1. Introduction
Colorectal cancer (CRC) is one of the most prevalent cancers worldwide. It is the second and third most common cancer in females and males, respectively, and is accompanied by a high rate of morbidity and mortality. Over 70% of CRC cases are sporadic, 20% of cases have an associated hereditary component, and less than 5% of cases are inherited (Lynch Syndrome, 2–5%) [
1]. Recently, the knowledge of the epidemiology, etiology, molecular biology, and clinical aspects of CRC has improved considerably. Nevertheless, 1.8 million new cases are diagnosed annually worldwide. CRC is often diagnosed at advanced clinical stages, and about 900,000 individuals die from this malignancy [
2]. A 60% increase in CRC cases is expected worldwide by 2030. The standard treatment for CRC patients is surgery, radiation, chemotherapy, or a combination of these therapies. Additionally, immunotherapy is becoming an attractive option compared with conventional chemotherapy for CRC. Many of the dependencies in CRC are still misunderstood. A better understanding of the mechanisms of this malignancy is essential for the development of modern, effective therapies. The search for new molecules involved in CRC development seems to be crucial [
3].
Many cancers represent a paradigm for the link between inflammation and oncogenesis, including CRC. Inflammation is associated with the accumulation of various immune cells and inflammatory mediators, such as cytokines, chemokines, and growth factors. Several studies suggest that chronic inflammation promotes tumor development and leads to the inclusion of inflammation in a characteristic component of tumorigenesis.
Until recently it was believed that the inflammatory chemokines constitute only an antitumor barrier, and these were viewed mainly as indispensable points of regular of immunity and inflammation. Updated information indicates that chemokines may play a very important role in tumor progression, being components of their microenvironment [
4]. Tumor cells via chemokine secretion adapt T cells, monocytes, myeloid cells, fibroblasts, and mesenchymal stromal cells (MSCs) from adipose tissue, and stimulate them by different mechanisms to become immunosuppressive T regulatory cells (Tregs), tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), cancer-associated fibroblasts (CAFs), and cancer-associated adipocytes [
5]. Tumor cells can take control of chemokine networks to support tumor progression. This phenomenon can be observed in the context of the CCL5/CCR5 axis.
CCL5 (RANTES) belongs to the C-C chemokine family and plays an active role in recruiting a variety of leukocytes into inflammatory sites, including T cells, macrophages, eosinophils, and basophils. CCL5 is a target gene of NF-κB activity and is expressed by T lymphocytes, macrophages, platelets, synovial fibroblasts, tubular epithelium, and certain types of tumor cells. The RANTES activity is mediated through its binding to CCR1, CCR3, and mainly CCR5.
The major functions of this chemokine in tumor development are poorly understood. CCL5 production is relevant to inducing proper immune responses against tumors, but, on the other hand, CCL5 is associated with cancer progression and metastasis [
6]. CCL5 by interaction with CCR5 can support tumor progression via pleiotropic effects, including by acting as growth factors, stimulating angiogenesis, enhancing tumor cell migration (metastasis formation, modulating the extracellular matrix, inducing the recruitment of additional stromal and inflammatory cells, decreasing the cytotoxicity of DNA-damaging agents, and taking part in immune evasion mechanisms via inducing the immunosuppressive polarization of macrophages [
5].
It has been reported that CCL5 is overexpressed in colorectal cancer and plays a crucial role in immune escape of tumor cells. Shengbo Zhang et al. examined that CCL5-deficiency could upregulate PD-1 and PD-L1 expression and reduce the resistance to anti-PD-1 antibody therapy in the CRC mouse model [
7]. That study also proved that knockdown of RANTES was associated with the reduction of tumor growth, metastasis and apoptosis of tumor-infiltrating CD8+ T cells. The proangiogenic activity of RANTES can be supported by increasing migration of endothelial cells, spreading, neovessel formation, and secretion of vascular endothelial growth factor (VEGF).
Many factors are involved in the regulation of CCL5 expression. Researchers have pointed out that there is a relationship between CCL5 levels and inflammatory molecules. TNF-α and IFN-γ have the potential to enhance CCL5 expression by TLR3 signaling. In this mechanism, TNF-α may activate NF-κB, in cooperation with TLR3 signaling. IFN-γ may stabilize CCL5 mRNA up-regulated by TLR3 [
8]. IFN-γ and TNF-α are among the most important pro-inflammatory cytokines involved in the recruitment of immune cells to the TME [
9,
10]. The TILs (tumor-infiltrating lymphocytes) parameter is associated with immune status and its increase is a positive prognostic factor in CRC [
11].
