Brain-Derived Neurotrophic Factor Levels in Cord Blood from Growth Restricted Fetuses with Doppler Alteration Compared to Adequate for Gestational Age Fetuses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Selection and Fetal Growing Assessment
2.2. Sample Collection, Initial Processing and Storage
2.3. Determination of BDNF in Umbilical Vein by ELISA Methodology
2.4. Statistical Analysys
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lees, C.C.; Stampalija, T.; Baschata, A.A.; Da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Ganzevoort, W. Building consensus and standards in fetal growth restriction studies. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status with Childhood Cognitive Outcomes. JAMA Pediatr. 2020, 174, 772. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, T.; Thilaganathan, B.; Hooper, R.; Khan, K.S.; Bhide, A. Neurodevelopmental delay in small babies at term: A systematic review. Ultrasound Obstet. Gynecol. 2012, 40, 267–275. [Google Scholar] [CrossRef]
- Baschat, A.A.; Gembruch, U.; Harman, C.R. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet. Gynecol. 2001, 18, 571–577. [Google Scholar] [CrossRef]
- Baschat, A.A. Neurodevelopment after Fetal Growth Restriction. Fetal Diagn. Ther. 2014, 36, 136–142. [Google Scholar] [CrossRef]
- Meher, S.; Hernandez-Andrade, E.; Basheer, S.N.; Lees, C. Impact of cerebral redistribution on neurodevelopmental outcome in small for gestational age or growth restricted babies: A systematic review. Ultrasound Obstet. Gynecol. 2015, 46, 398–404. [Google Scholar] [CrossRef]
- Benítez-Marín, M.J.; Marín-Clavijo, J.; Blanco-Elena, J.A.; Jiménez-López, J.; González-Mesa, E. Brain Sparing Effect on Neurodevelopment in Children with Intrauterine Growth Restriction: A Systematic Review. Children 2021, 8, 745. [Google Scholar] [CrossRef]
- Baschat, A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 2011, 37, 501–514. [Google Scholar] [CrossRef]
- Rees, S.; Harding, R.; Walker, D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int. J. Dev. Neurosci. 2011, 29, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Basilious, A.; Yager, J.; Fehlings, M.G. Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: A systematic review. Dev. Med. Child Neurol. 2015, 57, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wixey, J.A.; Chand, K.K.; Colditz, P.B.; Bjorkman, S.T. Review: Neuroinflammation in intrauterine growth restriction. Placenta 2017, 54, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, R.C.; Yang, Z.-J.; Lee, J.K.; Martin, L.J. Perinatal hypoxic-ischemic brain injury in large animal models: Relevance to human neonatal encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.A.; Faulkner, S.D.; Rumajogee, P.; Beldick, S.; Foltz, W.; Corrigan, J.; Basilious, A.; Jiang, S.; Thiyagalingam, S.; Yager, J.Y.; et al. The extent of intrauterine growth restriction determines the severity of cerebral injury and neurobehavioural deficits in rodents. PLoS ONE 2017, 12, e0184653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetin, I.; Barberis, B.; Brusati, V.; Brighina, E.; Mandia, L.; Arighi, A.; Radaelli, T.; Biondetti, P.; Bresolin, N.; Pardi, G.; et al. Lactate detection in the brain of growth-restricted fetuses with magnetic resonance spectroscopy. Am. J. Obstet. Gynecol. 2011, 205, 350.e1–350.e7. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cortés, M.; Simoes, R.V.; Bargalló, N.; Masoller, N.; Figueras, F.; Gratacos, E. Proton magnetic resonance spectroscopy assessment of fetal brain metabolism in late-onset ‘small for gestational age’ versus “intrauterine growth restriction” fetuses. Fetal Diagn. Ther. 2014, 37, 108–116. [Google Scholar] [CrossRef]
- Pascual Mancho, J.; Pintado-Recarte, P.; Romero-Román, C.; Morales-Camino, J.C.; Hernández-Martin, C.; Bujan, J.; Ortega, M.A.; De León-Luis, J. Influence of Cerebral Vasodilation on Blood Reelin Levels in Growth Restricted Fetuses. Diagnostics 2021, 11, 1036. [Google Scholar] [CrossRef]
- Giacobbo, B.L.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Ninomiya, M.; Numakawa, T.; Adachi, N.; Furuta, M.; Chiba, S.; Richards, M.; Shibata, S.; Kunugi, H. Cortical neurons from intrauterine growth retardation rats exhibit lower response to neurotrophin BDNF. Neurosci. Lett. 2010, 476, 104–109. [Google Scholar] [CrossRef]
