High Reoperation Rate in Mobile-Bearing Total Ankle Arthroplasty in Young Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Type and Population
2.2. Preoperative Preparation, Surgical Technique, Postoperative Care and Follow up
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Complications and Revisions
3.3. Survival Analysis and Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espinosa, N.; Klammer, G. Treatment of Ankle Osteoarthritis: Arthrodesis versus Total Ankle Replacement. Eur. J. Trauma Emerg. Surg. 2010, 36, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunfeld, R.; Aydogan, U.; Juliano, P. Ankle Arthritis: Review of Diagnosis and Operative Management. Med. Clin. N. Am. 2014, 98, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Norvell, D.C.; Ledoux, W.R.; Shofer, J.B.; Hansen, S.T.; Davitt, J.; Anderson, J.G.; Bohay, D.; Coetzee, J.C.; Maskill, J.; Brage, M.; et al. Effectiveness and Safety of Ankle Arthrodesis Versus Arthroplasty: A Prospective Multicenter Study. J. Bone Jt. Surg. Am. 2019, 101, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.E.; Kraszewski, A.P.; Ellis, S.J.; Queen, R.; Backus, S.I.; Hillstrom, H.; Demetracopoulos, C.A. Differences in Gait and Stair Ascent After Total Ankle Arthroplasty and Ankle Arthrodesis. Foot Ankle Int. 2020, 42, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lawton, C.D.; Butler, B.A.; Dekker, R.G.; Prescott, A.; Kadakia, A.R. Total Ankle Arthroplasty versus Ankle Arthrodesis—A Comparison of Outcomes over the Last Decade. J. Orthop. Surg. Res. 2017, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Seaworth, C.; Do, H.; Vulcano, E.; Mani, S.; Lyman, S.; Ellis, S. Epidemiology of Total Ankle Arthroplasty: Trends in New York State. Orthopedics 2016, 39, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Jeyaseelan, L.; Si-Hyeong Park, S.; Al-Rumaih, H.; Veljkovic, A.; Penner, M.J.; Wing, K.J.; Younger, A. Outcomes Following Total Ankle Arthroplasty: A Review of the Registry Data and Current Literature. Orthop. Clin. N. Am. 2019, 50, 539–548. [Google Scholar] [CrossRef]
