Which Clusters of Metabolic Syndrome Are the Most Associated with Serum Uric Acid?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
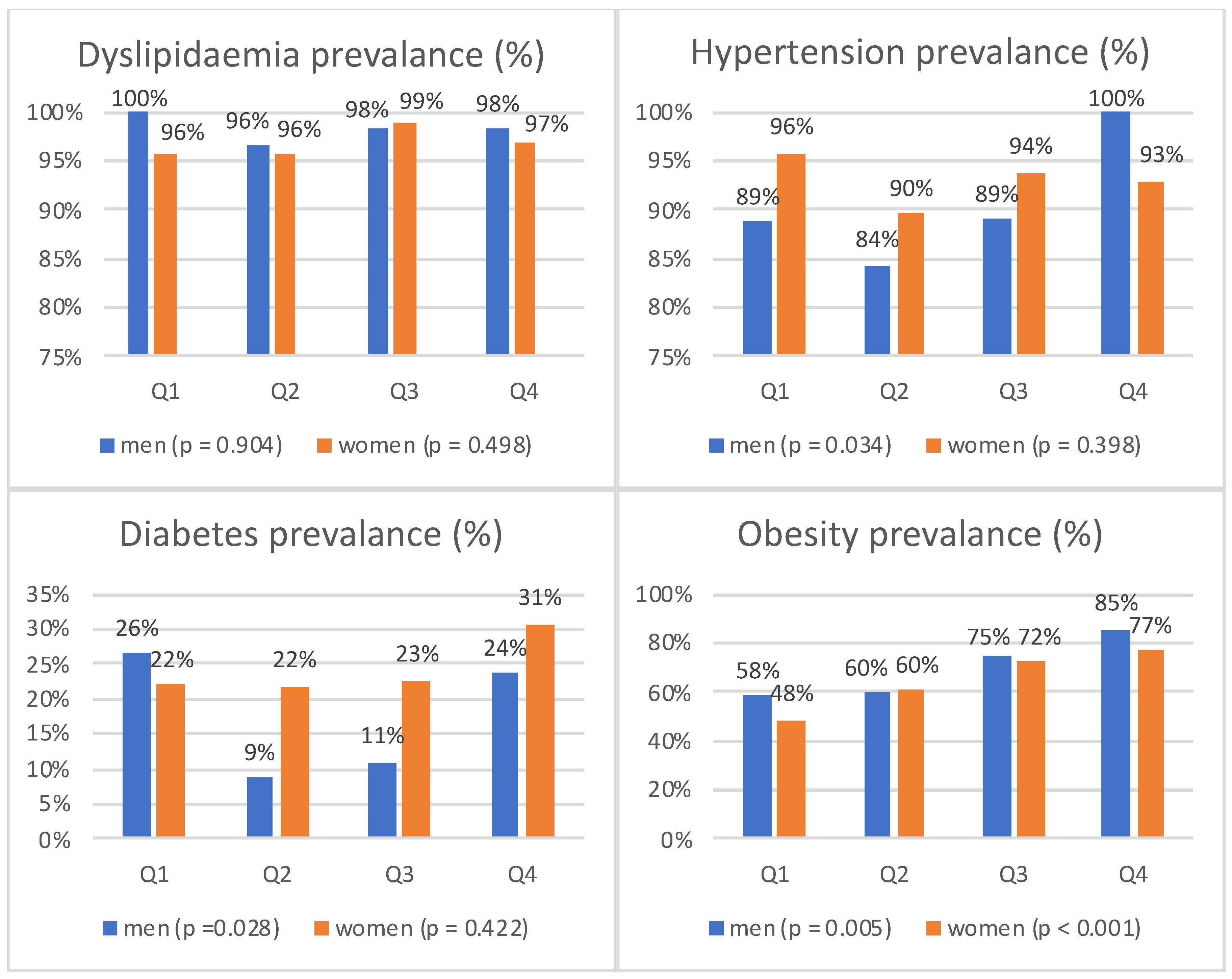
3.2. Prevalence of Major Cardiovascular Disease Risk Factors in the Study Population
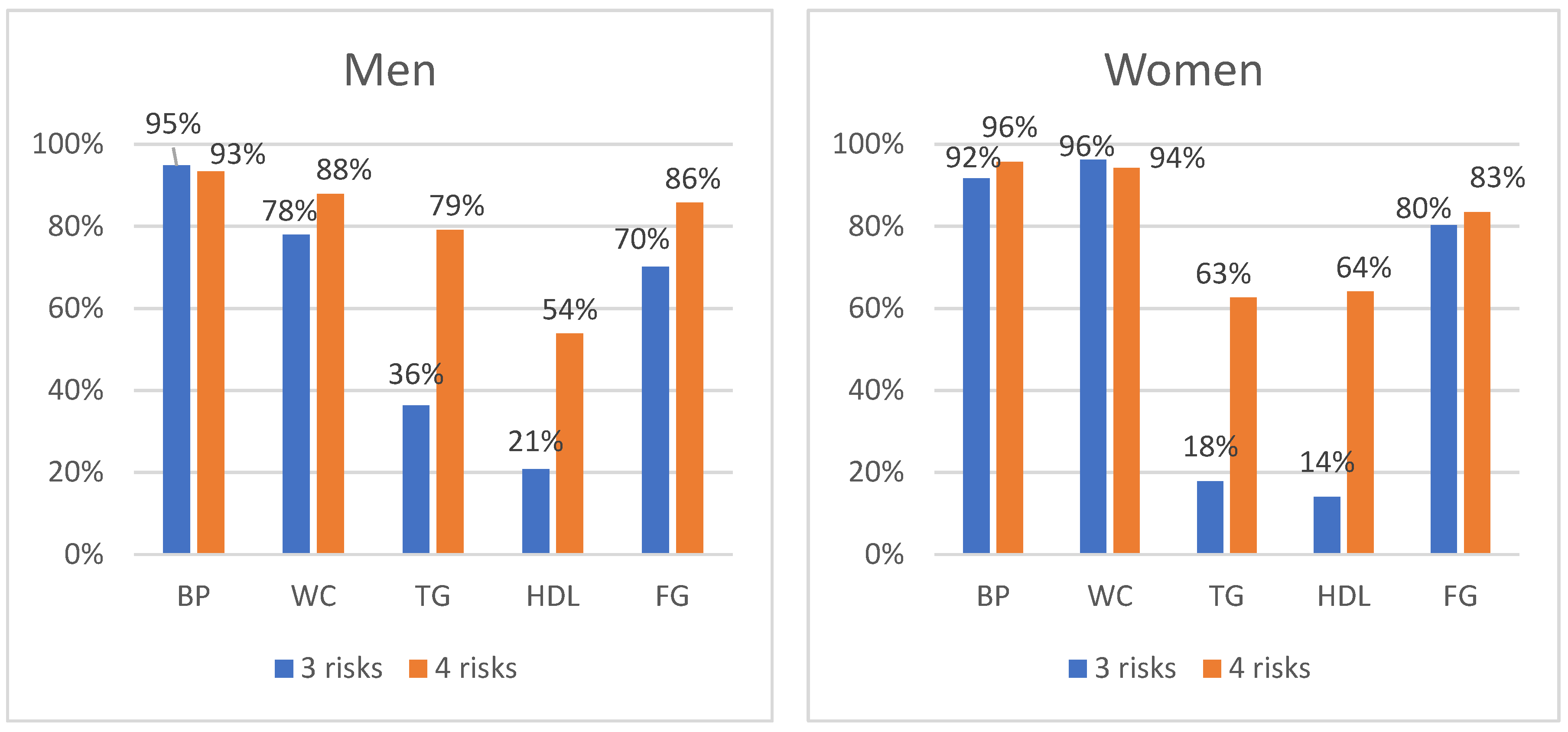
3.3. Distribution of Individual MetS Components
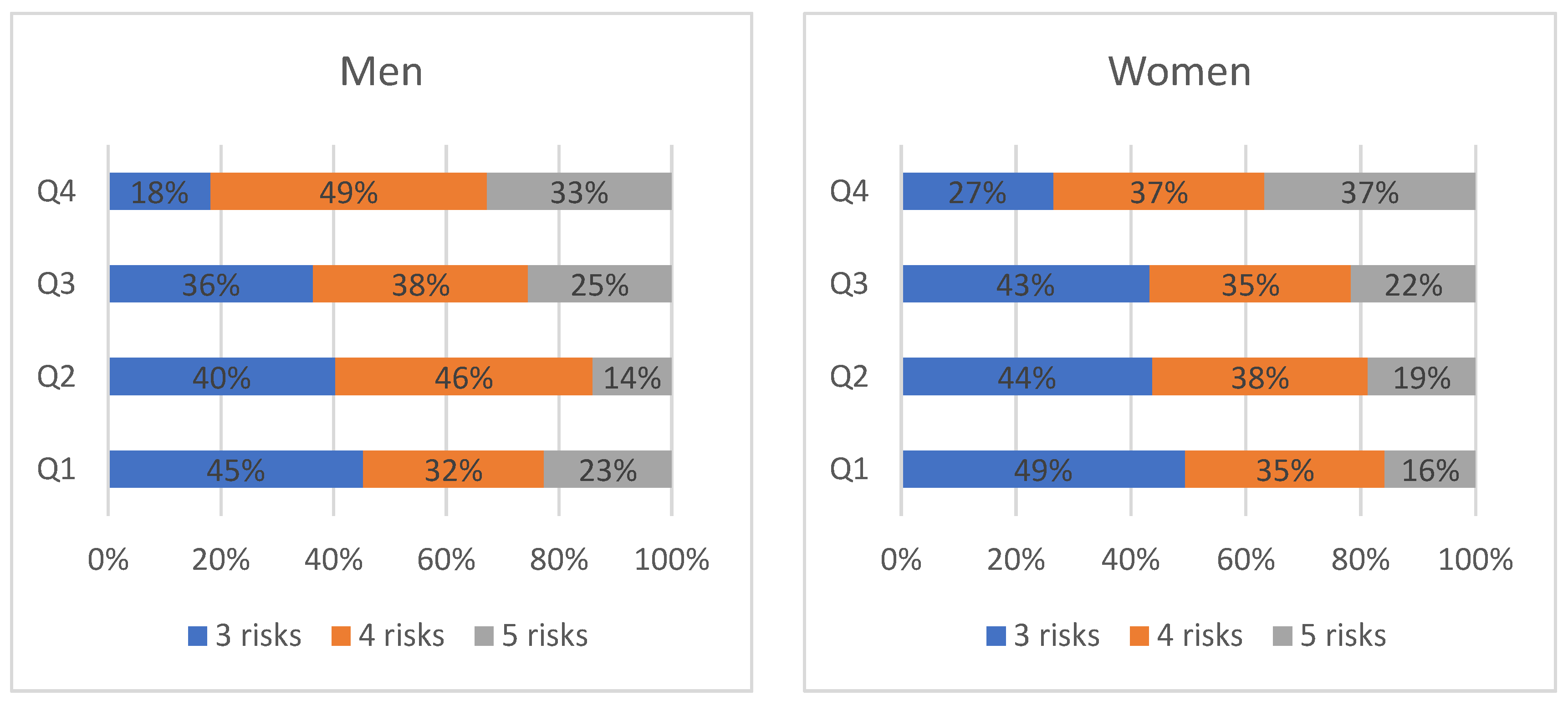
3.4. Prevalence of Clusters in MetS Components
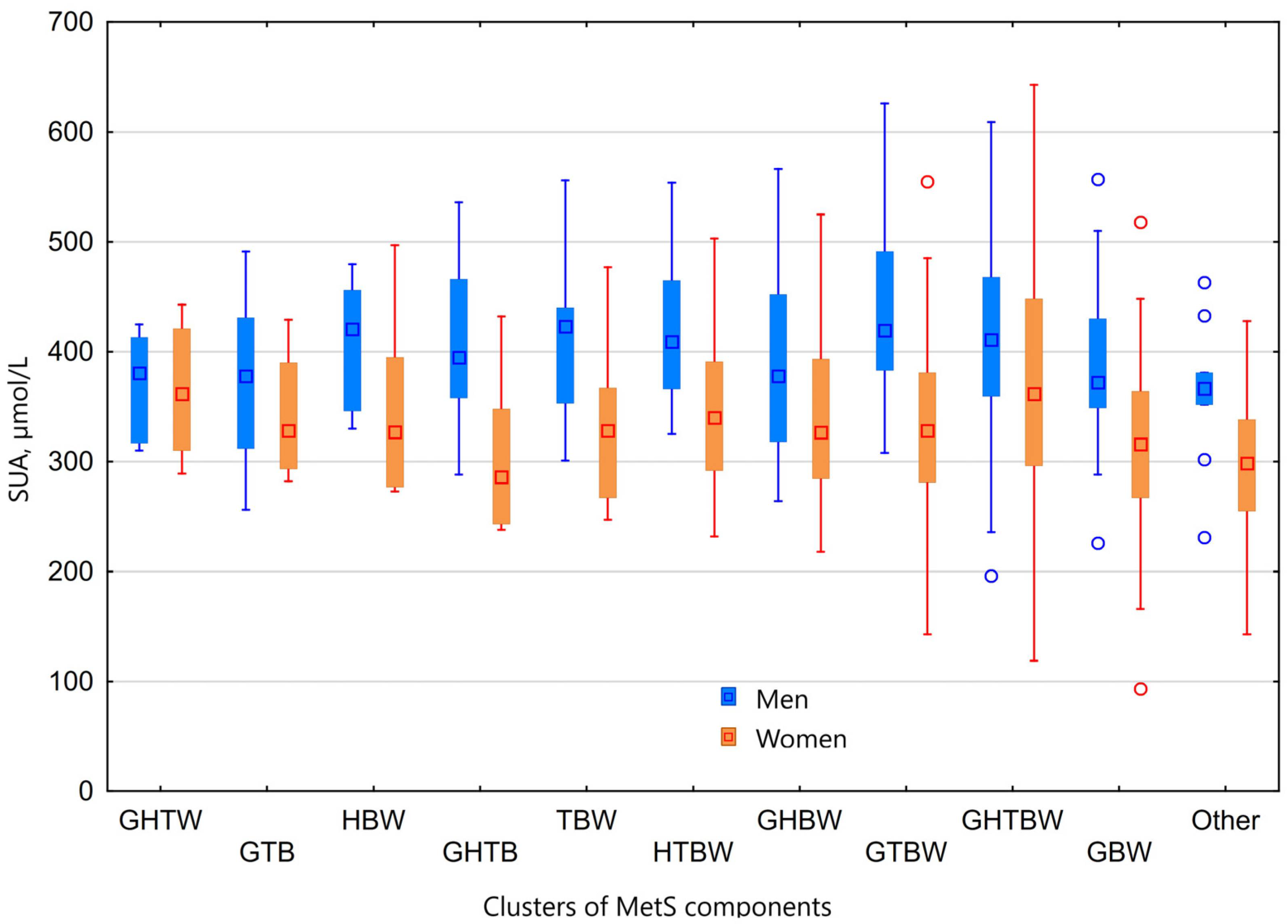
3.5. Hyperuricaemia and Clusters of MetS Components
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, A.; Igual, I.; Bedini, J.; Quint, L.; Conget, I. Uric acid concentration in subjects at risk of type 2 diabetes mellitus: Relationship to components of the metabolic syndrome. Metabolism 2002, 51, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Uyarel, H.; Hergenç, G.; Karabulut, A.; Albayrak, S.; Sarı, I.; Yazici, M.; Keleş, I.; Yazıcı, M. Serum Uric Acid Is a Determinant of Metabolic Syndrome in a Population-Based Study. Am. J. Hypertens. 2006, 19, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Meng, X.-F.; He, F.-F.; Chen, S.; Su, H.; Xiong, J.; Gao, P.; Tian, X.-J.; Liu, J.-S.; Zhu, Z.