- Coupé, B.; Dutriez-Casteloot, I.; Breton, C.; Lefèvre, F.; Mairesse, J.; Dickes-Coopman, A.; Silhol, M.; Tapia-Arancibia, L.; Lesage, J.; Vieau, D. Perinatal undernutrition modifies cell proliferation and brain-derived neurotrophic factor levels during critical time-windows for hypothalamic and hippocampal development in the male rat. J. Neuroendocrinol. 2009, 21, 40–48. [Google Scholar] [CrossRef]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: A bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, V.A.; Kilari, A.S.; Joshi, A.A.; Mehendale, S.S.; Pisal, H.M.; Joshi, S.R. Differential regulation of brain-derived neurotrophic factor in term and preterm preeclampsia. Reprod. Sci. 2014, 21, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Mashburn, C.B.; Mao, J.; Wadhwa, N.; Smith, G.M.; Desai, N.S. Brain-derived neurotrophic factor in infants. Pediatr. Res. 2009, 65, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachnas, M.A.; Mose, J.C.; Effendi, J.S.; Andonotopo, W. Influence of antenatal magnesium sulfate application on cord blood levels of brain-derived neurotrophic factor in premature infants. J. Perinat. Med. 2014, 42, 129–134. [Google Scholar] [CrossRef]
- Leviton, A.; Allred, E.N.; Yamamoto, H.; Fichorova, R.N.; Kuban, K.; O’Shea, T.M.; Dammann, O.; ELGAN Study Investigators. Antecedents and correlates of blood concentrations of neurotrophic growth factors in very preterm newborns. Cytokine 2017, 94, 21–28. [Google Scholar] [CrossRef]
- Chouthai, N.S.; Sampers, J.; Desai, N.; Smith, G.M. Changes in Neurotrophin Levels in Umbilical Cord Blood from Infants with Different Gestational Ages and Clinical Conditions. Pediatr. Res. 2003, 53, 965–969. [Google Scholar] [CrossRef] [Green Version]
- Harris, N.M.; Ritzel, R.; Mancini, N.S.; Jiang, Y.; Yi, X.; Manickam, D.S.; Banks, W.A.; Kabanov, A.V.; McCullough, L.D.; Verma, R. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol. Biochem. Behav. 2016, 150, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Dang, Y.; Liu, X.; Ren, J.; Wang, H. Exogenous brain-derived neurotrophic factor attenuates neuronal apoptosis and neurological deficits after subarachnoid hemorrhage in rats. Exp. Ther. Med. 2019, 18, 3837–3844. [Google Scholar] [CrossRef] [Green Version]
- Husson, I.; Rangon, C.-M.; Lelièvre, V.; Bemelmans, A.-P.; Sachs, P.; Mallet, J.; Kosofsky, B.E.; Gressens, P. BDNF-induced white matter neuroprotection and stage-dependent neuronal survival following a neonatal excitotoxic challenge. Cereb. Cortex 2005, 15, 250–261. [Google Scholar] [CrossRef]
- Karege, F.; Schwald, M.; Cisse, M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002, 328, 261–264. [Google Scholar] [CrossRef]
- Ghassabian, A.; Sundaram, R.; Chahal, N.; McLain, A.C.; Bell, E.; Lawrence, D.A.; Yeung, E.H. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev. Psychopathol. 2017, 29, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, H.; Liu, Y.; Hou, Z.; Yue, Y.; Zhang, Y.; Zhao, F.; Xu, Z.; Li, Y.; Mou, X.; et al. Combined serum levels of multiple proteins in tPA-BDNF pathway may. Sci. Rep. 2017, 7, 6871. [Google Scholar]
- Adachi, N. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. WJBC 2014, 5, 409. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.; Wright, A.; Syngelaki, A.; Wright, D.; Akolekar, R.; Nicolaides, K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019, 53, 465–472. [Google Scholar] [CrossRef]
- Gómez, O.; Figueras, F.; Fernández, S.; Bennasar, M.; Martínez, J.M.; Puerto, B.; Gratacós, E. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet. Gynecol. 2008, 32, 128–132. [Google Scholar] [CrossRef]
- Grupo de trabajo de la Guia de práctica clñinica de atención en el embarazo y puerperio, Ministerio de Sanidad, Servicios Sociales e Igualdad. Agencia de Evaluación de Tecnologías Sanitarias de Andalucía 2014. Guías de Práctica Clínica en el SNS. AETSA 2011/10. 2014, pp. 1–494. Available online: https://portal.guiasalud.es/wp-content/uploads/2018/12/GPC_533_Embarazo_AETSA_compl.pdf (accessed on 14 December 2021).
