Predictors of Clinical Outcome in Women with Pelvic Organ Prolapse Who Underwent Transvaginal Mesh Reconstruction Surgery
Abstract
1. Introduction
2. Methods
2.1. Operative Technique
2.1.1. Transobturator Mesh Fixation
2.1.2. Sacrospinous Mesh Fixation
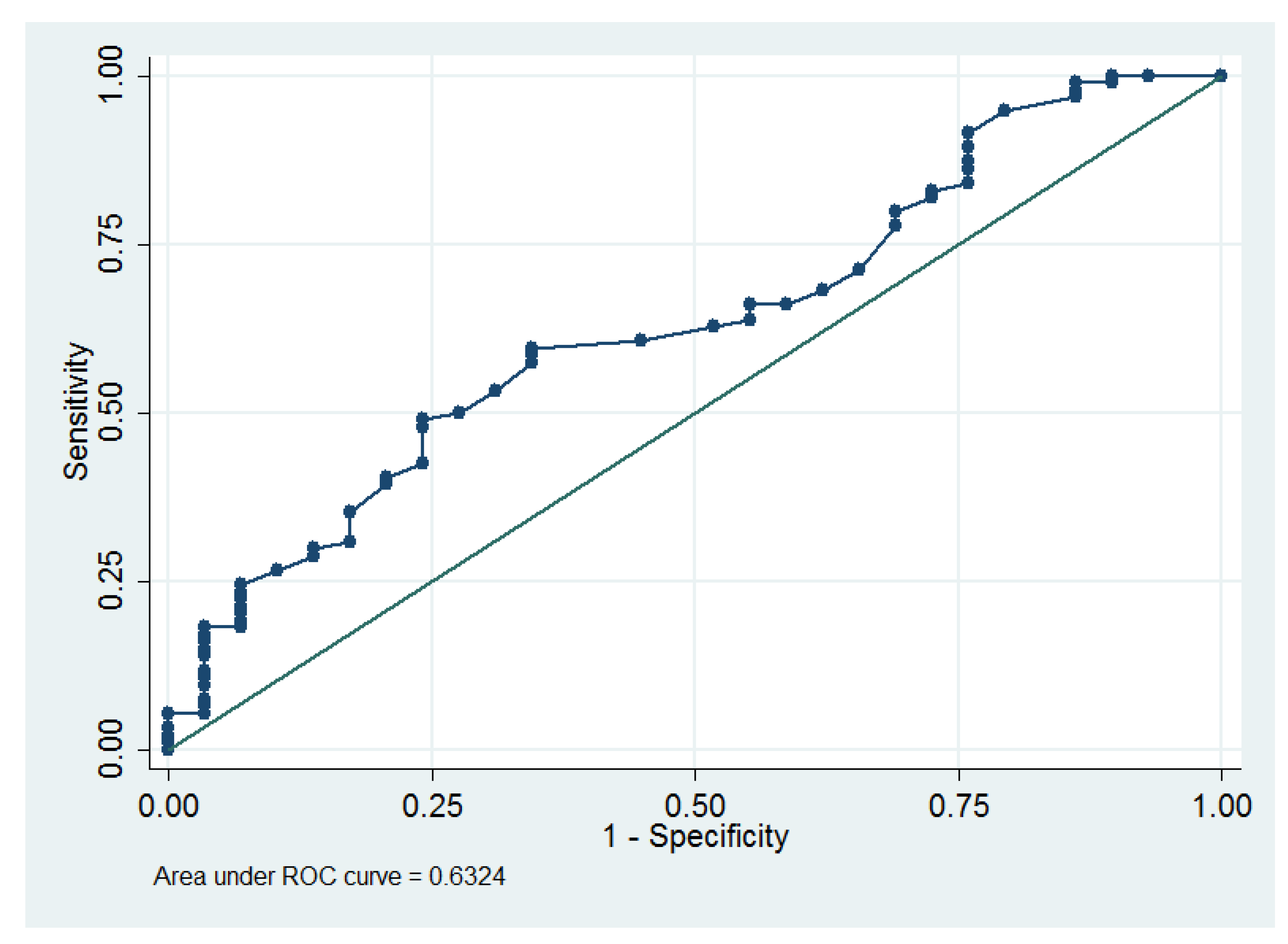
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rooney, K.; Kenton, K.; Mueller, E.R.; FitzGerald, M.P.; Brubaker, L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am. J. Obstet. Gynecol. 2006, 195, 1837–1840. [Google Scholar] [CrossRef]
- Elliott, C.S.; Yeh, J.; Comiter, C.V.; Chen, B.; Sokol, E.R. The Predictive Value of a Cystocele for Concomitant Vaginal Apical Prolapse. J. Urol. 2013, 189, 200–203. [Google Scholar] [CrossRef]
- Eilber, K.S.; Alperin, M.; Khan, A.; Wu, N.; Pashos, C.L.; Clemens, J.Q.; Anger, J.T. Outcomes of Vaginal Prolapse Surgery among Female Medicare Beneficiaries: The role of apical support. Obstet. Gynecol. 2013, 122, 981–987. [Google Scholar] [CrossRef]
- Cagnacci, A.; Palma, F.; Napolitano, A.; Xholli, A. Association between pelvic organ prolapse and climacteric symptoms in postmenopausal women. Maturitas 2017, 99, 73–78. [Google Scholar] [CrossRef]
- Rahkola-Soisalo, P.; Mikkola, T.; Altman, D.; Falconer, C.; Nordic TVM Group. Pelvic Organ Prolapse Repair Using the Uphold Vaginal Support System: 5-Year Follow-Up. Female Pelvic Med. Reconstr. Surg. 2019, 25, 200–205. [Google Scholar] [CrossRef]
- Altman, D.; Mikkola, T.S.; Bek, K.M.; Rahkola-Soisalo, P.; Gunnarsson, J.; Engh, M.E.; Falconer, C. Pelvic organ prolapse repair using the Uphold™ Vaginal Support System: A 1-year multicenter study. Int. Urogynecol. J. 2016, 27, 1337–1345. [Google Scholar] [CrossRef]
- Long, C.-Y.; Wang, C.-L.; Wu, M.-P.; Wu, C.-H.; Lin, K.-L.; Liu, C.-M.; Tsai, E.-M.; Shen, C.-J. Comparison of Clinical Outcomes Using “Elevate Anterior” versus “Perigee” System Devices for the Treatment of Pelvic Organ Prolapse. BioMed Res. Int. 2015, 2015, 479610. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Takes Action to Protect Women’s Health, Orders Manufacturers of Surgical Mesh Intended for Transvaginal Repair of Pelvic Organ Prolapse to Stop Selling All Devices. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal (accessed on 8 January 2022).
