Chosen Antioxidant Enzymes GPx4 and GPx8 in Human Colorectal Carcinoma: Study of the Slovak Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Experimental Groups
2.2. Immunohistochemical Detection of GPx4 and GPx8
2.3. Immunohistochemical Evaluation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, J.; Wu, J.; Wang, F.; Zhang, P.; Zhang, X. Semaphoring 4D is required for the induction of antioxidant stress and anti-inflammatory effects of dihydromyricetin in colon cancer. Int. Immunopharmacol. 2019, 67, 220–230. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, X.; Zhang, C.; Zhu, H.; Xu, Q.; Bu, Y.; Lei, Y. Redox Imbalance in the Development of Colorectal Cancer. J. Cancer 2017, 8, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruchniak, M.P.; Aražna, M.; Demkow, U. Biochemistry of Oxidative stress. Adv. Exp. Med. Biol. 2015, 35, 1058–1071. [Google Scholar]
- Nagornaja, N.V.; Chetverik, N.A. Oksidativnij stress: Vliyanie na organism cheloveka, metody ocenky. Zdorovje Rebenka 2010, 2, 140–145. [Google Scholar]
- Yoboue, E.D.; Rimessi, A.; Anelli, T.; Pinton, P.; Sitia, R. Regulation of Calcium Fluxes by GPX8, a Type-II Transmembrane Peroxidase Enriched at the Mitochondria-Associated Endoplasmic Reticulum Membrane. Antioxid. Redox Signal. 2017, 279, 583–595. [Google Scholar] [CrossRef]
- Strzelczyk, J.K.; Wielkoszyński, T.; Krakowczyk, L.; Adamek, B.; Zalewska-Ziob, M.; Gawron, K.; Kasperczyk, J.; Wiczkowski, A. The activity of antioxidant enzymes in colorectal adenocarcinoma and corresponding normal mucosa. Acta Biochim. Pol. 2012, 59, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Ramaratnam, R.S.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione Peroxidase 4: A New Player in Neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Martinez, C.S.; Peçanha, F.M.; Brum, D.S.; Santos, F.W.; Franco, J.L.; Zemolin, A.P.P.; Anselmo-Franci, J.A.; Junior, F.B.; Alonso, M.J.; Salaices, M.; et al. Reproductive Dysfunction After Mercury Exposure at Low Levels: Evidence for a Role of Glutathione Peroxidase (GPx) 1 and GPx4 in Male Rats. Reprod. Fertil. Dev. 2017, 29, 1803–1812. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutatione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gowder, S.J.T. Glutathione Peroxidase: A Potential Marker for the Most Common Diseases and Disorders. Recent Pat. Biomark. 