- Fleiss, B. Knowledge Gaps and Emerging Research Areas in Intrauterine Growth Restriction-Associated Brain Injury. Front. Endocrinol. 2019, 10, 188. [Google Scholar] [CrossRef] [Green Version]
- Briana, D.D.; Papastavrou, M.; Boutsikou, M.; Marmarinos, A.; Gourgiotis, D.; Malamitsi-Puchner, A. Differential expression of cord blood neurotrophins in gestational diabetes: The impact of fetal growth abnormalities. J. Matern. Fetal Neonatal Med. 2018, 31, 278–283. [Google Scholar] [CrossRef]
- Matoba, N.; Ouyang, F.; Mestan, K.K.L.; Porta, N.F.M.; Pearson, C.M.; Ortiz, K.M.; Bauchner, H.C.; Zuckerman, B.S.; Wang, X. Cord blood immune biomarkers in small for gestational age births. J. Dev. Orig. Health Dis. 2011, 2, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Malamitsi-Puchner, A.; Nikolaou, K.E.; Economou, E.; Boutsikou, M.; Boutsikou, T.; Kyriakakou, M.; Puchner, K.; Hassiakos, D. Intrauterine growth restriction and circulating neurotrophin levels at term. Early Hum. Dev. 2007, 83, 465–469. [Google Scholar] [CrossRef]
- Prince, C.S.; Maloyan, A.; Myatt, L. Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta 2017, 49, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-H.; Liu, T.-Y.; Chen, I.T.; Ou-Yang, M.-C.; Huang, L.-T.; Tsai, C.-C.; Chen, C.-C. Correlations between serum BDNF levels and neurodevelopmental outcomes in infants of mothers with gestational diabetes. Pediatr. Neonatol. 