-H.; et al. High Serum Uric Acid and Increased Risk of Type 2 Diabetes: A Systemic Review and Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2013, 8, e56864. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Ford, E.S. Prevalence of the Metabolic Syndrome in Individuals with Hyperuricemia. Am. J. Med. 2007, 120, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; Rosticci, M.; D’Addato, S.; Borghi, C. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci. Rep. 2018, 8, 11529. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Ryoo, J.-H.; Choi, J.-M.; Park, S.K. Serum Uric Acid Level and the Incidence of Metabolic Syndrome in Middle-aged Korean Men: A 5-Year Follow-up Study. J. Prev. Med. Public Health 2014, 47, 317–326. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Chen, S.; Shao, X.; Guo, J.; Liu, X.; Liu, A.; Zhang, Y.; Wang, H.; Li, B.; Deng, K.; et al. Association of Uric Acid with Metabolic Syndrome in Men, Premenopausal Women and Postmenopausal Women. Int. J. Environ. Res. Public Health 2014, 11, 2899–2910. [Google Scholar] [CrossRef]
- Nagahama, K.; Inoue, T.; Kohagura, K.; Ishihara, A.; Kinjo, K.; Ohya, Y. Hyperuricemia predicts future metabolic syndrome: A 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens. Res. 2013, 37, 232–238. [Google Scholar] [CrossRef]