- Chang, T.-C.; Hsiao, S.-M.; Chen, C.-H.; Wu, W.-Y.; Lin, H.-H. Clinical Outcomes and Urodynamic Effects of Tailored Transvaginal Mesh Surgery for Pelvic Organ Prolapse. BioMed Res. Int. 2015, 2015, 191258. [Google Scholar] [CrossRef]
- Zhu, L.; Lang, J.; Sun, Z.; Ren, C.; Liu, X.; Li, B. Pelvic reconstruction with mesh for advanced pelvic organ prolapse: A new economic surgical method. Menopause 2011, 18, 328–332. [Google Scholar] [CrossRef]
- Nishizawa, O.; Kato, H.; Ishizuka, O. Advantages of self-tailored mesh for vaginal prolapse. Int. J. Urol. 2012, 19, 494–495. [Google Scholar] [CrossRef][Green Version]
- Gonocruz, S.G.; Hayashi, T.; Tokiwa, S.; Sawada, Y.; Okada, Y.; Yoshio, Y.; Krisna, R.; Kitagawa, Y.; Shimizu, Y.; Nomura, M. Transvaginal surgery using self-cut mesh for pelvic organ prolapse: 3-year clinical outcomes. Int. J. Urol. 2019, 26, 731–736. [Google Scholar] [CrossRef]
- Rogowski, A.; Kluz, T.; Szafarowska, M.; Mierzejewski, P.; Sienkiewicz-Jarosz, H.; Samochowiec, J.; Bienkowski, P.; Baranowski, W. Efficacy and safety of the Calistar and Elevate anterior vaginal mesh procedures. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 239, 30–34. [Google Scholar] [CrossRef]
- Naumann, G.; Hüsch, T.; Mörgeli, C.; Kolterer, A.; Tunn, R. Mesh-augmented transvaginal repair of recurrent or complex anterior pelvic organ prolapse in accordance with the SCENIHR opinion. Int. Urogynecol. J. 2021, 32, 819–827. [Google Scholar] [CrossRef]
- Mateu-Arrom, L.; Gutiérrez-Ruiz, C.; Palou Redorta, J.; Errando-Smet, C. Pelvic organ prolapse repair with mesh: Description of surgical technique using the Surelift® Anterior Repair System. Urol. Int. 2021, 105, 137–142. [Google Scholar] [CrossRef]
- Skorupska, K.A.; Futyma, K.; Bogusiewicz, M.; Rechberger, E.; Ziętek-Strobl, A.; Miotła, P.; Wróbel, A.; Rechberger, T. Four-arm polypropylene mesh for vaginal vault prolapse-surgical technique and outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 203–210. [Google Scholar] [CrossRef]
- Weintraub, A.Y.; Neuman, M.; Reuven, Y.; Neymeyer, J.; Marcus-Braun, N. Efficacy and safety of skeletonized mesh implants for advanced pelvic organ prolapse: 12-month follow-up. World J. Urol. 2016, 34, 1491–1498. [Google Scholar] [CrossRef]
- Gon, L.M.; Riccetto, C.L.Z.; Neto, F.C.; Achermann, A.P.P.; Pereira, T.A.; Palma, P.C.R. Sacrospinous hysteropexy with an autologous rectus fascia sling for treatment of advanced apical pelvic organ prolapse. Int. Urogynecol. J. 2021, 32, 2291–2293. [Google Scholar] [CrossRef]
- Chaus, F.M.; Funk, J.T.; Pangilinan, J.; Lin, F.C.; Twiss, C.O. Total Autologous Fascia Lata Anterior and Apical Pelvic Organ Prolapse Repair: A New Technique and Initial Experience. Urology 2020, 137, 190–195. [Google Scholar] [CrossRef]
