Clinical, Laboratory, Histological, Radiological, and Metabolic Features and Prognosis of Malignant Pleural Mesothelioma
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Clinical and Laboratory Data Collection
2.3. Diagnostic Procedures
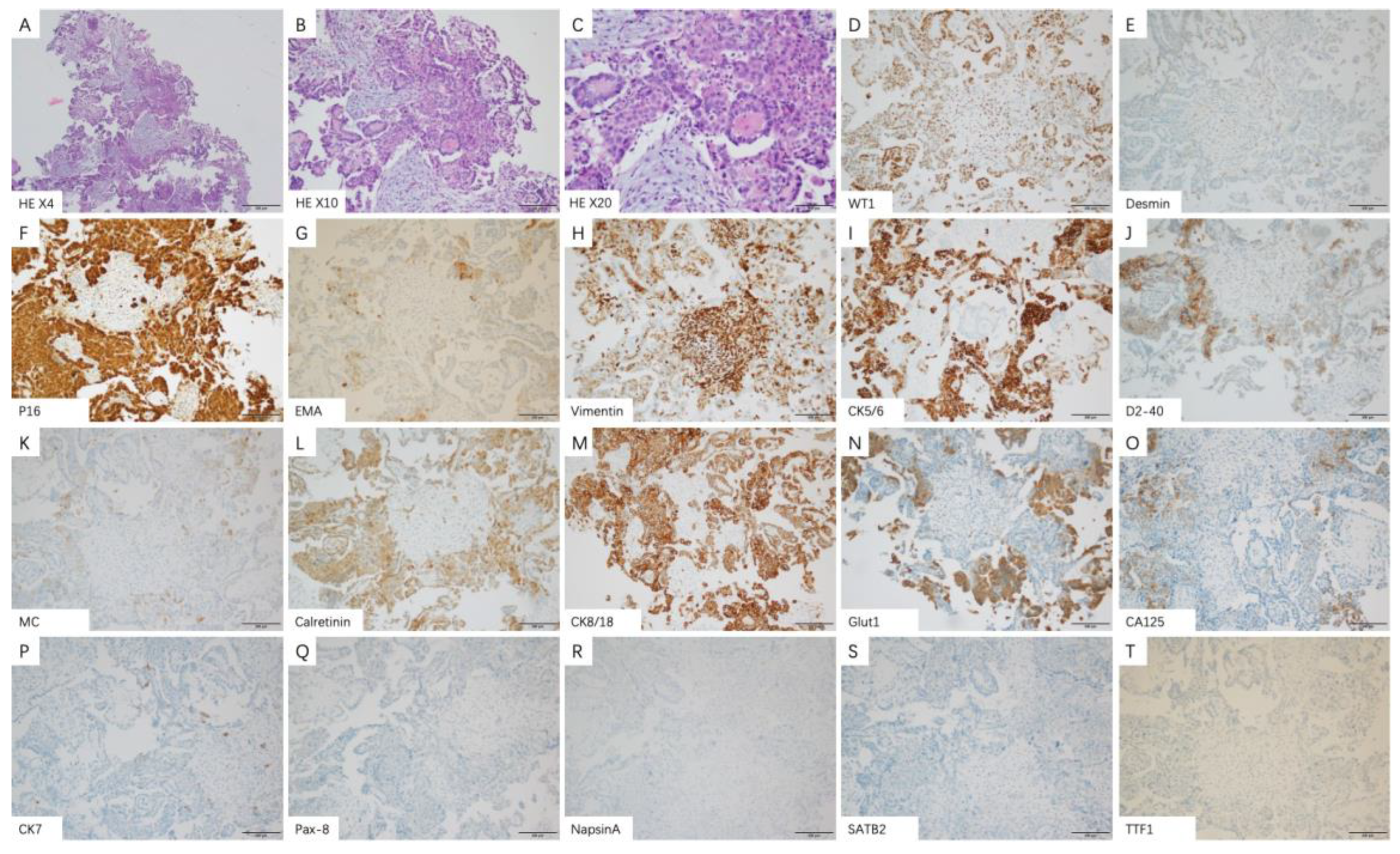