2021, 62, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.L.; Eke, A.C.; Vaidya, D.; Northington, F.J.; Everett, A.D.; Graham, E.M. Perinatal blood biomarkers for the identification of brain injury in very low birth weight growth-restricted infants. J. Perinatol. 2021, 41, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Antonakopoulos, N.; Iliodromiti, Z.; Mastorakos, G.; Iavazzo, C.; Valsamakis, G.; Salakos, N.; Papageorghiou, A.; Margeli, A.; Kalantaridou, S.; Creatsas, G.; et al. Association between Brain-Derived Neurotrophic Factor (BDNF) Levels in 2nd Trimester Amniotic Fluid and Fetal Development. Mediat. Inflamm. 2018, 2018, 8476217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eixarch, E.; Meler, E.; Iraola, A.; Illa, M.; Crispi, F.; Hernandez-Andrade, E.; Gratacos, E.; Figueras, F. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet. Gynecol. 2008, 32, 894–899. [Google Scholar] [CrossRef]
- Novak, I. Emergent Prophylactic, Reparative and Restorative Brain Interventions for Infants Born Preterm with Cerebral Palsy. Front. Physiol. 2019, 10, 15. [Google Scholar]
| GROUP A AGA N = 91 | GROUP B FGR N = 39 | p | |
|---|---|---|---|
| Maternal age (years) M (IQR) | 32 (7) | 34 (5) | NS |
| Gestational age (weeks) M (IQR) | 38 (4) | 35 (5) | <0.001 |
| Fetal sex (female) n (%) | 49 (54) | 15 (38) | NS |
| MgSO4 n (%) | 0 | 9 (23) | <0.001 |
| Lung maturation n (%) | 1 (1) | 16 (41) | <0.001 |
| Cesarean section n (%) | 11 (12) | 25 (64) | <0.001 |
| UtA PI > p95 n (%) | N/A | 18 (46) | N/A |
| Preeclampsia n (%) | 1 (1) | 6 (15) | 0.005 |
| Birth weight (g) M (IQR) | 3290 (650) | 1750 (870) | <0.001 |