- Vasudeva, P.; Tyagi, V.; Kumar, N.; Yadav, S.; Prasad, V.; Iyer, S.G. “Mesh free” autologous transobturator mid urethral sling placement for predominant stress urinary incontinence: A pilot study. Neurourol. Urodyn. 2021, 40, 659–665. [Google Scholar] [CrossRef]
- Cormio, L.; Mancini, V.; Liuzzi, G.; Lucarelli, G.; Carrieri, G. Cystocele Repair by Autologous Rectus Fascia Graft: The Pubovaginal Cystocele Sling. J. Urol. 2015, 194, 721–727. [Google Scholar] [CrossRef]
- Lo, T.-S.; Karim, N.B.; Cortes, E.F.M.; Wu, P.-Y.; Lin, Y.-H.; Tan, Y.L. Comparison between Elevate Anterior/Apical system and Perigee system in pelvic organ prolapse surgery: Clinical and sonographic outcomes. Int. Urogynecol. J. 2015, 26, 391–400. [Google Scholar] [CrossRef]
- Vu, M.K.; Letko, J.; Jirschele, K.; Gafni-Kane, A.; Nguyen, A.; Du, H.; Goldberg, R.P. Minimal mesh repair for apical and anterior prolapse: Initial anatomical and subjective outcomes. Int. Urogynecol. J. 2012, 23, 1753–1761. [Google Scholar] [CrossRef]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall: London, UK, 1991; pp. 403–405. [Google Scholar]
- Kato, K.; Suzuki, S.; Ishiyama, A.; Kawanishi, H.; Matsui, H.; Kato, T.; Hirabayashi, H.; Hattori, R. Mesh exposure after transvaginal mesh prolapse surgery: Out of permissible range? Int. J. Urol. 2021, 28, 202–207. [Google Scholar] [CrossRef]
- Vergeldt, T.F.M.; van Kuijk, S.; Notten, K.J.B.; Kluivers, K.B.; Weemhoff, M. Anatomical Cystocele Recurrence: Development and internal validation of a prediction model. Obstet Gynecol. 2016, 127, 341–347. [Google Scholar] [CrossRef]
- Friedman, T.; Eslick, G.D.; Dietz, H.P. Risk factors for prolapse recurrence: Systematic review and meta-analysis. Int. Urogynecol. J. 2018, 29, 13–21. [Google Scholar] [CrossRef]
- Long, C.-Y.; Lo, T.-S.; Wang, C.-L.; Wu, C.-H.; Liu, C.-M.; Su, J.-H. Risk factors of surgical failure following transvaginal mesh repair for the treatment of pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 161, 224–227. [Google Scholar] [CrossRef]
- Price, N.; Slack, A.; Jwarah, E.; Jackson, S. The incidence of reoperation for surgically treated pelvic organ prolapse: An 11-year experience. Menopause Int. 2008, 14, 145–148. [Google Scholar] [CrossRef]
- Nüssler, E.; Eskildsen, J.K.; Bixo, M.; Löfgren, M. Impact of surgeon experience on routine prolapse operations. Int. Urogynecol. J. 2018, 29, 297–306. [Google Scholar] [CrossRef]
- Khrucharoen, U.; Ramart, P.; Choi, J.; Kang, D.; Kim, J.-H.; Raz, S. Clinical predictors and risk factors for vaginal mesh extrusion. World J. Urol. 2018, 36, 299–304. [Google Scholar] [CrossRef]