2.4. Histology and Immunohistochemical Staining
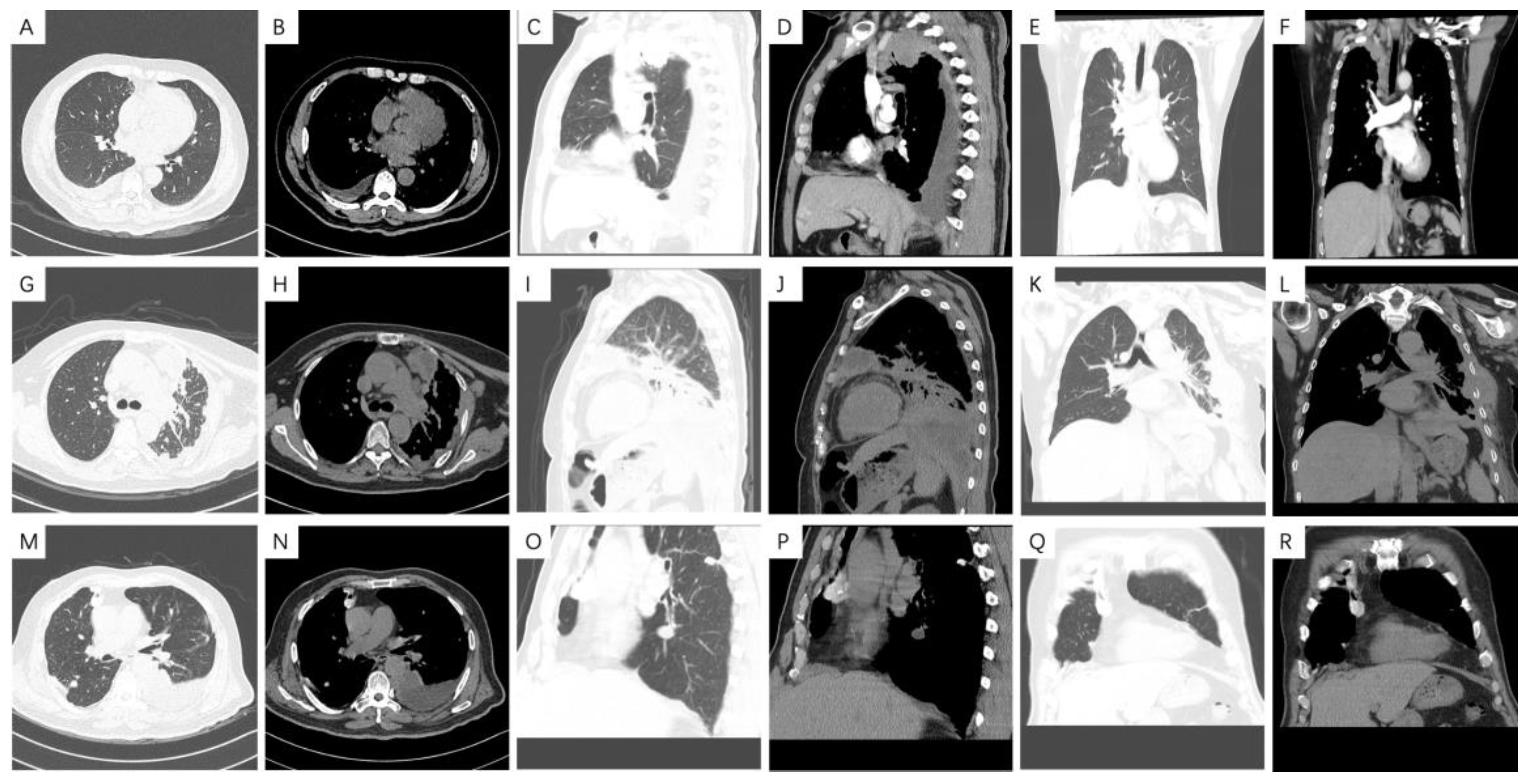
2.5. CT Technique and Image Analysis
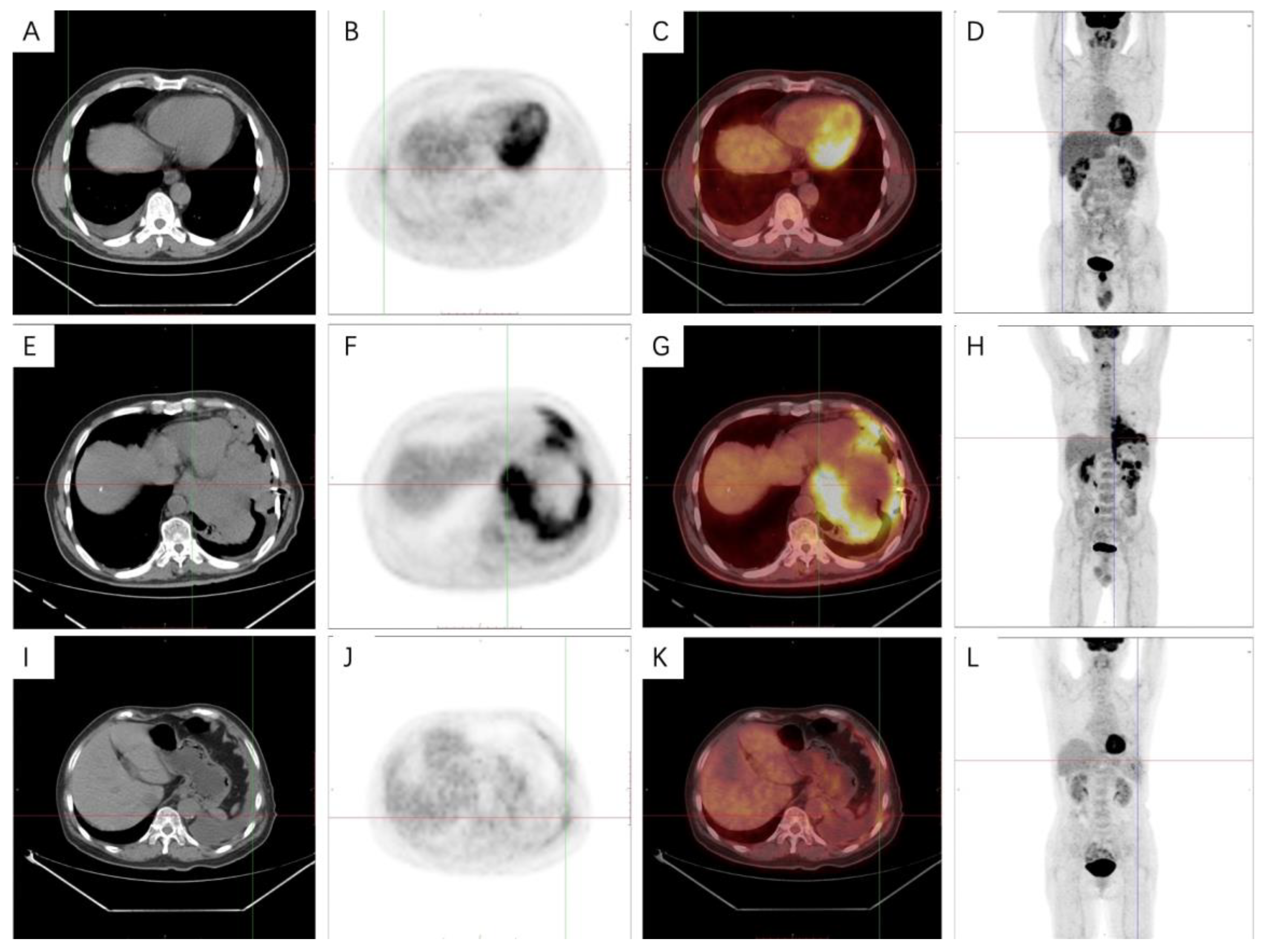