- McKenna, B.J.; Cook, J.; Cook, E.A.; Crafton, J.; Knabel, M.; Swenson, E.; Miner, S.; Manning, E.; Basile, P. Total Ankle Arthroplasty Survivorship: A Meta-Analysis. J. Foot Ankle Surg. 2020, 59, 1040–1048. [Google Scholar] [CrossRef]
- Vakhshori, V.; Sabour, A.F.; Alluri, R.K.; Hatch, G.F.I.; Tan, E.W. Patient and Practice Trends in Total Ankle Replacement and Tibiotalar Arthrodesis in the United States From 2007 to 2013. JAAOS—J. Am. Acad. Orthop. Surg. 2019, 27, e77–e84. [Google Scholar] [CrossRef]
- Krause, F.G.; Schmid, T. Ankle Arthrodesis versus Total Ankle Replacement: How Do I Decide? Foot Ankle Clin. 2012, 17, 529–543. [Google Scholar] [CrossRef]
- Pedowitz, D.I.; Kane, J.M.; Smith, G.M.; Saffel, H.L.; Comer, C.; Raikin, S.M. Total Ankle Arthroplasty versus Ankle Arthrodesis. Bone Jt. J. 2016, 98, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Daniels, T.R.; Parker, K. Gait Analysis and Functional Outcomes Following Ankle Arthrodesis for Isolated Ankle Arthritis. JBJS 2006, 88, 526–535. [Google Scholar] [CrossRef]
- SooHoo, N.F.; Zingmond, D.S.; Ko, C.Y. Comparison of Reoperation Rates Following Ankle Arthrodesis and Total Ankle Arthroplasty. J. Bone Jt. Surg. Am. 2007, 89, 2143–2149. [Google Scholar] [CrossRef]
- Dekker, T.J.; Walton, D.; Vinson, E.N.; Hamid, K.S.; Federer, A.E.; Easley, M.E.; DeOrio, J.K.; Nunley, J.A.; Adams, S.B. Hindfoot Arthritis Progression and Arthrodesis Risk After Total Ankle Replacement. Foot Ankle Int. 2017, 38, 1183–1187. [Google Scholar] [CrossRef]
- Ross, B.J.; Savage-Elliott, I.; Wu, V.J.; Rodriguez, R.F. Complications Following Total Ankle Arthroplasty Versus Ankle Arthrodesis for Primary Ankle Osteoarthritis. Foot Ankle Spec. 2021, 1938640020987741. [Google Scholar] [CrossRef]
- Stavrakis, A.I.; SooHoo, N.F. Trends in Complication Rates Following Ankle Arthrodesis and Total Ankle Replacement. JBJS 2016, 98, 1453–1458. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.-W.; Seon, J.; Kim, N.-S.; Lee, K.-B. Comparison of Intermediate-Term Outcomes of Total Ankle Arthroplasty in Patients Younger and Older Than 55 Years. Foot Ankle Int. 2019, 40, 762–768. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Muras, J.; Martín Oliva, X.; Amado, P. Total Ankle Replacement in Patients under the Age of 50. Should the Indications Be Revised? Foot Ankle Surg. 2013, 19, 229–233. [Google Scholar] [CrossRef]