- Berglund, A.-L.; Eisemann, M.; Lalos, A.; Lalos, O. Social adjustment and spouse relationships among women with stress incontinence before and after surgical treatment. Soc. Sci. Med. 1996, 42, 1537–1544. [Google Scholar] [CrossRef]
- Hsiao, S.-M.; Lin, H.-H. Impact of the mid-urethral sling for stress urinary incontinence on female sexual function and their partners’ sexual activity. Taiwan J. Obstet. Gynecol. 2018, 57, 853–857. [Google Scholar] [CrossRef]
- Huang, A.J.; Subak, L.L.; Thom, D.H.; Eeden, S.K.V.D.; Ragins, A.I.; Kuppermann, M.; Shen, H.; Brown, J.S. Sexual Function and Aging in Racially and Ethnically Diverse Women. J. Am. Geriatr. Soc. 2009, 57, 1362–1368. [Google Scholar] [CrossRef]
- Ehsani, N.; Ghafar, M.A.; Antosh, D.D.; Tan-Kim, J.; Warner, W.B.; Mamik, M.M.; Brown, H.; Chung, C.P.; Segal, S.; Abed, H.; et al. Risk Factors for Mesh Extrusion After Prolapse Surgery: A case-control study. Female Pelvic Med. Reconstr. Surg. 2012, 18, 357–361. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Narimoto, K.; Urata, S.; Takeyama, M.; Kadono, Y.; Mizokami, A. Predictors of persistent stress urinary incontinence after transvaginal mesh repair. BMC Womens Health 2018, 18, 174. [Google Scholar] [CrossRef]
- Seki, N.; Shahab, N.; Hara, R.; Takei, M.; Yamaguchi, A.; Naito, S. Voiding dynamics in women with stress urinary incontinence and high-stage cystocele. Int. J. Urol. 2011, 18, 219–224. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Narimoto, K.; Urata, S.; Kawaguchi, S.; Kuribayashi, M.; Namiki, M. High urinary flow in women with stress incontinence: Corrected flow–age nomogram evaluation after a transobturator tape procedure. Int. Urogynecol. J. 2016, 27, 1075–1080. [Google Scholar] [CrossRef]
- Pilsetniece, Z.; Vjaters, E. The role of conventional urodynamic in diagnosing specific types of urinary incontinence in women. Turk. J. Urol. 2020, 46, 134–139. [Google Scholar] [CrossRef]
- de Boer, T.A.; Kluivers, K.B.; Withagen, M.I.J.; Milani, A.L.; Vierhout, M.E. Predictive factors for overactive bladder symptoms after pelvic organ prolapse surgery. Int. Urogynecol. J. 2010, 21, 1143–1149. [Google Scholar] [CrossRef]
- de Boer, T.A.; Vierhout, M.E. Predictors for overactive bladder symptoms after pelvic organ prolapse surgery. Curr. Opin. Obstet. Gynecol. 2011, 23, 366–370. [Google Scholar] [CrossRef]
- Tomoe, H. Improvement of overactive bladder symptoms after tension-free vaginal mesh operation in women with pelvic organ prolapse: Correlation with preoperative urodynamic findings. Int. J. Urol. 2015, 22, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Barber, M.D.; Parasio, M.F.R.; Walters, M.D.; Weidner, A.C.; Amundsen, C.L. A prospective assessment of overactive bladder symptoms in a cohort of elderly women who underwent transvaginal surgery for advanced pelvic organ prolapse. Am. J. Obstet. Gynecol. 2007, 197, 82.e1–82.e4. [Google Scholar] [CrossRef] [PubMed]