- Wang, H.-J.; Shi, L.-Z.; Liu, C.-F.; Liu, S.-M.; Shi, S.-T. Association between uric acid and metabolic syndrome in elderly women. Open Med. 2018, 13, 172–177. [Google Scholar] [CrossRef]
- Wang, T.T.; Lin, B.; Cui, W.X.; Zhang, M.Z.; Zhang, Y.H.; Zhang, S.Y. Clustering of Cardiovascular Risk Factors and Diabetes: A Prospective Cohort Study on the Inner Mongolian Population in China. Biomed. Environ. Sci. 2018, 31, 749–756. [Google Scholar]
- Filho, Z.A.d.S.; Ferreira, A.A.; dos Santos, J.; Meira, K.; Pierin, A. Cardiovascular risk factors with an emphasis on hypertension in the Mura Indians from Amazonia. BMC Public Health 2018, 18, 1251. [Google Scholar]
- van Sloten, T.T.; Tafflet, M.; Périer, M.-C.; Dugravot, A.; Climie, R.E.D.; Singh-Manoux, A.; Empana, J.-P. Association of Change in Cardiovascular Risk Factors with Incident Cardiovascular Events. JAMA 2018, 320, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Ardern, C.I. Age and Sex Differences in the Clustering of Metabolic Syndrome Factors. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.; Rodríguez-Mañas, L.; Raso, F.U.M.; Muiesan, M.L.; Ryliškytė, L.; Rietzschel, E.; et al. Metabolic syndrome across Europe: Different clusters of risk factors. Eur. J. Prev. Cardiol. 2014, 22, 486–491. [Google Scholar] [CrossRef]