- Cottom, J.M.; Graney, C.T.; Douthett, S.M.; Sisovsky, C.; McConnell, K.K.; Plemmons, B.S. Age-Related Outcomes in Total Ankle Arthroplasty: An Analysis of 112 Patients. J. Foot Ankle Surg. 2020, 59, 739–742. [Google Scholar] [CrossRef]
- Gaugler, M.; Krähenbühl, N.; Barg, A.; Ruiz, R.; Horn-Lang, T.; Susdorf, R.; Dutilh, G.; Hintermann, B. Effect of Age on Outcome and Revision in Total Ankle Arthroplasty. Bone Jt. J. 2020, 102, 925–932. [Google Scholar] [CrossRef]
- Tenenbaum, S.; Bariteau, J.; Coleman, S.; Brodsky, J. Functional and Clinical Outcomes of Total Ankle Arthroplasty in Elderly Compared to Younger Patients. Foot Ankle Surg. 2017, 23, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Demetracopoulos, C.A.; Adams, S.B.; Queen, R.M.; DeOrio, J.K.; Nunley, J.A.; Easley, M.E. Effect of Age on Outcomes in Total Ankle Arthroplasty. Foot Ankle Int. 2015, 36, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Consul, D.W.; Chu, A.; Langan, T.M.; Hyer, C.F.; Berlet, G. Total Ankle Arthroplasty Survivorship, Complication, and Revision Rates in Patients Younger Than 55 Years. Foot Ankle Spec. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cianni, L.; Bocchi, M.B.; Vitiello, R.; Greco, T.; De Marco, D.; Masci, G.; Maccauro, G.; Pitocco, D.; Perisano, C. Arthrodesis in the Charcot Foot: A Systematic Review. Orthop. Rev. 2020, 12, 8670. [Google Scholar] [CrossRef]
- Colombier, J.A.; Judet, T.; Bonnin, M.; Gaudot, F. Techniques and Pitfalls with the Salto Prosthesis: Our Experience of the First 15 Years. Foot Ankle Clin. 2012, 17, 587–605. [Google Scholar] [CrossRef]
- Bonnin, M.; Judet, T.; Colombier, J.A.; Buscayret, F.; Graveleau, N.; Piriou, P. Midterm Results of the Salto Total Ankle Prosthesis. Clin. Orthop. Relat. Res. 2004, 424, 6–18. [Google Scholar] [CrossRef]