- Ugianskiene, A.; Kjærgaard, N.; Larsen, T.; Glavind, K. What happens to urinary incontinence after pelvic organ prolapse surgery? Int. Urogynecol. J. 2019, 30, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
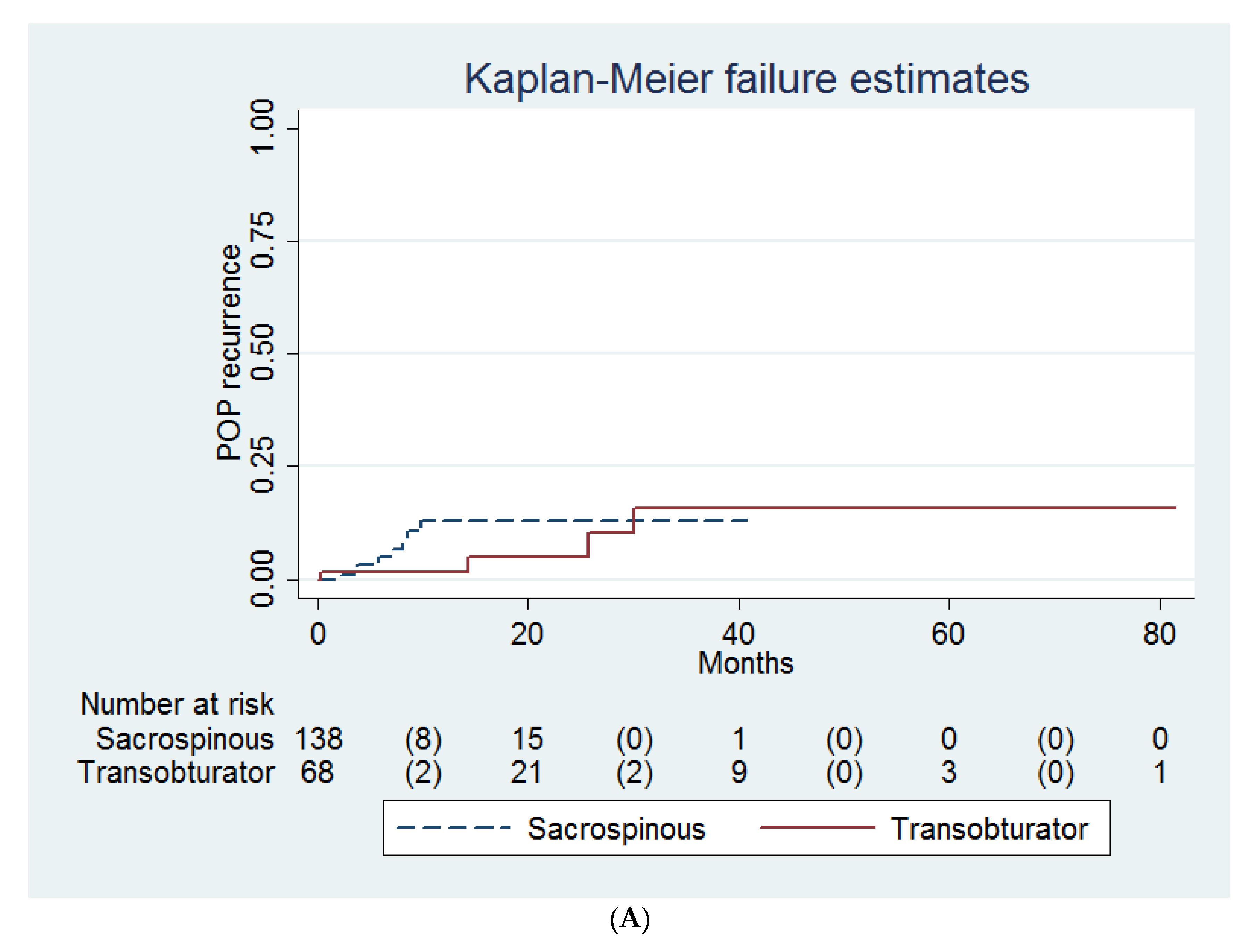
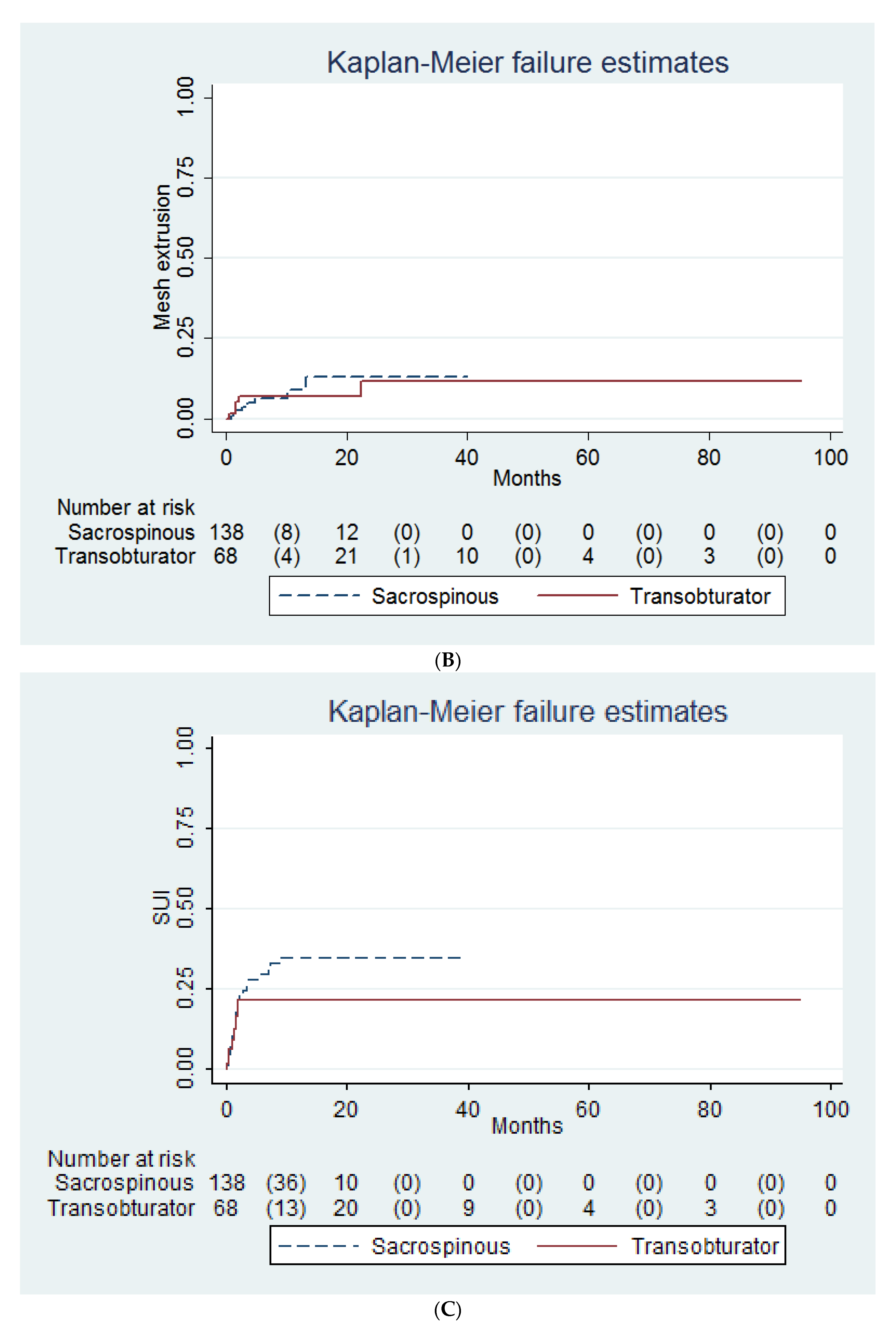
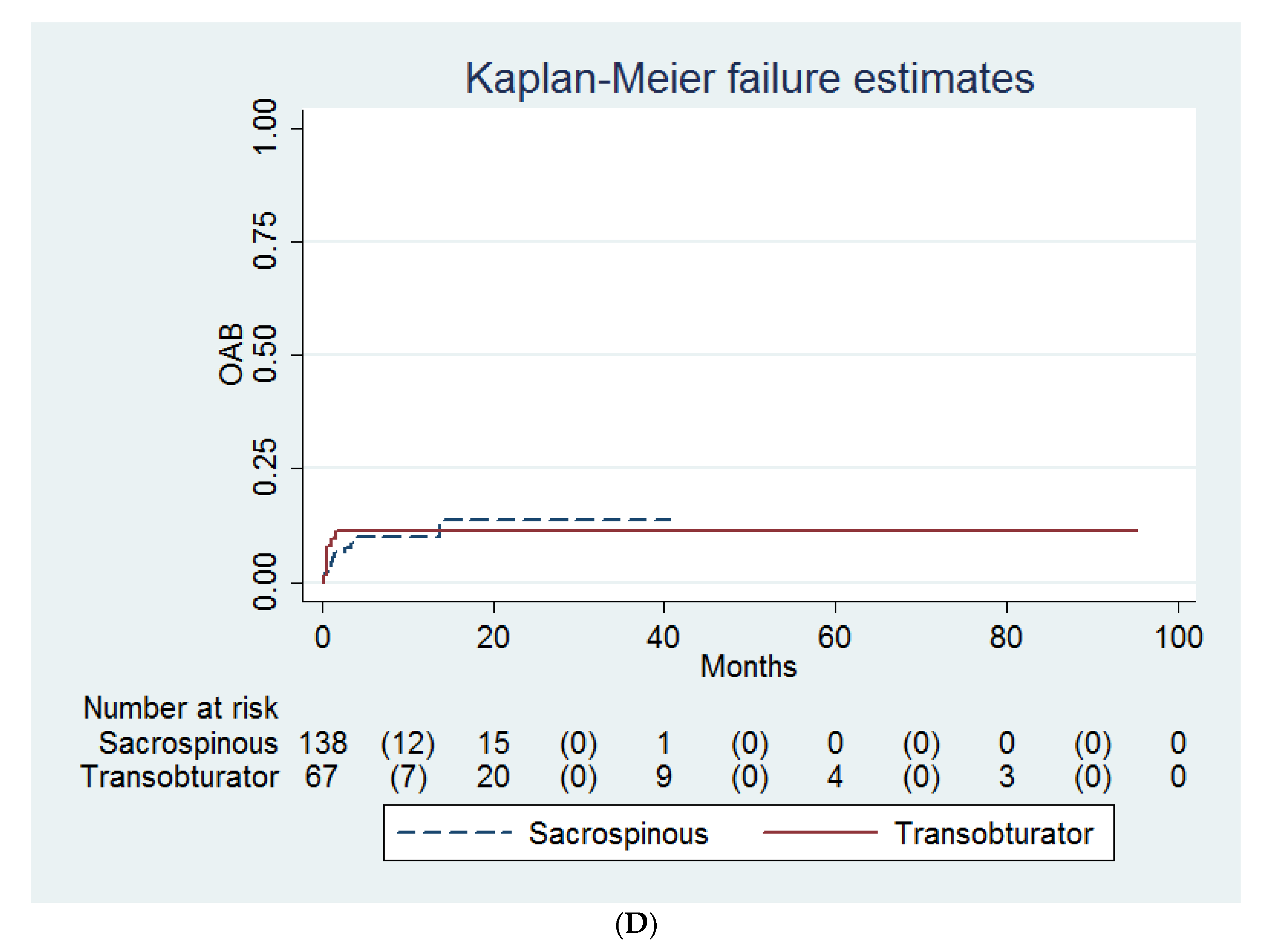
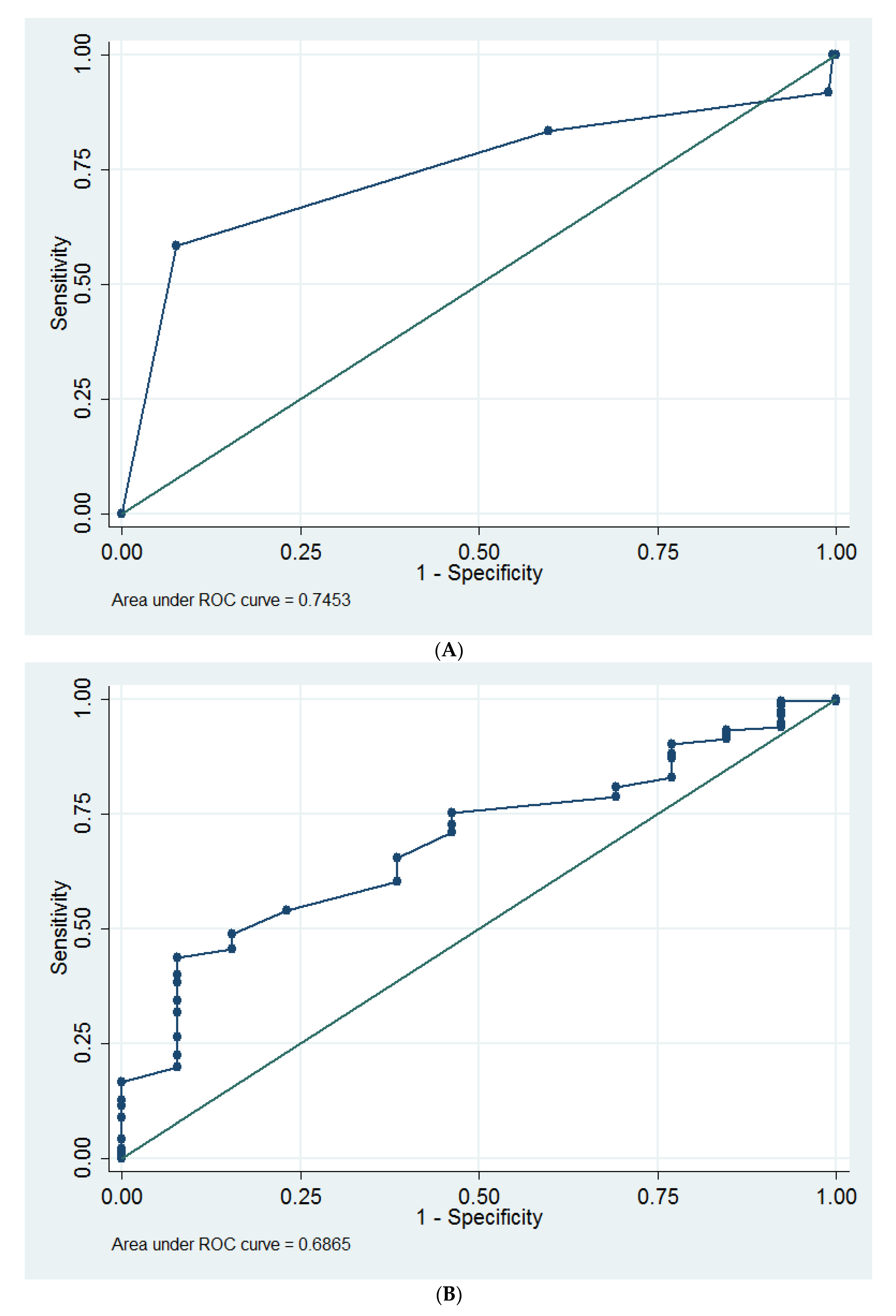
| Variables | Transobturator (n = 68) | Sacrospinous (n = 138) | † p |
|---|---|---|---|
| Age (years) | 64.1 ± 8.0 | 64.4 ± 9.9 | 0.56 |
| Menopause | 65 (96) | 120 (87) | 0.08 |
| BMI (kg/m2) | 25.0 ± 3.0 | 24.8 ± 3.7 | 0.50 |
| Parity | 3.1 ± 1.2 | 3.1 ± 1.2 | 0.93 |
| Diabetes | 15 (22) | 42 (30) | 0.21 |
| Hypertension | 41 (60) | 64 (46) | 0.06 |
| Prior hysterectomy | 10 (15) | 14 (10) | 0.34 |
| Prior POP surgery | 6 (9) | 10 (7) | 1.00 |
| ≥stage II cystocele | 67 (99) | 131 (95) | 0.67 |
| ≥stage II uterine prolapse | 47 (69) | 126 (91) | 0.005 |
| ≥stage II rectocele | 30 (44) | 88 (65) | 0.005 |
| Pad weight (g) | 17.5 ± 39.5 | 24.8 ± 52.9 | 0.48 |
| Qmax (mL/s) | 21.9 ± 10.9 | 19.8 ± 11.5 | 0.26 |
| Voided volume (mL) | 292 ± 164 | 261 ± 156 | 0.25 |
| Post-void residual (mL) | 121 ± 84 | 113 ± 81 | 0.62 |
| PdetQmax (cmH2O) | 27.0 ± 17.1 | 35.1 ± 19.2 | 0.01 |
| Detrusor overactivity | 14 (21) | 18 (13) | 0.052 |
| MUCP (cmH2O) | 60.1 ± 32.0 | 72.3 ± 44.8 | 0.10 |
| SUI | 28 (37) | 62 (45) | 0.76 |
| OAB | 13 (19) | 35 (24) | 0.64 |
| VD | 15 (22) | 45 (33) | 0.12 |
| VTH | 29 (43) | 39 (28) | <0.001 |
| MUS | 7(10) | 20 (14) | 0.40 |
| Posterior colporrhaphy | 37 (54) | 109 (79) | <0.001 |
| Operative time (mins) | 108 ± 39 | 119 ± 36 | 0.65 |
| Blood loss (mL) | 101 ± 104 | 142 ± 211 | 0.17 |
| Perioperative Complications | |||
| Bladder perforation | 0 (0) | 1 (1) | 1.00 |
| Massive bleeding | 0 (0) | 1 (1) | 1.00 |
| Clavien-Dindo classification | |||
| Grade II | 0 (0) | 1 (1) | 1.00 |
| Grade IIIb | 0 (0) | 1 (1) | 1.00 |
| Follow-up interval (months) | 19.1 ± 23.3 | 8.2 ± 8.7 | 0.01 |
| Recurrence of POP | 4 (6) | 8 (2) | 0.42 ‡ |
| Mesh extrusion | 5 (7) | 8 (9) | 0.97 ‡ |
| Dysuria/UTI | 7 (10) | 9 (2) | 0.34 |
| De novo dyspareunia | 1 (1) | 1(1) | 0.55 |
| Surgeon | |||
| A | 19 (28) | 86 (62) | <0.001 |
| B | 33 (49) | 26 (19) | |
| C | 7 (10) | 23 (17) | |
| D | 9 (13) | 0 (0) | |
| E | 0 (0) | 3 (2) |
| Recurrence of POP | Mesh Extrusion | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| Variables | HR (95% CI) | p † | HR (95% CI) | p ‡ | HR (95% CI) | p † | HR (95% CI) | p ‡ |