| Weight centile M (IQR) | 65 (45) | 0 (1) | <0.001 |
| pH AU M (IQR) | 7.29 (0.11) | 7.26 (0.09) | NS |
| Cord blood leukocytes (number/uL) M (IQR) | 15,600 (6500) | 12,700 (7500) | NS |
| Neonatal care admission n (%) | 6 (6.6) | 25 (64) | <0.001 |
| Intraventricular hemorrhage n (%) | 0 | 4 (10) | 0.007 |
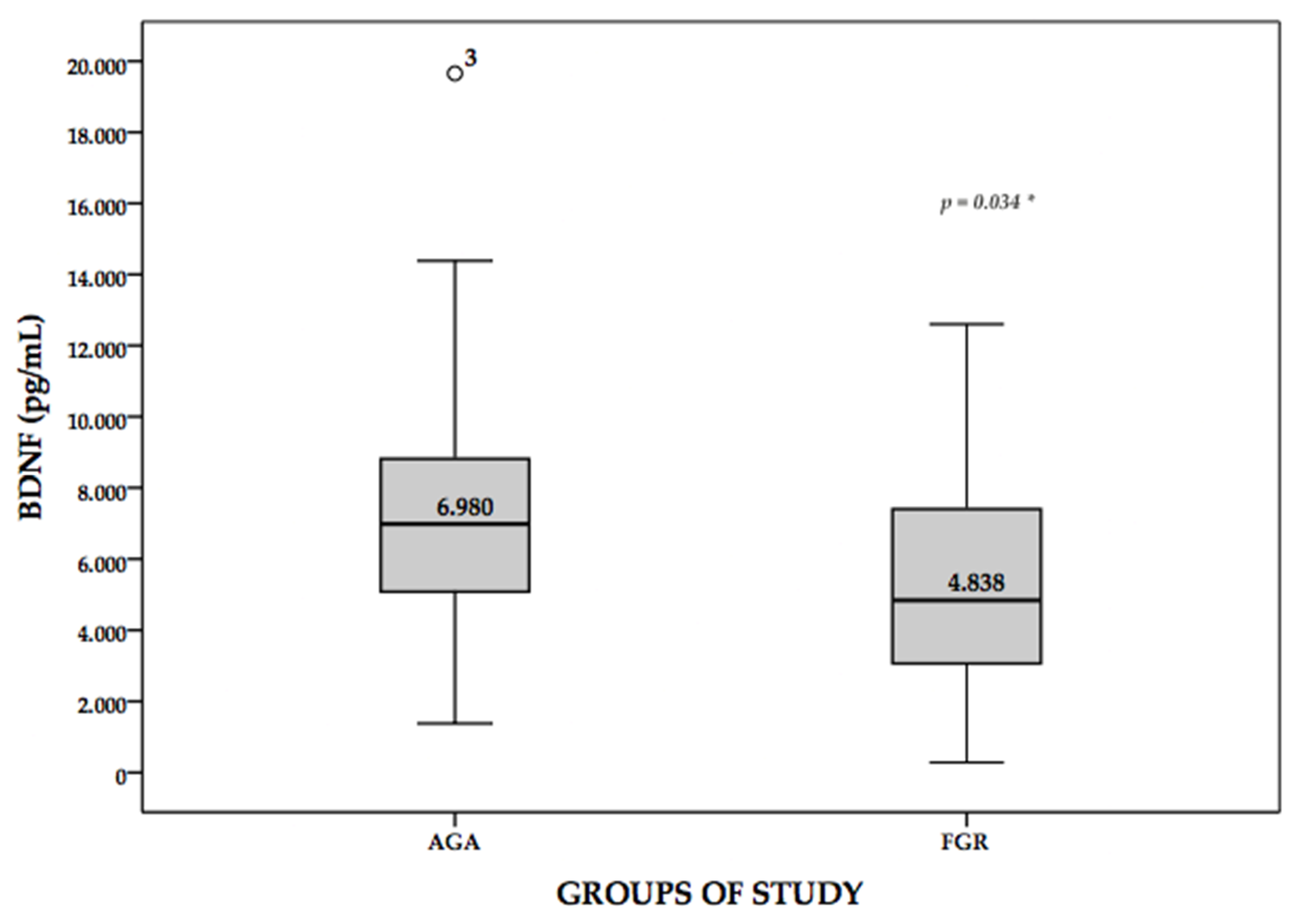
| BDNF (pg/mL) M (IQR) | 6980 (3735) | 4838 (4724) | 0.001 |
| AGA N = 91 | FGR with MCA PI < 5th Exclusively N = 16 | p | |
|---|---|---|---|
| Maternal age (years) M (SD) | 32 (28–35) | 34 (31–35) | NS |
| Gestational age (weeks) M (IQR) | 38 (36–40) | 36 (35–38) | 0.027 |
| Fetal sex (female) n (%) | 49 (54) | 9 (56) | NS |
| MgSO4 n (%) | 0 | 3 (19) | 0.003 |
| Lung maturation n (%) | 1 (1) | 3 (19) | 0.01 |
| Cesarean section n (%) | 11 (12) | 10 (63) | <0.001 |
| UtA PI > p95 n (%) | N/A | 4 (25) | N/A |
| Preeclampsia n (%) | 1 (1.3) | 4 (25) | NS |
| Birth weight (g) m (SD) | 3302 (442) | 2069 (412) | <0.001 |
| Weight centile M (IQR) | 65 (43–88) | 1 (1–3) | <0.001 |
| pH AU M (IQR) | 7.29 (0.11) | 7.27 (0.08) | NS |
| Cord blood leukocytes (number/uL) M (IQR) | 15,600 (6500) | 14,700 (7225) | NS |
| Neonatal care admission n (%) | 6 (6.6) | 6 (37.5) | 0.002 |
| Intraventricular hemorrhage n (%) | 0 | 0 | NS |
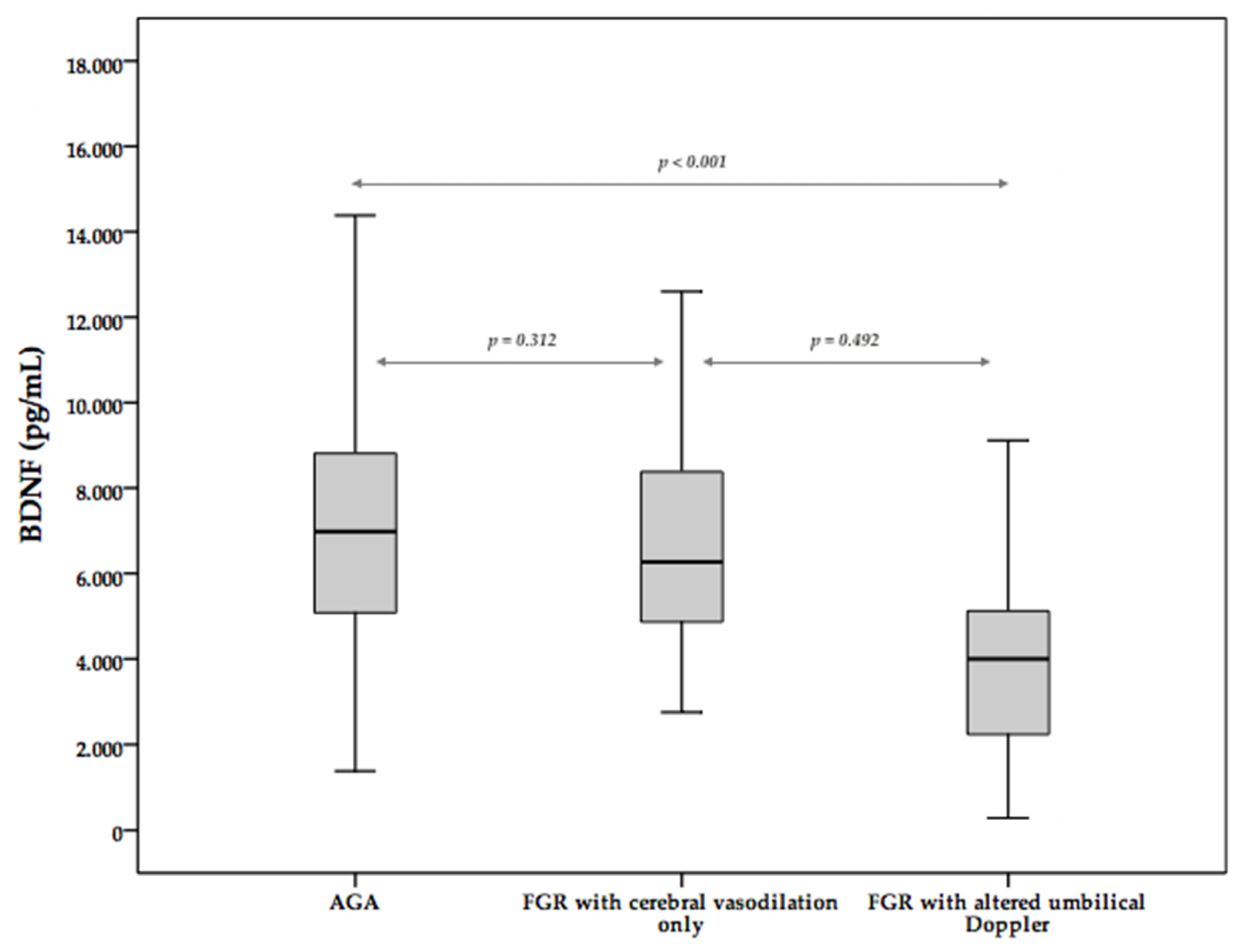