- Henricson, A.; Carlsson, Å.; Rydholm, U. What Is a Revision of Total Ankle Replacement? Foot Ankle Surg. 2011, 17, 99–102. [Google Scholar] [CrossRef]
- Saltzman, C. Total Ankle Arthroplasty: State of the Art. Instr. Course Lect. 1999, 48, 263–268. [Google Scholar]
- Samaila, E.M.; Bissoli, A.; Argentini, E.; Negri, S.; Colò, G.; Magnan, B. Total Ankle Replacement in Young Patients. Acta Biomed. 2020, 91, 31–35. [Google Scholar] [CrossRef]
- Hintermann, B.; Knupp, M.; Zwicky, L.; Barg, A. Total Ankle Replacement for Treatment of End-Stage Osteoarthritis in Elderly Patients. J. Aging Res. 2012, 2012, 345237. [Google Scholar] [CrossRef] [Green Version]
- Pitta, M.; Esposito, C.I.; Li, Z.; Lee, Y.; Wright, T.M.; Padgett, D.E. Failure After Modern Total Knee Arthroplasty: A Prospective Study of 18,065 Knees. J. Arthroplast. 2018, 33, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Bottle, A.; Parikh, S.; Aylin, P.; Loeffler, M. Risk Factors for Early Revision after Total Hip and Knee Arthroplasty: National Observational Study from a Surgeon and Population Perspective. PLoS ONE 2019, 14, e0214855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, B.; Bordini, B.; Caputo, D.; Neri, T.; Stea, S.; Toni, A. What Are the Influencing Factors on Hip and Knee Arthroplasty Survival? Prospective Cohort Study on 63619 Arthroplasties. Orthop. Traumatol. Surg. Res. 2019, 105, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The Effect of Patient Age at Intervention on Risk of Implant Revision after Total Replacement of the Hip or Knee: A Population-Based Cohort Study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Namba, R.S.; Cafri, G.; Khatod, M.; Inacio, M.C.S.; Brox, T.W.; Paxton, E.W. Risk Factors for Total Knee Arthroplasty Aseptic Revision. J. Arthroplast. 2013, 28, 122–127. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Shepherd, E.F.; Dorey, F.J.; Jackson, W.O.; dela Rosa, M.; Fa’vae, F.; McKellop, H.A.; McClung, C.D.; Martell, J.; Moreland, J.R.; et al. The John Charnley Award. Wear Is a Function of Use, Not Time. Clin. Orthop. Relat. Res. 2000, 381, 36–46. [Google Scholar] [CrossRef]