| Transobturator method | 0.60 (0.17, 2.11) | 0.43 | - | - | 0.98 (0.31, 3.08) | 0.97 | - | - |
| Age (years) | 1.03 (0.97, 1.09) | 0.40 | - | - | 0.94 (0.89, 1.00) | 0.04 | 0.94 (0.89, 1.00) | 0.04 |
| Menopause | 6.94 × 1015 (0, infinity) | 1.00 | - | - | 1.17 (0.15, 9.09) | 0.88 | - | - |
| Parity | 1.17 (0.76, 1.80) | 0.48 | - | - | 1.04 (0.68, 1.61) | 0.84 | - | - |
| BMI (kg/m2) | 1.07 (0.92, 1.24) | 0.39 | - | - | 0.98 (0.84, 1.14) | 0.80 | - | - |
| Hypertension | 1.41 (0.45, 4.46) | 0.56 | - | - | 1.64 (0.53, 5.01) | 0.39 | - | - |
| Diabetes | 1.04 (0.28, 3.86) | 0.95 | - | - | 1.31 (0.40, 4.26) | 0.66 | - | - |
| Prior hysterectomy | 0.57 (0.07, 4.46) | 0.59 | - | - | 0.61 (0.08, 4.72) | 0.64 | - | - |
| Prior POP surgery | 1.21 (0.16, 9.43) | 0.85 | - | - | 1.10 (0.14, 8.46) | 0.93 | - | - |
| Cystocele stage | 6.17 (2.25, 16.91) | <0.001 | 8.80 (2.15, 36.09) | 0.003 | 1.01 (0.44, 2.29) | 0.99 | - | - |
| Apical prolapse stage § | 2.78 (1.44, 5.33) | 0.002 | - | - | 0.74 (0.46, 1.19) | 0.22 | - | - |
| Pad weight (g) | 1.00 (0.99, 1.02) | 0.45 | - | - | 1.00 (0.99, 1.01) | 0.90 | - | - |
| Qmax (mL/s) | 1.03 (0.98, 1.08) | 0.29 | - | - | 0.99 (0.94, 1.04) | 0.71 | - | - |
| Voided volume (mL) | 1.00 (1.00, 1.00) | 0.99 | - | - | 1.00 (1.00, 1.00) | 0.30 | - | - |
| Post-void residual (mL) | 1.01 (1.00, 1.01) | 0.16 | - | - | 1.01 (1.00, 1.01) | 0.11 | - | - |
| PdetQmax (cmH2O) | 0.99 (0.95, 1.05) | 0.81 | - | - | 0.98 (0.93, 1.02) | 0.26 | - | - |
| Detrusor overactivity | 0.93 (0.20, 4.36) | 0.92 | - | - | 0.89 (0.19, 4.14) | 0.89 | - | - |
| MUCP(cmH2O) | 1.01 (0.99, 1.02) | 0.28 | - | - | 1.00 (0.98, 1.01) | 0.74 | - | - |
| SUI | 0.87 (0.26, 2.88) | 0.81 | - | - | 2.03 (0.68, 6.04) | 0.21 | - | - |
| OAB | 1.06 (0.29, 3.94) | 0.93 | - | - | 0.96 (0.26, 3.47) | 0.95 | - | - |
| VD | 3.16 (1.02, 9.84) | 0.047 | 2.77 (0.76, 10.10) | 0.12 | 0.81 (0.22, 2.94) | 0.75 | - | - |
| VTH | 0.24 (0.03, 1.86) | 0.17 | - | - | 0.49 (0.11, 2.23) | 0.36 | - | - |
| Mid-urethral sling | 1.31 (0.29, 6.03) | 0.73 | - | - | 0.54 (0.07, 4.15) | 0.55 | - | - |
| Surgeon ¶ | ||||||||
| A (reference) | 1.00 | - | 1.00 | - | 1.00 | - | - | - |
| B | 9.,95 (1.19, 83.1) | 0.03 | 3.90 (0.44, 34.43) | 0.22 | 1.79 (0.52, 6.19) | 0.36 | - | - |
| C | 14.1 (1.57, 127.08) | 0.02 | 2.90 (0.21, 39.88) | 0.43 | 1.90 (0.45, 7.98) | 0.38 | - | - |
| D | 8.66 × 10−19 (-, -) | - | 9.89 × 10−19 (-, -) | - | 2.01 × 10−15 (0, infinity) | 1.00 | - | - |
| E | 36.07 (2.20, 591.54) | 0.01 | 804.60 (21.63, 29,924.48) | <0.001 | 2.00 × 10−15 (0, infinity) | 1.00 | - | - |