- Guize, L.; Thomas, F.; Pannier, B.; Bean, K.; Jego, B.; Benetos, A. All-Cause Mortality Associated with Specific Combinations of the Metabolic Syndrome According to Recent Definitions. Diabetes Care 2007, 30, 2381–2387. [Google Scholar] [CrossRef]
- Scuteri, A.; Najjar, S.S.; Orru, M.; Usala, G.; Piras, M.G.; Ferrucci, L.; Cao, A.; Schlessinger, D.; Uda, M.; Lakatta, E. The central arterial burden of the metabolic syndrome is similar in men and women: The SardiNIA Study. Eur. Heart J. 2009, 31, 602–613. [Google Scholar] [CrossRef]
- Franco, O.H.; Massaro, J.M.; Civil, J.; Cobain, M.R.; O’Malley, B.; D’Agostino, R.B. Trajectories of entering the metabolic syndrome: The framingham heart study. Circulation 2009, 120, 1943–1950. [Google Scholar] [CrossRef]
- Laucevičius, A.; Kasiulevičius, V.; Jatužis, D.; Žaneta, P.; Ryliškytė, L.; Rinkūnienė, E.; Badarienė, J.; Ypienė, A.; Gustienė, O.; Šlapikas, R. Lithuanian High Cardiovascular Risk (LitHiR) primary prevention programme-rationale and design. Semin. Cardiovasc. Med. 2012, 18, 1–6. [Google Scholar] [CrossRef]
- ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Kumar, A.U.A.; Browne, L.D.; Li, X.; Adeeb, F.; Perez-Ruiz, F.; Fraser, A.D.; Stack, A.G. Temporal trends in hyperu- ricaemia in the Irish health system from 2006-2014: A cohort study. PLoS ONE 2018, 13, e0198197. [Google Scholar] [CrossRef]
- Roman, Y.M. The Daniel K. Inouye College of Pharmacy Scripts: Perspectives on the Epidemiology of Gout and Hyperuricemia. Hawaii J. Med. Public Health 2019, 78, 71–76. [Google Scholar]
- You, L.; Liu, A.; Wuyun, G.; Wu, H.; Wang, P. Prevalence of Hyperuricemia and the Relationship between Serum Uric Acid and Metabolic Syndrome in the Asian Mongolian Area. J. Atheroscler. Thromb. 2014, 21, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, E.; Bennett, M.; Chen, L. Aging, not menopause, is associated with higher prevalence of hyperuricemia among older women. Menopause 2014, 21, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Suh, Y.J. Association between Serum Uric Acid and Metabolic Syndrome in Koreans. J. Korean Med. Sci. 2019, 34, e307. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://osp.stat.gov.lt/lietuvos-gyventoju-sveikata-2020/apie-leidini (accessed on 16 January 2022).