- Chakravarty, R.; Elmallah, R.D.K.; Cherian, J.J.; Kurtz, S.M.; Mont, M.A. Polyethylene Wear in Knee Arthroplasty. J. Knee Surg. 2015, 28, 370–375. [Google Scholar] [CrossRef]
- Naudie, D.D.R.; Ammeen, D.J.; Engh, G.A.; Rorabeck, C.H. Wear and Osteolysis Around Total Knee Arthroplasty. JAAOS—J. Am. Acad. Orthop. Surg. 2007, 15, 53–64. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Szuszczewicz, E.S.; Northfield, M.R.; Akizuki, K.H.; Frankel, R.E.; Belcher, G.; Amstutz, H.C. Quantitative Assessment of Walking Activity after Total Hip or Knee Replacement. J. Bone Jt. Surg. Am. 1998, 80, 54–59. [Google Scholar] [CrossRef]
- Cherian, J.J.; Jauregui, J.J.; Banerjee, S.; Pierce, T.; Mont, M.A. What Host Factors Affect Aseptic Loosening After THA and TKA? Clin. Orthop. Relat. Res. 2015, 473, 2700–2709. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.S.; Smyth, N.A.; Fraser, E.J.; Hogan, M.V.; Seaworth, C.M.; Ross, K.A.; Kennedy, J.G. Investigating the Relationship Between Ankle Arthrodesis and Adjacent-Joint Arthritis in the Hindfoot: A Systematic Review. JBJS 2015, 97, e43. [Google Scholar] [CrossRef] [PubMed]
- Morasiewicz, P.; Dejnek, M.; Urbański, W.; Dragan, S.Ł.; Kulej, M.; Dragan, S.F. Radiological Evaluation of Ankle Arthrodesis with Ilizarov Fixation Compared to Internal Fixation. Injury 2017, 48, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Zhang, Y. Epidemiology of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.J.; Roddy, E.; Zhang, W.; Menz, H.B.; Hannan, M.T.; Peat, G.M. The Population Prevalence of Foot and Ankle Pain in Middle and Old Age: A Systematic Review. Pain 2011, 152, 2870–2880. [Google Scholar] [CrossRef] [PubMed]

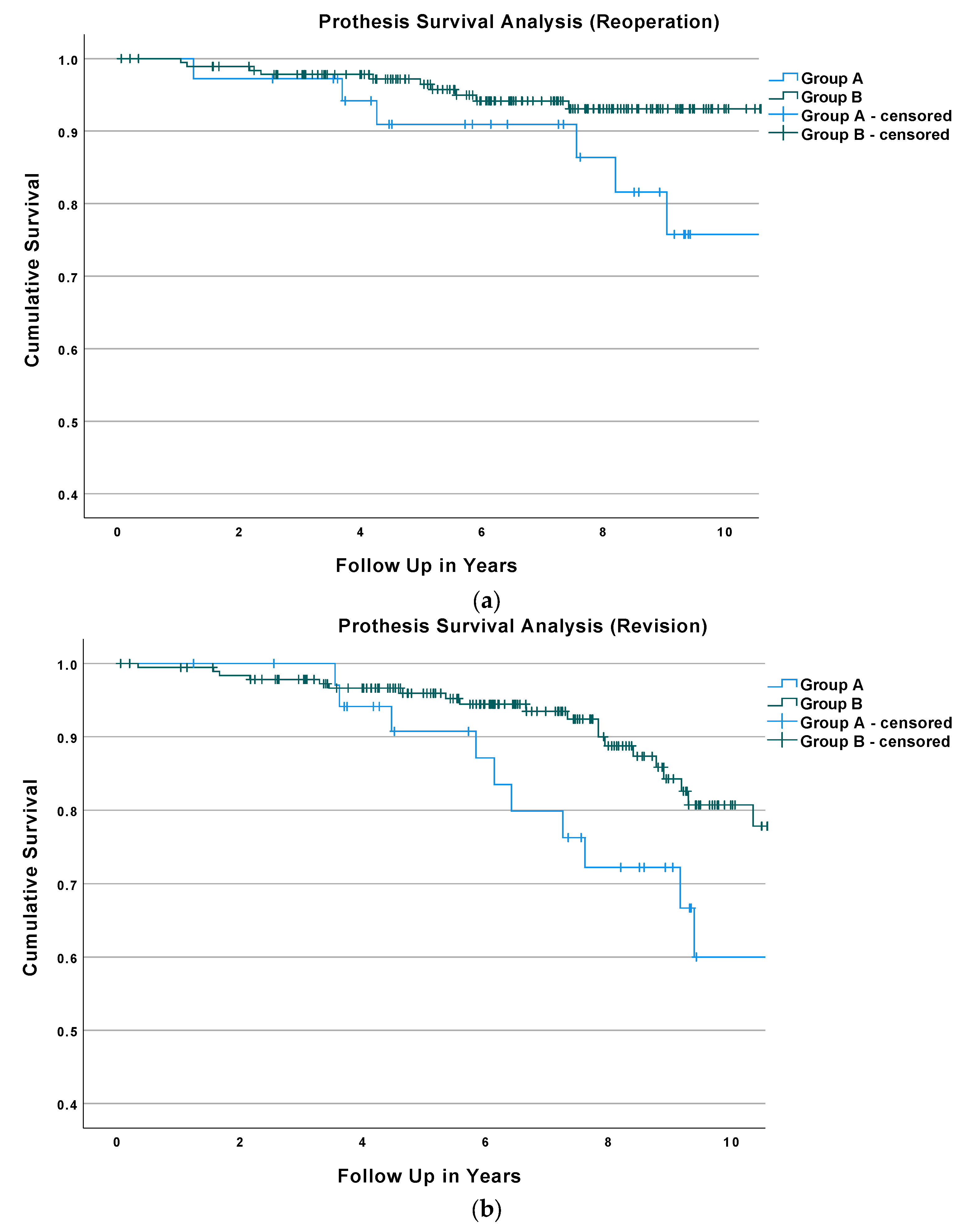
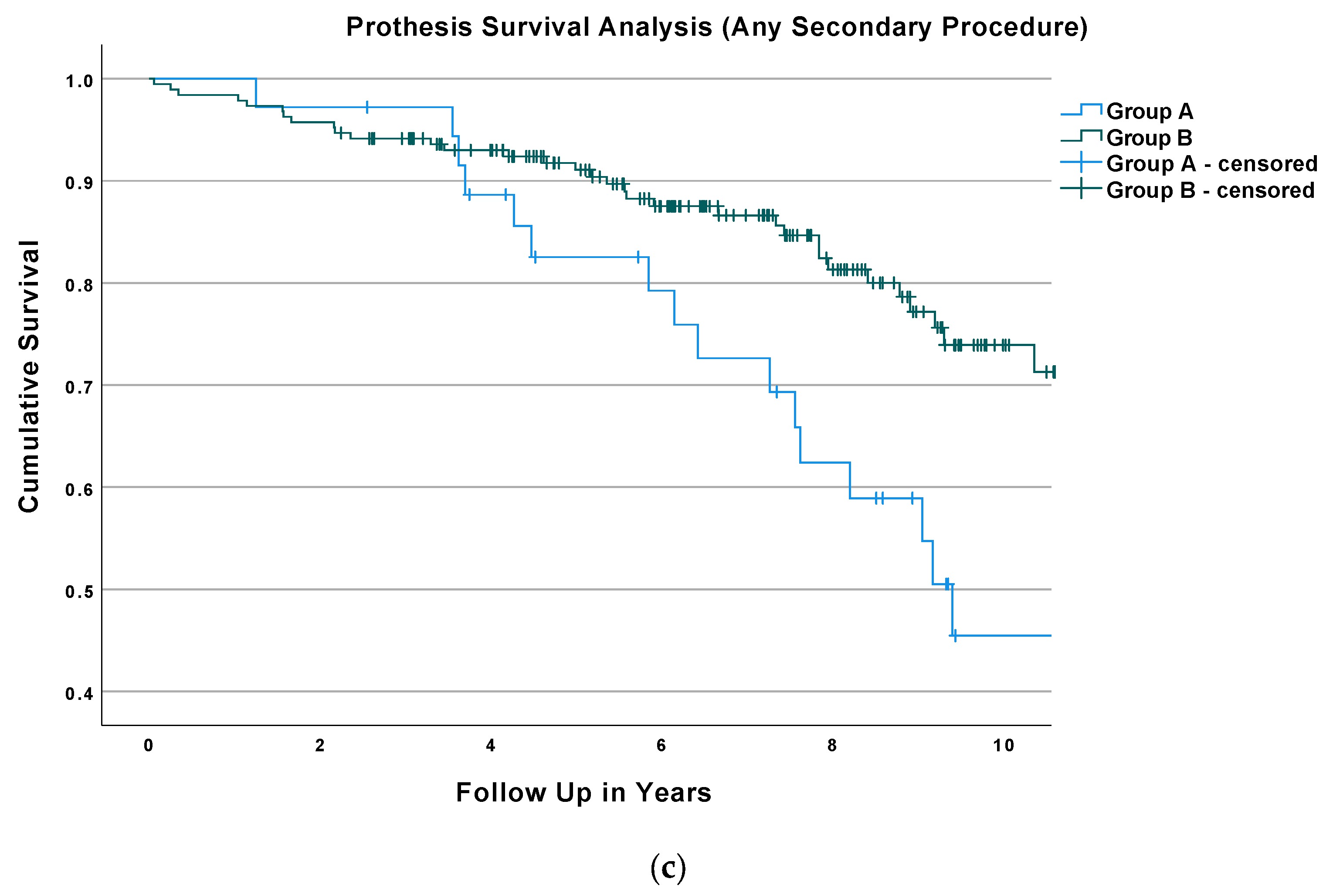
| Baseline Characteristics of the Study Population | Overall (n = 224) | Group A (n = 36) | Group B (n = 188) | p-Value |
|---|---|---|---|---|
| Mean follow up (years) | 7.1 ± 3.2 | 7.8 ± 3.5 | 7.0 ± 3.1 | 0.187 |
| Mean age (years) | 61.4 ± 11.8 | 41.7 ± 6.7 | 65.2 ± 8.3 | <0.001 |
| Female | 107 | 13 | 94 | 0.147 |