| Postoperative SUI | Postoperative OAB | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| Variables | HR (95% CI) | p † | HR (95% CI) | p ‡ | HR (95% CI) | p † | HR (95% CI) | p ‡ |
| Transobturator fixation | 0.69 (0.36, 1.29) | 0.24 | - | - | 1.17 (0.46, 2.98) | 0.75 | - | - |
| Age (years) | 1.01 (0.98, 1.04) | 0.74 | - | - | 1.02 (0.97, 1.07) | 0.47 | - | - |
| Menopause | 0.91 (0.36, 2.29) | 0.84 | - | - | 1.95 (0.26, 14.63) | 0.52 | - | - |
| Parity | 0.92 (0.72, 1.17) | 0.48 | - | - | 0.91 (0.63, 1.33) | 0.64 | - | - |
| BMI (kg/m2) | 1.04 (0.96, 1.12) | 0.33 | - | - | 0.93 (0.81, 1.07) | 0.31 | - | - |
| Hypertension | 0.98 (0.56, 1.72) | 0.96 | - | - | 0.57 (0.23, 1.45) | 0.24 | - | - |
| Diabetes | 1.40 (0.77, 2.55) | 0.27 | - | - | 0.49 (0.14, 1.69) | 0.26 | - | - |
| Prior hysterectomy | 1.48 (0.66, 3.28) | 0.34 | - | - | 3.30 (1.18, 9.19) | 0.02 | 2.58 (0.91, 7.28) | 0.07 |
| Prior POP surgery | 0.80 (0.25, 2.57) | 0.71 | - | - | 1.56 (0.36, 6.74) | 0.56 | - | - |
| Cystocele stage | 1.09 (0.73, 1.61) | 0.68 | - | - | 0.86 (0.45, 1.64) | 0.64 | - | - |
| Apical prolapse stage | 0.93 (0.73, 1.19) | 0.56 | - | - | 0.84 (0.57, 1.24) | 0.38 | - | - |
| Pad weight (g) | 0.99 (0.98, 1.00) | 0.12 | - | - | 1.00 (0.99, 1.01) | 0.45 | - | - |
| Qmax (mL/s) | 1.02 (1.00, 1.05) | 0.07 | 1.03 (1.00, 1.07) | 0.04 | 1.01 (0.97, 1.06) | 0.59 | - | - |
| Voided volume (mL) | 1.00 (1.00, 1.00) | 0.48 | - | - | 1.00 (1.00, 1.00) | 0.46 | - | - |
| Post-void residual (mL) | 1.00 (1.00, 1.00) | 0.74 | - | - | 1.00 (0.99, 1.00) | 0.54 | - | - |
| PdetQmax (cmH2O) | 0.97 (0.95, 1.00) | 0.02 | 0.98 (0.96, 1.01) | 0.15 | 0.99 (0.96, 1.02) | 0.53 | - | - |
| Detrusor overactivity | 0.43 (0.15, 1.21) | 0.11 | - | - | 1.83 (0.64, 5.28) | 0.26 | - | - |
| MUCP (cmH2O) | 0.99 (0.98, 1.00) | 0.01 | 0.99 (0.98, 1.01) | 0.38 | 1.00 (0.99, 1.01) | 0.90 | - | - |
| SUI | 1.12 (0.64, 2.01) | 0.68 | - | - | 1.63 (0.66, 4.01) | 0.29 | - | - |
| OAB | 1.06 (0.55, 2.04) | 0.86 | - | - | 3.13 (1.27, 7.71) | 0.01 | 3.22 (1.30, 7.96) | 0.01 |
| VD | 1.38 (0.77, 2.49) | 0.28 | - | - | 1.17 (0.44, 3.07) | 0.75 | - | - |
| VTH | 0.59 (0.28, 1.25) | 0.17 | - | - | 0.16 (0.02, 1.22) | 0.08 | 0.19 (0.02, 1.46) | 0.11 |
| Mid-urethral sling | 0.13 (0.02, 0.92) | 0.04 | 0.15 (0.02, 1.17) | 0.07 | 1.85 (0.61, 5.58) | 0.27 | - | - |
| Surgeon § | ||||||||
| A (reference) | 1.00 | - | 1.00 | - | 1.00 | - | - | |
| B | 0.98 (0.54, 1.78) | 0.94 | 0.99 (0.44, 2.02) | 0.98 | 0.70 (0.25, 1.99) | 0.51 | - | - |
| C | 0.31 (0.09, 1.02) | 0.054 | 0.23 (0.03, 1.79) | 0.16 | 0.56 (0.12, 2.50) | 0.45 | - | - |
| D | 6.28 × 10−17 (0, infinity) | 1.00 | - | - | 1.69 × 10−16 (0, infinity) | 1.00 | - | - |