Transforming growth factor-β1 (TGF-β1) is a pleiotropic cytokine that also regulates CCL5 levels. TGF-β1 inhibits RANTES expression mediated by β-catenin-triggered blockade of NF-κB signaling [
12]. Epithelial to mesenchymal transition (EMT) of tumor cells is reflected by budding and is considered an additional prognostic factor in CRC [
13]. Tumor budding is described as the presence of a single cancer cell or clusters consisting of four cells or less at the tumor invasive front [
14].
In this study, we wanted to investigate the correlations between RANTES and selected factors in the context of immune response and angiogenesis processes in colorectal cancer. Additionally, our aim was to investigate expression of RANTES and PD-L1 in selected cases with the use of immunostaining to determine which cells in the tumor environment produce these proteins. We also assessed whether the levels of RANTES, TNFα, IFNγ, TGF- β1, PD-L1, VEGF-A, and VEGF-C were associated with some histopathological parameters: MVD (microvessel density), budding, and tumor-infiltrating lymphocytes. MVD has been reported to be an independent prognostic factor in many cancers, but in CRC the results are inconclusive [
8].
The levels of RANTES, TNFα, IFNγ, TGF- β1, PD-L1, VEGF-A, and VEGF-C were measured using ELISA tests; for 24 randomly selected cases RANTES and PD-L1 immunostaining was performed. These data and other histopathological parameters (MVD, TILs, budding) were correlated with the patients’ clinical features.
2. Materials and Methods
The samples from 49 patients obtained during surgery due to CRC were enrolled in the study. The patients were treated in the 1st Specialistic Hospital in Bytom, Poland (with the approval of the Research Ethics Committee PCN/0022/KB1/42/VI/14/16/18/19/20) between March 2019 and April 2020. The collected specimens included colorectal tumor tissues and surgical margin tissues. Inclusion criteria involved colorectal adenocarcinoma and surgical “tumor-free” margin tissue confirmed by histological examination, patients’ age >18 years, and signed consent. Exclusion criteria included tumors other than adenocarcinoma, presence of margin infiltration, patients’ age <18 years, lack of signed consent, and history of chemo- or radiotherapy. To classify the cancer stage, the TNM staging system and grading were used. The characteristics of the study sample are provided in
Table 1.
2.1. Preparation of RANTES, PD-L1, IFN-γ, TNF-α, TGF-β, VEGF-A, and VEGF-C Samples for the Evaluation
Fragments of the tumor tissue and surgical margin tissue were weighted and homogenized using a PRO 200 homogenizer (PRO Scientific Inc., Oxford, CT, USA) at 10,000 rpm in nine volumes of phosphate-buffered saline (BIOMED, Lublin, Poland). The suspensions were sonicated with an ultrasonic cell disrupter (UP 100, Hilscher, Ultrasonics GmbH, Teltow, Germany). Subsequently, the homogenates were centrifuged at 12,000 rpm for 5 min at 4 °C. The total protein level was determined using a universal microplate spectrophotometer (μQUANT, Biotek Inc., Winooski, VT, USA).
2.2. Evaluation of RANTES, PD-L1, IFN-γ, TNF-α, TGF-β, VEGF-A, and VEGF-C Levels
To assess the levels of the investigated proteins, an enzyme-linked immunosorbent assay (ELISA) was used according to the manufacturer’s instructions. RANTES level was assayed using a human RANTES ELISA kit (Cloud Clone, Wuhan, China) with a sensitivity of 0.059 ng/mL. PD-L1 level was evaluated with a human PD-L1 ELISA kit (Cloud Clone, Wuhan, China) with a sensitivity of 0.057 ng/mL. INF-γ level was determined with a human INFγ level ELISA kit (KHC4022, Invitrogen, Waltham, MA, USA) with a sensitivity of 4 pg/mL. TNF-α level was determined using a human tumor necrosis factor alpha (TNFa) ELISA kit (Cloud Clone, China) with a sensitivity of 5.9 pg/mL. TGF-β level was assessed using a human TGF-β1 ELISA kit (Diaclone, Besancon Cedex, France) with a sensitivity of 8.6 pg/mL. VEGF-A level was determined with a human VEGF-A ELISA kit (Cloud Clone, China) with a sensitivity of 6.2 pg/mL. VEGF-C level was assayed using a human VEGF-C ELISA kit (Biovendor, Brno, Czech Republic) with a sensitivity of 0.057 ng/mL. The absorbance of the samples was determined using a universal microplate spectrophotometer (μQUANT, Biotek Inc., Winooski, VT, USA). The measurement was conducted at a wavelength of 450 nm. The results obtained were recalculated to the corresponding total protein level and presented as ng/mg of protein.