| Male | 117 | 23 | 94 | 0.147 |
| Right Ankle | 130 | 22 | 108 | 0.716 |
| Left Ankle | 94 | 14 | 80 | 0.716 |
| Mean body weight (kg) | 80.9 ± 17.0 | 83.5 ± 13.9 | 80.4 ± 17.5 | 0.318 |
| Mean body height (cm) | 169.6 ± 14.7 | 174.4 ± 8.1 | 167.6 ± 18.9 | 0.002 |
| Mean BMI | 28.0 ± 4.3 | 27.4 ± 3.7 | 28.2 ± 4.4 | 0.312 |
| Mean ASA Score | 1.9 ± 0.5 | 1.5 ± 0.6 | 2.0 ± 0.5 | <0.001 |
| Mean size tibial component | 1.9 ± 0.8 | 2.0 ± 0.8 | 1.9 ± 0.8 | 0.412 |
| Mean size talar component | 1.6 ± 0.6 | 1.7 ± 0.6 | 1.5 ± 0.6 | 0.140 |
| Mean size polyethylene inlay | 5.5 ± 1.1 | 5.3 ± 1.2 | 5.5 ± 1.1 | 0.237 |
| Indications for Total Ankle Arthroplasty | Overall (n = 224) | Group A (n = 36) | Group B (n = 188) | |
| Posttraumatic osteoarthritis | 144 | 27 | 117 | 0.143 |
| Primary osteoarthritis | 53 | 1 | 52 | 0.001 |
| Chronic inflammatory diseases | 16 | 4 | 12 | 0.313 |
| Prior infection | 5 | 2 | 3 | 0.016 |
| Aseptic osteonecrosis | 3 | 2 | 1 | 0.141 |
| Haemochromatosis | 3 | 0 | 3 | 0.445 |
| Complication | Group A (n = 21) | Group B (n = 39) | Overall (n = 60) |
|---|---|---|---|
| Osteolytic cysts | 10 | 10 | 20 |
| Inlay fracture | 6 | 13 | 19 |
| Ossifications | 10 | 7 | 17 |
| Wear | 5 | 6 | 11 |
| Soft tissue impingement | 3 | 5 | 8 |
| Contracture/ROM-Limitation | 3 | 2 | 5 |
| Acute infection | 0 | 4 | 4 |
| Deep wound infection | 0 | 2 | 2 |
| Inlay luxation | 0 | 2 | 2 |
| Aseptic osteonecrosis | 0 | 1 | 1 |
| Talonavicular OA | 0 | 1 | 1 |
| Instability | 0 | 1 | 1 |
| Achilles’ tendon rupture | 0 | 1 | 1 |
| No information available | 1 | 1 | 2 |
| Group A (n = 21) | Group B (n = 39) | Overall (n = 60) | |
|---|---|---|---|
| Inlay replacement | 18 | 27 | 45 |
| Synovectomy | 10 | 14 | 24 |
| Filling of osteolytic cysts | 9 | 8 | 17 |
| Removal of ossifications | 10 | 7 | 17 |
| Explantation | 6 | 10 | 16 |
| Arthrodesis | 5 | 9 | 14 |
| Revision prothesis | 1 | 1 | 2 |
| Achilles’ tendon lengthening | 2 | 2 | 4 |
| Corrective osteotomy | 0 | 3 | 3 |
| Ligamentous release | 1 | 1 | 2 |
| Lateral ligament repair | 0 | 1 | 1 |