2014, 4, 43–52. [Google Scholar] [CrossRef]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal. 2018, 29, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Heirman, I.; Ginneberge, D.; Brigelius-Flohé, R.; Hendrickx, N.; Agostinis, P.; Brouckaert, P.; Rottiers, P.; Grooten, J. Blocking tumor cell eicosanoid synthesis by GP x 4 impedes tumor growth and malignancy. Free Radic. Biol. Med. 2006, 40, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in Ferroptosis and Its Pharmacological Implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Shah, R.; Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489–498. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.K.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Toppo, S.; Flohé, L.; Ursini, F.; Vanin, S.; Maiorino, M. Catalytic mechanisms and specificities of glutathione peroxidases: Variations of a basic scheme. Biochim. Biophys. Acta 2009, 1790, 1486–1500. [Google Scholar] [CrossRef]
- Bosello Travain, V.; Miotto, G.; Vučković, A.M.; Cozza, G.; Roveri, A.; Toppo, S.; Ursini, F.; Venerando, R.; Zaccarin, M.; Maiorino, M. Lack of glutathione peroxidase-8 in the ER impacts on lipid composition of HeLa cells microsomal membranes. Free Radic. Biol. Med. 2020, 47, 80–89. [Google Scholar] [CrossRef]
- Bosello-Travain, V.; Forman, H.J.; Roveri, A.; Toppo, S.; Ursini, F.; Venerando, R.; Warnecke, C.; Zaccarin, M.; Maiorino, M. Glutathione peroxidase 8 is transcriptionally regulated by HIFα and modulates growth factor signaling in HeLa cells. Free Radic. Biol. Med. 2015, 81, 58–68. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Mohamed, A.A.; Ali, H. Pulmonary apoptotic and oxidative damaging effects of Triclosan alone or in combination with Fluoride in Sprague Dawley rats. Acta Histochem. 2017, 119, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [Green Version]
- Pastornická, A.; Rybárová, S.; Drahošová, S.; Mihalik, J.; Kreheľová, A.; Pavliuk-Karachevtseva, A.; Hodorová, I. Influence of Paclitaxel and Doxorubicin Therapy of ßIII-Tubulin, Carbonic Anhydrase IX, and Survivin in Chemically Induced Breast Cancer in Female Rat. Int. J. Mol. Sci. 2021, 22, 6363. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Faivre, S.; Raymond, E. The role of integrins in colorectal cancer. Oncology 2007, 21, 21–24. [Google Scholar] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopčević, K.R.; Rovčanin, B.R.; Tatič, S.B.; Krivokapić, Z.V.; Gajić, M.M.; Dragutinović, V.V. Activity of Superoxide Dismutase, Catalase, Glutathione Peroxidase, and Glutathione Reductase in Different Stages of Colorectal Carcinoma. Dig. Dis. Sci. 2013, 58, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, E.H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, I.; Kang, Y.N.; Choi, I.J.; Kim, D.K. Genetic Characteristics of Mitochondrial DNA Was Associated with Colorectal Carcinogenesis and Its Prognosis. PLoS ONE 2015, 10, e0118612. [Google Scholar] [CrossRef]