| E | 6.27 × 10−17 (0, infinity) | 1.00 | 8.30 × 10−19 (0, infinity) | 1.00 | 1.70 × 10−16 (0, infinity) | 1.00 | - | - |
| Univariate | ||
|---|---|---|
| Variables ‡ | Odds Ratio (95% CI) | p † |
| Transobturator fixation | 0.28 (0.03, 2.32) | 0.24 |
| Age (years) | 1.02 (0.95, 1.11) | 0.57 |
| Parity | 1.05 (0.57, 1.93) | 0.89 |
| BMI (kg/m2) | 0.90 (0.72, 1.13) | 0.35 |
| Hypertension | 0.96 (0.23, 3.95) | 0.96 |
| Diabetes | 1.60 (0.37, 6.92) | 0.53 |
| Prior hysterectomy | 1.09 (0.13, 9.24) | 0.94 |
| Prior POP surgery | 1.74 (0.20, 15.12) | 0.61 |
| Cystocele stage | 0.76 (0.26, 2.26) | 0.62 |
| Apical prolapse stage | 1.50 (0.72, 3.13) | 0.27 |
| Pad weight (g) | 1.01 (1.00, 1.02) | 0.26 |
| Qmax (mL/s) | 0.97 (0.90, 1.05) | 0.42 |
| Voided volume (mL) | 1.00 (0.99, 1.00) | 0.18 |
| Post-void residual (mL) | 1.00 (0.99, 1.01) | 0.46 |
| PdetQmax (cmH2O) | 1.02 (0.98, 1.06) | 0.39 |
| Detrusor overactivity | 1.61 (0.30, 8.72) | 0.58 |
| MUCP (cmH2O) | 1.00 (0.98, 1.02) | 0.94 |
| SUI | 1.79 (0.43, 7.37) | 0.42 |
| OAB | 2.04 (0.47, 8.87) | 0.34 |
| VD | 2.54 (0.61, 10.49) | 0.20 |
| VTH | 0.46 (0.06, 3.83) | 0.47 |
| Mid-urethral sling | 2.31 (0.44, 12.06) | 0.32 |
| Surgeon § | ||
| A (reference) | 1.00 | - |
| B | 0.70 (0.13, 3.73) | 0.68 |
| C | 0.69 (0.08, 6.14) | 0.74 |
| D | - | - |
| E | - | - |
| Transobturator (n = 68) | Sacrospinous (n = 138) | |||||
|---|---|---|---|---|---|---|
| Variables | Baseline | After Surgery | p † | Baseline | After Surgery | p † |
| SUI ‡ | 20 (33) | 13 (21) | 0.11 | 36 (31) | 35 (30) | 0.89 |
| OAB | 13 (19) | 8 (12) | 0.20 | 35 (25) | 12 (9) | 0.0001 |
| VD | 15 (22) | 1 (1) | 0.0005 | 45 (33) | 7 (5) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-H.; Tu, F.-C.; Lin, H.-H.; Hsiao, S.-M. Predictors of Clinical Outcome in Women with Pelvic Organ Prolapse Who Underwent Transvaginal Mesh Reconstruction Surgery. Medicina 2022, 58, 148. https://doi.org/10.3390/medicina58020148
Lin T-H, Tu F-C, Lin H-H, Hsiao S-M. Predictors of Clinical Outcome in Women with Pelvic Organ Prolapse Who Underwent Transvaginal Mesh Reconstruction Surgery. Medicina. 2022; 58(2):148. https://doi.org/10.3390/medicina58020148
Chicago/Turabian StyleLin, Ting-Hsuan, Fung-Chao Tu, Ho-Hsiung Lin, and Sheng-Mou Hsiao. 2022. "Predictors of Clinical Outcome in Women with Pelvic Organ Prolapse Who Underwent Transvaginal Mesh Reconstruction Surgery" Medicina 58, no. 2: 148. https://doi.org/10.3390/medicina58020148
APA StyleLin, T.-H., Tu, F.-C., Lin, H.-H., & Hsiao, S.-M. (2022). Predictors of Clinical Outcome in Women with Pelvic Organ Prolapse Who Underwent Transvaginal Mesh Reconstruction Surgery. Medicina, 58(2), 148. https://doi.org/10.3390/medicina58020148