| Achilles’ tendon repair | 0 | 1 | 1 |
| Talonavicular arthrodesis | 0 | 1 | 1 |
| Flap Surgery | 0 | 1 | 1 |
| Wound revision | 0 | 1 | 1 |
| Subtalar arthrodesis | 0 | 1 | 1 |
| No information available | 0 | 1 | 1 |
| Characteristic | Reoperation | Revision | Any Sec. Procedure |
|---|---|---|---|
| Age (years) | 0.94 (0.90–0.99; p = 0.009) | 0.94 (0.90–0.97; p = 0.001) | 0.93 (0.90–0.96; p > 0.001) |
| Sex (male) | 1.38 (0.32–5.90; p = 0.664) | 1.24 (0.42–3.68; p = 0.705) | 1.25 (0.49–3.20; p = 0.639) |
| Body height (cm) | 1.09 (0.70–1.70; p = 0.703) | 1.01 (0.92–1.18; p = 0.796) | 1.02 (0.92–1.14; p = 0.698) |
| Body weight (kg) | 0.89 (0.56–1.42; p = 0.603) | 1.01 (0.90–1.15; p = 0.838) | 0.99 (0.87–1.12; p = 0.830) |
| Body Mass Index | 1.27 (0.33–4.93; p = 0.726) | 0.94 (0.65–1.36; p = 0.747) | 0.98 (0.68–1.42; p = 0.918) |
| ASA-Score | 1.23 (0.47–3.20; p = 0.678) | 1.48 (0.69–3.18; p = 0.320) | 1.27 (0.65–2.48; p = 0.490) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadler, C.; Luger, M.; Stevoska, S.; Gahleitner, M.; Pisecky, L.; Gotterbarm, T.; Klasan, A.; Klotz, M.C. High Reoperation Rate in Mobile-Bearing Total Ankle Arthroplasty in Young Patients. Medicina 2022, 58, 288. https://doi.org/10.3390/medicina58020288
Stadler C, Luger M, Stevoska S, Gahleitner M, Pisecky L, Gotterbarm T, Klasan A, Klotz MC. High Reoperation Rate in Mobile-Bearing Total Ankle Arthroplasty in Young Patients. Medicina. 2022; 58(2):288. https://doi.org/10.3390/medicina58020288
Chicago/Turabian StyleStadler, Christian, Matthias Luger, Stella Stevoska, Manuel Gahleitner, Lorenz Pisecky, Tobias Gotterbarm, Antonio Klasan, and Matthias C. Klotz. 2022. "High Reoperation Rate in Mobile-Bearing Total Ankle Arthroplasty in Young Patients" Medicina 58, no. 2: 288. https://doi.org/10.3390/medicina58020288
APA StyleStadler, C., Luger, M., Stevoska, S., Gahleitner, M., Pisecky, L., Gotterbarm, T., Klasan, A., & Klotz, M. C. (2022). High Reoperation Rate in Mobile-Bearing Total Ankle Arthroplasty in Young Patients. Medicina, 58(2), 288. https://doi.org/10.3390/medicina58020288