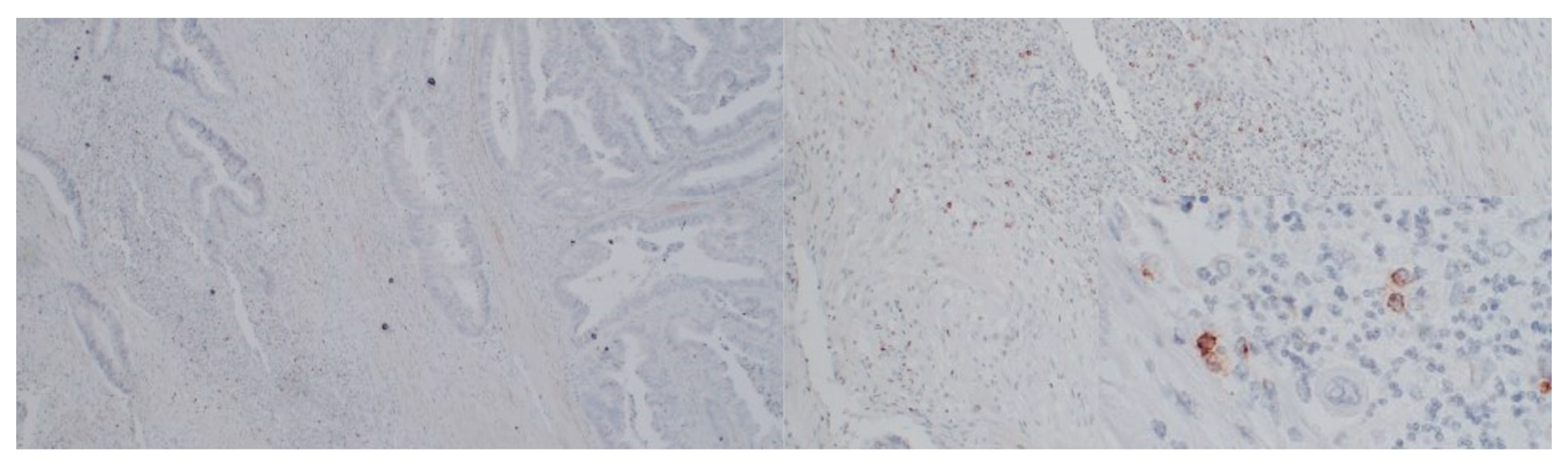
2.3. Immunostaining
RANTES and PD-L1 immunostaining was performed in 24 randomly selected cases and CD34 immunostaining was performed in the same 23 cases. The tissue samples were derived from formalin-fixed paraffin-embedded tissue blocks with CRC primary and tumor-free margin specimens. Then the samples underwent deparaffinization and rehydration. Afterward, we performed antigen retrieval by cooking slides in EnVision Flex Target Retrieval Solution High pH (Dako, Carpinteria, CA, USA) for 20 min at 95 °C. The prepared samples were incubated with peroxidase-blocked reagent (Dako, Carpinteria, CA, USA) and then incubated with CD34 antibody (clone: QBEnd/10 Cell Marque, Rocklin, CA, USA; incubation time: 30′; dilution: 1:150), RANTES antibody (clone: A-4, Santa Cruz biotechnology, Heidelberg, Germany; incubation time 60′; dilution 1:200), and PD-L1 (clone ZR3, Cell Marque, Rocklin, CA, USA; incubation time 30′, dilution 1:100). In the next step, the samples were put in EnVision FLEX HRP (Dako, Carpinteria, CA, USA). Then, antigen–antibody complexes were stained using 3,3′-diaminobenzidine. Finally, tissue sections were counterstained with hematoxylin, dehydrated, and covered with coverslips for further analysis.