- Da Silva, H.A.; Carraro, J.C.C.; Bressan, J.; Hermsdorff, H.H.M. Relation between uric acid and metabolic syndrome in subjects with cardiometabolic risk. Einstein São Paulo 2015, 13, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mukhopadhyay, P.; Pandit, K.; Chatterjee, P.; Majhi, B.; Chowdhury, S. Uric acid and its correlation with various metabolic parameters: A population-based study. Indian J. Endocrinol. Metab. 2019, 23, 134–139. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Moreto, F.; Silveira, L.V.d.A.; Burini, R.C. Dietary, anthropometric, and biochemical determinants of uric acid in free-living adults. Nutr. J. 2013, 12, 11. [Google Scholar] [CrossRef]
- Tao, M.; Pi, X.; Ma, X.; Shi, Y.; Zhang, Y.; Gu, H.; Chi, Y.; Zhuang, S.; Liu, N. Relationship between serum uric acid and clustering of cardiovascular disease risk factors and renal disorders among Shanghai population: A multicentre and cross-sectional study. BMJ Open 2019, 9, e025453. [Google Scholar] [CrossRef]
- Nan, H.; Qiao, Q.; Söderberg, S.; Pitkäniemi, J.; Zimmet, P.; Shaw, J.; Alberti, G.; Uusitalo, U.; Pauvaday, V.; Chitson, P.; et al. Serum uric acid and incident diabetes in Mauritian Indian and Creole populations. Diabetes Res. Clin. Pract. 2008, 80, 321–327. [Google Scholar] [CrossRef]
- Lin, K.-C.; Tsai, S.-T.; Lin, H.-Y.; Chou, P. Different progressions of hyperglycemia and diabetes among hyperuricemic men and women in the kinmen study. J. Rheumatol. 2004, 31, 1159–1165. [Google Scholar] [PubMed]
- Higa, S.; Yoshida, M.; Shima, D.; Ii, Y.; Kitazaki, S.; Yamamoto, Y.; Fujimoto, Y. A Retrospective, Cross-Sectional Study on the Prevalence of Hyperuricemia Using a Japanese Healthcare Database. Arch. Rheumatol. 2020, 35, 41–51. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Jungner, I.; Robinson, D.; Kolar, W.; Pearl, A.; Hale, A. Serum Urate, Serum Glucose and Diabetes. Ann. Clin. Biochem. Int. J. Lab. Med. 1992, 29, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.C.; Miname, M.H.; Santos, R.D. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis 2009, 202, 11–17. [Google Scholar] [CrossRef]
- Reunanen, A.; Takkunen, H.; Knekt, P.; Aromaa, A. Hyperuricemia as a Risk Factor for Cardiovascular Mortality. Acta Medica Scand. 2009, 212, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 2016, 6, srep19520. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Lonardo, A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016, 46, 1074–1087. [Google Scholar] [CrossRef]
