.6 | 945 | 23.3 | 676 | 13.7 | 11,974 | 434 |
| 2009 | 430 | 9.6 | 1104 | 27.1 | 719 | 14.4 | 12,651 | 460.1 |
| 2010 | 464 | 10.5 | 993 | 23.9 | 715 | 14.1 | 12,531 | 457.3 |
| 2011 | 482 | 10.2 | 882 | 22.1 | 695 | 14 | 12,819 | 469.7 |
| 2012 | 453 | 10.2 | 986 | 23.2 | 722 | 13 | 12,358 | 456.6 |
| 2013 | 553 | 11.3 | 863 | 20.3 | 692 | 13.4 | 12,456 | 462.3 |
| 2014 | 517 | 12 | 836 | 20.1 | 748 | 14.4 | 12,571 | 468.5 |
| 2015 | 543 | 12 | 777 | 18.1 | 702 | 13.9 | 14,582 | 497.6 |
| 2016 | 721 | 19 | 1239 | 33.8 | 973 | 24.8 | 19,115 | 614.6 |
| 2017 | 715 | 18.7 | 1124 | 30.3 | 990 | 25.6 | 19,535 | 507.8 |
| 2018 | 690 | 18.5 | 1057 | 28.1 | 861 | 22 | 22,066 | 648.7 |
| Segment | Lower Endpoint | Upper Endpoint | APC | Lower CI | Upper CI | Test Statistic (t) | Prob > |t| |
|---|---|---|---|---|---|---|---|
| Uterine Cancer | |||||||
| Count | |||||||
| ASR W* | |||||||
| 1 | 2003 | 2014 | 0.7 | −1.8 | 3.2 | 0.6 | 0.564 |
| 2 | 2014 | 2018 | 16.8 * | 4.0 | 31.1 | 2.9 | 0.014 |
| Cervical Cancer | |||||||
| Count | |||||||
| ASR W | |||||||
| 1 | 2003 | 2015 | −0.2 | −3.4 | 3.1 | −0.1 | 0.887 |
| 2 | 2015 | 2018 | 14.2 | −13.3 | 50.5 | 1.1 | 0.311 |
| Ovarian Cancer | |||||||
| Count | |||||||
| ASR | |||||||
| 1 | 2003 | 2012 | −0.6 | −3.2 | 2.1 | −0.5 | 0.645 |
| 2 | 2012 | 2018 | 12.1 * | 6.7 | 17.8 | 5.1 | <0.001 |
| All Cancers | |||||||
| Count | |||||||
| ASR W | |||||||
| 1 | 2003 | 2014 | 1.2 | −0.0 | 2.5 | 2.1 | 0.055 |
| 2 | 2014 | 2018 | 6.9 * | 0.9 | 13.3 | 2.5 | 0.028 |
| Cancer Site | Age Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–19 | 20–39 | 40–59 | 60–79 | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| CC * | 4 | 0.03 | 2027 | 12.94 | 7749 | 49.46 | 5887 | 37.58 | 15,667 | 100 |
| UC § | 1 | 0.01 | 255 | 1.63 | 4200 | 26.81 | 7307 | 46.64 | 11,763 | 100 |
| OC ** | 50 | 0.32 | 705 | 4.50 | 3200 | 20.43 | 4414 | 28.17 | 8369 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, M.M.; Rancic, N.K.; Andjelković Apostolović, M.R.; Ignjatović, A.M.; Stojanovic, D.R.; Mitic Lakusic, V.R.; Ilic, M.V. Temporal Changes in Incidence Rates of the Most Common Gynecological Cancers in the Female Population in Central Serbia. Medicina 2022, 58, 306. https://doi.org/10.3390/medicina58020306
Stojanovic MM, Rancic NK, Andjelković Apostolović MR, Ignjatović AM, Stojanovic DR, Mitic Lakusic VR, Ilic MV. Temporal Changes in Incidence Rates of the Most Common Gynecological Cancers in the Female Population in Central Serbia. Medicina. 2022; 58(2):306. https://doi.org/10.3390/medicina58020306
Chicago/Turabian StyleStojanovic, Miodrag M., Natasa K. Rancic, Marija R. Andjelković Apostolović, Aleksandra M. Ignjatović, Dijana R. Stojanovic, Vesna R. Mitic Lakusic, and Mirko V. Ilic. 2022. "Temporal Changes in Incidence Rates of the Most Common Gynecological Cancers in the Female Population in Central Serbia" Medicina 58, no. 2: 306. https://doi.org/10.3390/medicina58020306
APA StyleStojanovic, M. M., Rancic, N. K., Andjelković Apostolović, M. R., Ignjatović, A. M., Stojanovic, D. R., Mitic Lakusic, V. R., & Ilic, M. V. (2022). Temporal Changes in Incidence Rates of the Most Common Gynecological Cancers in the Female Population in Central Serbia. Medicina, 58(2), 306. https://doi.org/10.3390/medicina58020306