- Matosevic, P.; Klepac-Pulanic, T.; Kinda, E.; Augustin, G.; Brcic, I.; Jakic-Razumovic, J. Immunohistochemical expression of 8-oxo-7,8-dihydro-2′-deoxyguanosine in cytoplasm of tumour and adjacent normal mucosa cells in patients with colorectal cancer. World J. Surg. Oncol. 2015, 13, 241. [Google Scholar] [CrossRef] [Green Version]
- Skrzydlewska, E.; Sulkowski, S.; Koda, M.; Zalewski, B.; Kanczuga-Koda, L.; Sulkowska, M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403–406. [Google Scholar] [CrossRef]
- Janion, K.; Szczepańska, E.; Nowakowska-Zajdel, E.; Walkiewicz, K.; Strzelczyk, J. Lipid peroxidation and total oxidant/antioxidant status in colorectal cancer patients. J. Biol. Regul. Homeost. Agents 2020, 34, 239–244. [Google Scholar] [PubMed]
- Kekec, Y.; Paydas, S.; Tuli, A.; Zorludemir, S.; Sakman, G.; Seydaoglu, G. Antioxidant enzyme levels in cases with gastrointesinal cancer. Eur. J. Intern. Med. 2009, 20, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Yagublu, V.; Arthur, J.R.; Babayeva, S.N.; Nicol, F.; Post, S.; Keese, M. Expression of Selenium-containing Proteins in Human Colon Carcinoma Tissue. Anticancer Res. 2011, 31, 2693–2698. [Google Scholar] [PubMed]
- Cole-Ezea, P.; Swan, D.; Shanley, D.; Hesketh, J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free Radic. Biol. Med. 2012, 53, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C.; Hughes, D.J.; Pardini, B.; Naccarati, A.; Soucek, P.; Vodickova, L.; Hlavatá, I.; Vrána, D.; Vodicka, P.; Hesketh, J.E. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis 2010, 31, 1074–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermano, G.; Pagmantidis, V.; Holloway, N.; Kadri, S.; Mowat, N.A.G.; Shiel, R.S.; Arthur, J.R.; Mathers, J.C.; Daly, A.K.; Broom, J.; et al. Evidence that a polymorphism within the 3’UTR of glutathione peroxidase 4 is functional and is associated with susceptibility to colorectal cancer. Genes Nutr. 2007, 2, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Blarigan, E.L.; Ma, J.; Kenfield, S.A.; Stampfer, M.J.; Sesso, H.D.; Giovannucci, E.L.; Witte, J.S.; Erdman, J.W., Jr.; Chan, J.M.; Penney, K.L. Plasma antioxidants, genetic variation in SOD2, CAT, GPX1, GPX4, and prostate cancer survival. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Du, J.; Zhang, Y.; Sun, W.; Smith, B.J.; Oberley, L.W.; Cullen, J.J. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum. Gene Ther. 2006, 17, 105–116. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, D.; Li, Y.; Wang, H.; Cheng, C.; Yao, Y.; Jia, J. Glutathione Peroxidase 8 as a Prognostic Biomarker of Gastric Cancer: An Analysis of The Cancer Genome Atlas (TCGA) Data. Med. Sci. Monit. 2020, 26, 921775. [Google Scholar] [CrossRef]