| Women (n = 386) | Men (n = 220) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 <283 μmol/L | Q2 283–329 μmol/L | Q3 329–385 μmol/L | Q4 ≥385 μmol/L | p | Q1 <353 μmol/L | Q2 353–398 μmol/L | Q3 398–460 μmol/L | Q4 ≥460 μmol/L | p | |
| n | 95 | 96 | 97 | 98 | 53 | 57 | 55 | 55 | ||
| SUA, μmol/L | 242.35 ± 36.05 | 304.79 ± 14.85 | 354.67 ± 16.21 | 443.41 ± 55.96 | <0.001 | 312.06 ± 34.50 | 374.70 ± 12.60 | 423.98 ± 17.28 | 511.45 ± 42.73 | <0.001 |
| Age, years | 57.89 ± 3.95 | 57.13 ± 4.47 | 57.90 ± 4.27 | 57.66 ± 4.01 | 0.577 | 48.36 ± 3.67 | 47.84 ± 4.20 | 46.22 ± 4.39 | 45.62 ± 3.75 | 0.001 |
| Weight, kg | 80.62 ± 11.84 | 85.55 ± 13.63 | 89.04 ± 14.14 | 92.31 ±14.20 | <0.001 | 98.09 ±10.21 | 101.37 ± 13.32 | 103.36 ± 10.34 | 109.80 ± 12.19 | <0.001 |
| BMI, kg/m2 | 30.28 ± 4.47 | 31.51 ± 4.34 | 33.22 ± 4.94 | 34.06 ± 4.82 | <0.001 | 30.86 ± 3.00 | 31.08 ± 3.31 | 32.03 ± 3.25 | 33.60 ± 3.89 | <0.001 |
| WC, cm | 97.37 ± 9.05 | 101.04 ± 10.33 | 103.36 ± 11.73 | 104.22 ± 9.66 | <0.001 | 105.49 ± 6.30 | 106.82 ± 7.81 | 109.02 ± 7.50 | 110.94 ± 8.14 | <0.001 |
| TC, mmol/L | 6.09 ± 1.46 | 6.03 ± 1.25 | 6.15 ± 1.25 | 6.12 ± 1.41 | 0.885 | 5.92 ± 2.19 | 6.08 ± 1.38 | 5.89 ± 1.16 | 5.99 ± 1.41 | 0.409 |
| LDL-C, mmol/L | 3.83 ± 1.24 | 3.87 ± 1.07 | 3.96 ± 1.02 | 3.75 ± 1.14 | 0.4 | 3.48 ± 1.16 | 3.71 ± 1.01 | 3.60 ± 1.02 | 3.70 ± 1.25 | 0.825 |
| HDL-C, mmol/L | 1.41 ± 0.34 | 1.34 ± 0.27 | 1.32 ± 0.25 | 1.25 ± 0.29 | 0.004 | 1.01 ± 0.22 | 1.11 ± 0.25 | 1.04 ± 0.19 | 1.03 ± 0.23 | 0.211 |
| TG, mmol/L | 1.88 ± 1.21 | 1.92 ± 1.87 | 1.89 ± 0.89 | 2.42 ± 1.49 | 0.002 | 3.99 ± 9.17 | 2.63 ± 1.73 | 2.76 ± 2.10 | 2.86 ± 1.43 | 0.017 |
| Plasma glucose, mmol/L | 6.66 ± 2.00 | 6.65 ± 2.18 | 6.58 ± 1.47 | 6.76 ± 1.66 | 0.197 | 7.32 ± 2.62 | 6.37 ± 1.82 | 6.05 ± 0.55 | 6.41 ± 1.08 | 0.039 |
| MetS components | 3.66 ± 0.74 | 3.75 ± 0.75 | 3.78 ± 0.78 | 4.10 ± 0.79 | <0.001 | 3.77 ± 0.80 | 3.74 ± 0.70 | 3.89 ± 0.79 | 4.15 ± 0.70 | 0.019 |
| SBP, mmHg | 134.60 ± 13.65 | 136.47 ± 15.96 | 141.07 ± 15.90 | 138.69 ± 15.16 | 0.022 | 137.87 ± 13.05 | 134.18 ± 13.15 | 137.02 ± 14.18 | 141.44 ± 14.48 | 0.036 |
| DBP, mmHg | 79.67 ± 8.82 | 80.39 ± 9.80 | 82.02 ± 8.60 | 81.56 ± 9.24 | 0.224 | 84.81 ± 8.48 | 84.14 ± 9.38 | 84.56 ± 10.83 | 87.18 ± 9.09 | 0.259 |
| Risk Factors Combination | Men (n = 220) | Women (n = 386) | ||||
|---|---|---|---|---|---|---|
| n | % | SUA, μmol/L | n | % | SUA, μmol/L | |
| 5 risks factors | ||||||
| GHTBW | 52 | 23.6 | 418.92 ± 84.49 | 90 | 23.3 | 373.12 ± 100.31 |
| 4 risks factors | ||||||
| GTBW | 42 | 19.1 | 436.76 ± 76.51 | 50 | 13.0 | 327.68 ± 77.73 |