2.4. Histological Evaluation
Histological evaluation was performed by two independent pathologists using an Olympus BX51 microscope.
MVD was assessed on CD34-stained specimens by two independent pathologists using a light microscope in the tumor invasive front regions counting the highest numbers of microvessels per area [
15]. Initially, tumor sections were assessed at low magnification to detect tumor invasive front, then three hot spots with high vascularization were chosen. Microvessels were counted in three fields of view under ×20 magnification. MVD was presented as the mean count of microvessels in the assessed view fields; the number was adjusted by the normalization factor (1.210).
TIL assessment was performed in 30 specimens. The percentage of tumor-associated lymphatic infiltration was estimated semi-quantitatively in a four-grade scale on the same H&E-stained slides by the two pathologists, according to the criteria defined by Salgado et al. in breast cancer [
16]. These include intratumoral lymphocytes with cell-to-cell contact between lymphocyte and tumor cell and stromal TILs in tumor tissue located dispersed in the stroma within the tumor cells without direct contact, including TILs at the invasive margin. According to the recommendations, stromal TILs were scored as a percentage of the stromal area alone, excluding areas occupied by carcinoma cells. Lymphatic infiltrates outside the tumor borders were not included in the evaluation. Lymphocyte infiltration area lower than 5% was considered TILs 1, whereas 5–25%, 25–50%, and 50–75% of lymphocytes in the stroma were defined as TILs 2, TILs 3 and TILs 4, respectively. More than 75% was defined as TILs 5.
Tumor budding was assessed in the same 30 specimens. Tumor buds were estimated in one FOV at a hotspot area in the invasive front under ×20 magnification. The number of buds was adjusted by the normalization factor (1.210) as described. Budding was reported in the following manner: low budding: 0–4 buds; intermediate budding: 5–9 buds; high budding: >10 buds. The mean number of buds per FOV was also used in the statistical analysis.
Two independent pathologists assessed RANTES and PD-L1 immunostaining in 24 cases. PD-L1 and RANTES expression was assessed both in tumor cells and in tumor-infiltrating lymphocytes (TILs). PD-L1 and RANTES were considered positive in the tumor cells when staining was present in 1% or more cancer cells. RANTES and PD-L1 expression in TILs was assessed semi-quantitatively in a five-grade scale. An area of lymphocyte infiltration with RANTES or PD-L1 expression lower than 5% was considered as Grade 0, whereas 5–25%, 25–50%, and 50–75% of lymphocytes expressing RANTES or PD-L1 in the stroma were defined as Grade 1, 2, 3 and 4, respectively. More than 75% was defined as Grade 5.
2.5. Statistical Analyses
Data distribution was assessed using the Shapiro–Wilk test. The log transformation of the levels of the examined molecules provided a better fit to the Gaussian distribution. Data are presented as mean ± SD for the variables with normal distribution and as median with interquartile range for the variables with non-normal distribution. To compare the tumor and margin levels, the paired Student’s t-test (for variables with the normal distribution) and Mann–Whitney U test (for variables with non-normal distribution) were used. Independent variables were also compared using the Student’s t-test. To assess the association between RANTES and PD-L1 levels, linear regression was performed. Pearson’s coefficient was used to assess the relationships between the examined variables (including variables with normal distribution). Tau–Kendall’s tau rank correlation coefficient was used for variables with non-normal distribution. Fisher’s exact test was used to test the association between the data obtained from IHC staining for RANTES and PD-L1 expression and clinicopathological parameters of patients. p values < 0.05 were considered statistically significant. Statistical analysis was performed using STATISTICA 13 software (Statsoft, Tulsa, OK, USA).) and the ggplot2-R package dedicated to data visualization in RStudio software (Integrated Development for R. RStudio, PBC, Boston, MA, USA). A heatmap with hierarchic clustering was generated using Seaborn (Python data visualization library).
5. Conclusions
RANTES, PD-L1, IFN-γ, TNF-α, TGF-β, VEGF-A, and VEGF-C are upregulated in CRC tissues. RANTES activity might be associated with angiogenesis, lymphogenesis, and immune escape in CRC. Additionally, RANTES might potentially be a good candidate as a therapeutic target in CRC, due to its pleiotropic effect on tumors.
The future directions of research regarding the role of the RANTES in CRC should focus on its impact on TME modulation, EMT promotion, angiogenesis, and lymphangiogenesis.