- Khatib, A.; Balakrishnan, S.; Ben-Yosef, M.; Oren, G.; Rmaileh, A.A.; Schlesinger, M.; Axelrod, J.H.; Lichtenstein, M.; Shaul, Y.D. Glutathione Peroxidase 8 (GPX8)-IL6 axis is essential in maintaining breast cancer mesenchymal stem-like state and aggressive phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 21420–21431. [Google Scholar] [CrossRef]
- Ovchinnikov, D.A. Macrophages in the embryo and beyond: Much more than just giant phagocytes. Genesis 2008, 46, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Isidro, R.A.; Appleyard, C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Physiol. Gastrointest. Liver Physiol. 2016, 311, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Akaike, T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry 1998, 63, 854–865. [Google Scholar]
- Hsu, J.L.; Chou, J.W.; Chen, T.F.; Hsu, J.T.; Su, F.Y.; Lan, J.L.; Wu, P.C.; Hu, C.M.; Lee, E.Y.; Lee, W.H. Glutathione peroxidase 8 negatively regulates caspase-4/11 to protect against colitis. EMBO Mol. Med. 2020, 12, 9386. [Google Scholar] [CrossRef]
- Mihalik, J.; Krehel’ová, A.; Kovaříková, V.; Solár, P.; Domoráková, I.; Pavliuk-Karachevtseva, A.; Hladová, A.; Rybárová, S.; Hodorová, I. GPx8 Expression in Rat Oocytes, Embryos, and Female Genital Organs During Preimplantation Period of Pregnancy. Int. J. Mol. Sci. 2020, 21, 6313. [Google Scholar] [CrossRef]
- Santos de Oliveira, P.V.; Garcia-Rosab, S.; Azevedo Sachettod, A.T.; Soares Morettia, A.I.; Debbasa, V.; Coralie De Bessaa, T.; Tenguan Silvaa, N.; Da Costa Pereirae, A.; Martins-de-Souzab, D.; Larami Santorod, M.; et al. Protein disulfide isomerase plasma levels in healthy humans reveal proteomic signatures involved in contrasting endothelial phenotypes. Redox Biol. 2019, 22, 101142. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Saaranen, M.J.; Karala, A.R.; Lappi, A.K.; Wang, L.; Raykhel, I.B.; Alanen, H.I.; Salo, K.E.H.; Wang, C.; Ruddock, L.W. Two Endoplasmic Reticulum PDI Peroxidases Increase the Efficiency of the Use of Peroxide during Disulfide Bond Formation. J. Mol. Biol. 2011, 406, 503–515. [Google Scholar] [CrossRef]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef]

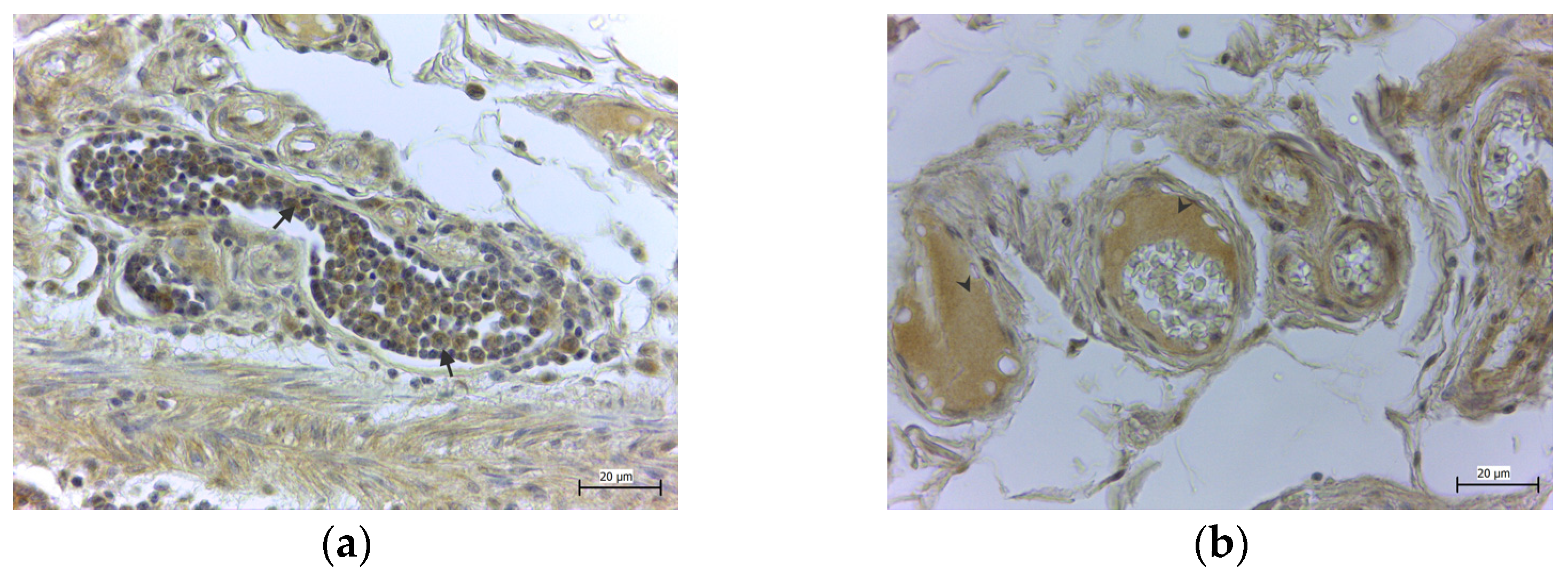

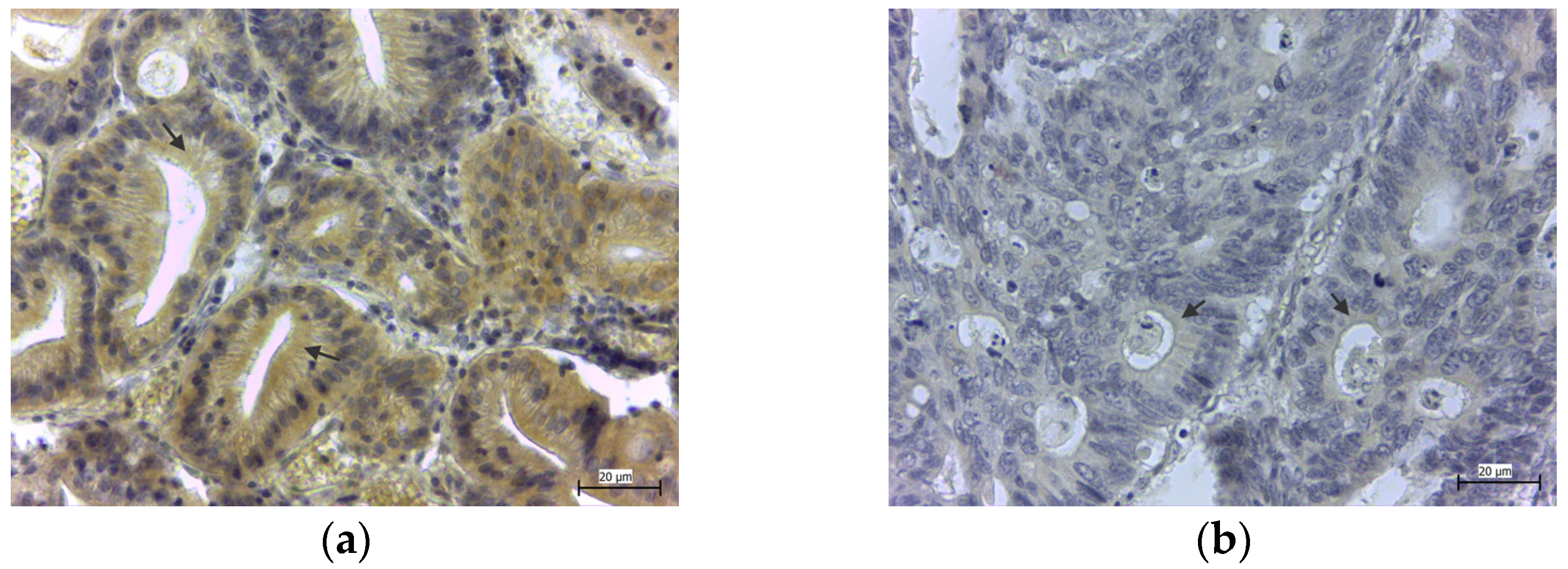
| Characteristics | No. |
|---|---|
| All patients | 58 |
| Male | 35 |
| Female | 23 |
| Age ≤60 years | 10 |
| 60–70 years | 25 |
| ≥70 years | 23 |
| Tumor grade I | 37 |
| II | 8 |
| III | 2 |
| Unknown | 11 |
| KRAS + | 8 |
| − | 4 |
| Analysis has not been completed | 46 |