| GHBW | 19 | 8.6 | 395.32 ± 92.73 | 52 | 13.5 | 342.90 ± 74.47 |
| HTBW | 13 | 5.9 | 413.92 ± 67.39 | 23 | 6.0 | 347.35 ± 69.92 |
| GHTB | 11 | 5.0 | 400.91 ± 75.49 | 8 | 2.1 | 303.00 ± 71.47 |
| GHTW | 6 | 2.7 | 371.00 ± 49.67 | 6 | 1.6 | 364.33 ± 65.56 |
| 3 risks factors | ||||||
| GBW | 37 | 16.8 | 384.00 ± 68.86 | 111 | 28.8 | 313.83 ± 67.57 |
| TBW | 13 | 5.9 | 411.31 ± 74.65 | 17 | 4.4 | 331.71 ± 63.30 |
| HBW | 6 | 2.7 | 408.83 ± 62.59 | 11 | 2.8 | 345.09 ± 76.04 |
| GTB | 11 | 5.0 | 368.91 ± 70.86 | 4 | 1.0 | 341.75 ± 64.86 |
| GHW | 2 | 0.9 | 366.50 ± 20.51 | 6 | 1.6 | 321.00 ± 67.36 |
| GHB | 4 | 1.8 | 318.50 ± 66.78 | 1 | 0.3 | 255.00 |
| HTW | 2 | 0.9 | 400.00 ± 46.67 | 3 | 0.8 | 304.67 ± 15.18 |
| GTW | - | - | - | 3 | 0.8 | 221.67 ± 76.14 |
| HTB | 2 | 0.9 | 408.00 ± 77.78 | - | - | - |
| GHT | - | - | - | 1 | 0.3 | 348.00 |
| MetS Components Combination * | Crude | ||
|---|---|---|---|
| OR | 95% C.I. | p-Value | |
| 5 risks | |||
| GHTBW | 1.982 | 1.351–2.906 | <0.001 |
| 4 risks | |||
| GHTB | 0.475 | 0.156–1.450 | 0.191 |
| GHTW | 0.601 | 0.161–2.243 | 0.449 |
| GTBW | 0.927 | 0.585–1.469 | 0.748 |
| GHBW | 1.058 | 0.632–1.769 | 0.831 |
| HTBW | 1.030 | 0.511–2.076 | 0.935 |
| 3 risks | |||
| GBW | 0.653 | 0.436–0.978 | 0.039 |
| GTB | 0.655 | 0.206–2.082 | 0.473 |
| HBW | 1.282 | 0.481–3.418 | 0.619 |
| TBW | 1.056 | 0.493–2.262 | 0.889 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikolaitytė, J.; Badarienė, J.; Puronaitė, R.; Čypienė, A.; Rutkauskienė, I.; Dadonienė, J.; Laucevičius, A. Which Clusters of Metabolic Syndrome Are the Most Associated with Serum Uric Acid? Medicina 2022, 58, 297. https://doi.org/10.3390/medicina58020297
Mikolaitytė J, Badarienė J, Puronaitė R, Čypienė A, Rutkauskienė I, Dadonienė J, Laucevičius A. Which Clusters of Metabolic Syndrome Are the Most Associated with Serum Uric Acid? Medicina. 2022; 58(2):297. https://doi.org/10.3390/medicina58020297
Chicago/Turabian StyleMikolaitytė, Jurgita, Jolita Badarienė, Roma Puronaitė, Alma Čypienė, Irma Rutkauskienė, Jolanta Dadonienė, and Aleksandras Laucevičius. 2022. "Which Clusters of Metabolic Syndrome Are the Most Associated with Serum Uric Acid?" Medicina 58, no. 2: 297. https://doi.org/10.3390/medicina58020297
APA StyleMikolaitytė, J., Badarienė, J., Puronaitė, R., Čypienė, A., Rutkauskienė, I., Dadonienė, J., & Laucevičius, A. (2022). Which Clusters of Metabolic Syndrome Are the Most Associated with Serum Uric Acid? Medicina, 58(2), 297. https://doi.org/10.3390/medicina58020297