| BDNF (pg/mL) m (SD) | 6980 (3735) | 6268 (3539) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-Mancho, J.; Pintado-Recarte, P.; Morales-Camino, J.C.; Romero-Román, C.; Hernández-Martin, C.; Bravo, C.; Bujan, J.; Alvarez-Mon, M.; Ortega, M.A.; De León-Luis, J. Brain-Derived Neurotrophic Factor Levels in Cord Blood from Growth Restricted Fetuses with Doppler Alteration Compared to Adequate for Gestational Age Fetuses. Medicina 2022, 58, 178. https://doi.org/10.3390/medicina58020178
Pascual-Mancho J, Pintado-Recarte P, Morales-Camino JC, Romero-Román C, Hernández-Martin C, Bravo C, Bujan J, Alvarez-Mon M, Ortega MA, De León-Luis J. Brain-Derived Neurotrophic Factor Levels in Cord Blood from Growth Restricted Fetuses with Doppler Alteration Compared to Adequate for Gestational Age Fetuses. Medicina. 2022; 58(2):178. https://doi.org/10.3390/medicina58020178
Chicago/Turabian StylePascual-Mancho, Jara, Pilar Pintado-Recarte, Jorge C. Morales-Camino, Carlos Romero-Román, Concepción Hernández-Martin, Coral Bravo, Julia Bujan, Melchor Alvarez-Mon, Miguel A. Ortega, and Juan De León-Luis. 2022. "Brain-Derived Neurotrophic Factor Levels in Cord Blood from Growth Restricted Fetuses with Doppler Alteration Compared to Adequate for Gestational Age Fetuses" Medicina 58, no. 2: 178. https://doi.org/10.3390/medicina58020178
APA StylePascual-Mancho, J., Pintado-Recarte, P., Morales-Camino, J. C., Romero-Román, C., Hernández-Martin, C., Bravo, C., Bujan, J., Alvarez-Mon, M., Ortega, M. A., & De León-Luis, J. (2022). Brain-Derived Neurotrophic Factor Levels in Cord Blood from Growth Restricted Fetuses with Doppler Alteration Compared to Adequate for Gestational Age Fetuses. Medicina, 58(2), 178. https://doi.org/10.3390/medicina58020178