| Mucinous adenocarcinoma | 7 |
| Adenocarcinoma | 51 |
| Invasion into lymph nodes | |
| Diagnosed | 18 |
| Not found or absent | 44 |
| Quantity of Expression | GPx4 | % | GPx8 | % |
|---|---|---|---|---|
| +++ | 4 | 6.89 | 4 | 6.89 |
| ++ | 20 | 34.48 | 13 | 22.41 |
| + | 15 | 25.86 | 15 | 25.86 |
| − | 19 | 32.77 | 26 | 44.84 |
| Number of positive samples | 24 | 41.4 | 17 | 29.3 |
| Number of negative samples | 34 | 58.6 | 41 | 70.7 |
| Tumor Grade I | Tumor Grade II | Tumor Grade III | Unknown | Fisher’s Test | |
|---|---|---|---|---|---|
| GPx4 + | 14 | 2 | 1 | 7 | p > 0.05 (p = 0.22) |
| GPx4 − | 21 | 8 | 1 | 4 | |
| GPx8 + | 16 | 3 | 1 | 5 | p > 0.05 (p = 1.00) |
| GPx8 − | 21 | 4 | 2 | 6 |
| Adenocarcinoma | Mucinous Adenocarcinoma | Fisher’s Test | |
|---|---|---|---|
| GPx4 + | 19 | 5 | p > 0.05 (p = 0.11) |
| GPx4 − | 32 | 2 | |
| GPx8 + | 16 | 1 | p > 0.05 (p = 0.66) |
| GPx8 − | 35 | 6 |
| Males | Females | Fisher’s Test | |
|---|---|---|---|
| GPx4 + | 14 | 10 | p > 0.05 (p = 0.78) |
| GPx4 − | 22 | 12 | |
| GPx8 + | 11 | 6 | p > 0.05 (p = 1.00) |
| GPx8 − | 26 | 15 |
| <60 Years | 60–70 Years | >70 Years | Fisher’s Test | |
|---|---|---|---|---|
| GPx4 + | 5 | 10 | 9 | p > 0.05 (p = 0.88) |
| GPx4 − | 5 | 15 | 14 | |
| GPx8 + | 2 | 8 | 7 | p > 0.05 (p = 0.86) |
| GPx8 − | 8 | 17 | 16 |
| Invasion into Lymph Nodes + | Invasion into Lymph Nodes − | Fisher’s Test | |
|---|---|---|---|
| GPx4 + | 5 | 19 | p > 0.05 (p = 0.25) |
| GPx4 − | 13 | 21 | |
| GPx8 + | 8 | 9 | p > 0.05 (p = 0.12) |
| GPx8 − | 10 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavliuk-Karachevtseva, A.; Mihalik, J.; Biel, R.; Rybárová, S.; Hodorová, I. Chosen Antioxidant Enzymes GPx4 and GPx8 in Human Colorectal Carcinoma: Study of the Slovak Population. Medicina 2022, 58, 298. https://doi.org/10.3390/medicina58020298
Pavliuk-Karachevtseva A, Mihalik J, Biel R, Rybárová S, Hodorová I. Chosen Antioxidant Enzymes GPx4 and GPx8 in Human Colorectal Carcinoma: Study of the Slovak Population. Medicina. 2022; 58(2):298. https://doi.org/10.3390/medicina58020298
Chicago/Turabian StylePavliuk-Karachevtseva, Andriana, Jozef Mihalik, Róbert Biel, Silvia Rybárová, and Ingrid Hodorová. 2022. "Chosen Antioxidant Enzymes GPx4 and GPx8 in Human Colorectal Carcinoma: Study of the Slovak Population" Medicina 58, no. 2: 298. https://doi.org/10.3390/medicina58020298
APA StylePavliuk-Karachevtseva, A., Mihalik, J., Biel, R., Rybárová, S., & Hodorová, I. (2022). Chosen Antioxidant Enzymes GPx4 and GPx8 in Human Colorectal Carcinoma: Study of the Slovak Population. Medicina, 58(2), 298. https://doi.org/10.3390/medicina58020298