2.6. 18F-FDG PET-CT Technique and Image Analysis
2.7. Clinical Outcome Evaluation
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
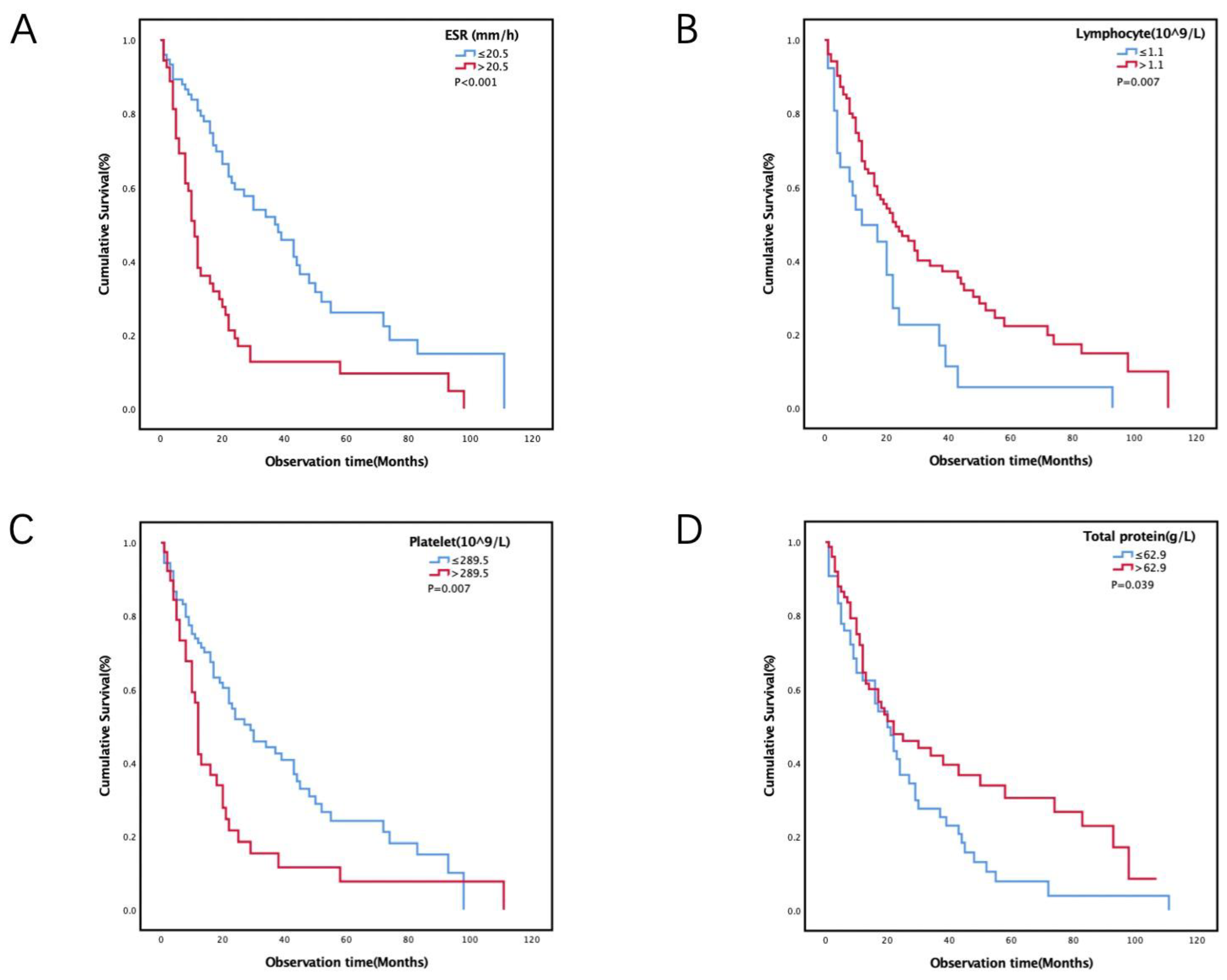
3.2. Cut-off Values for Laboratory Variables and Survival Analysis
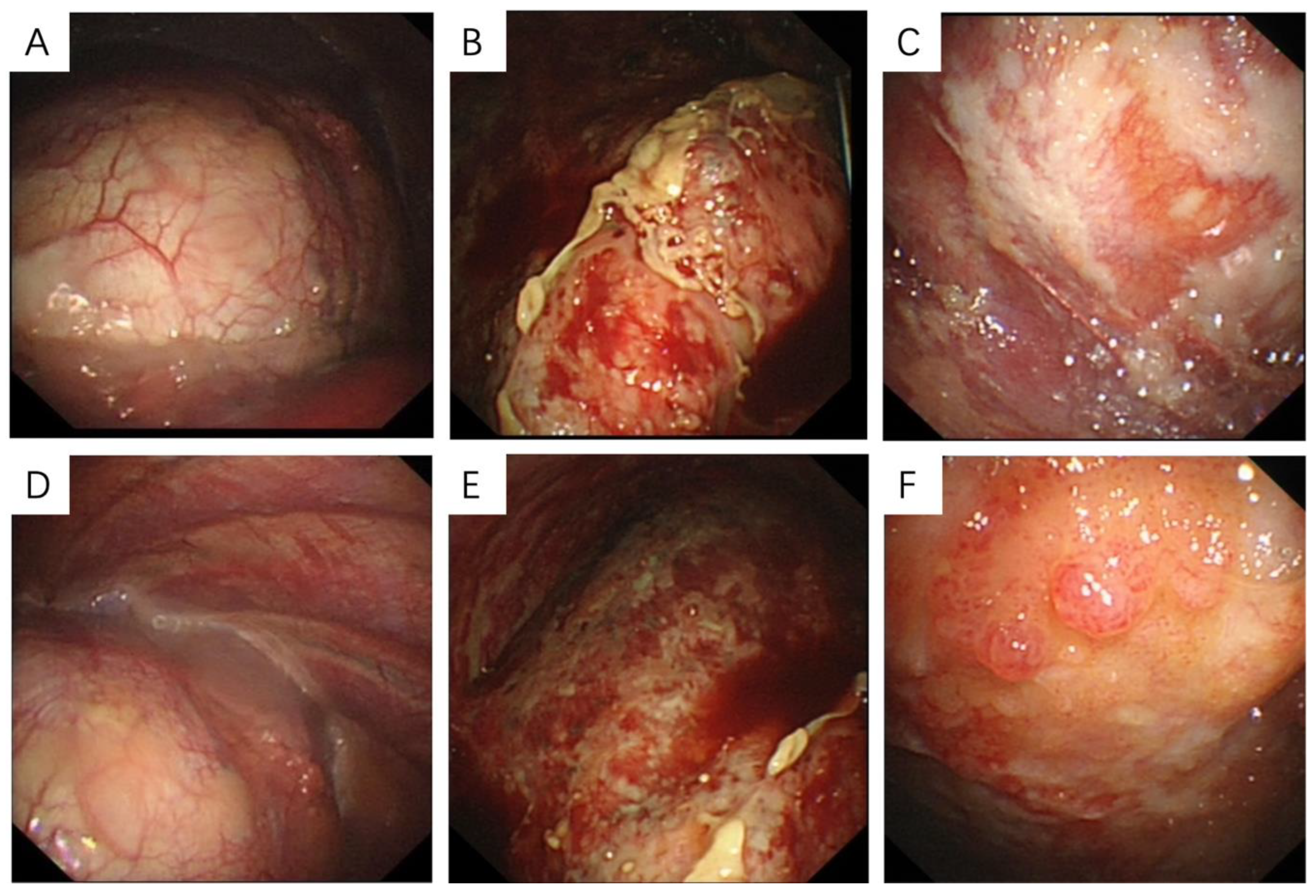
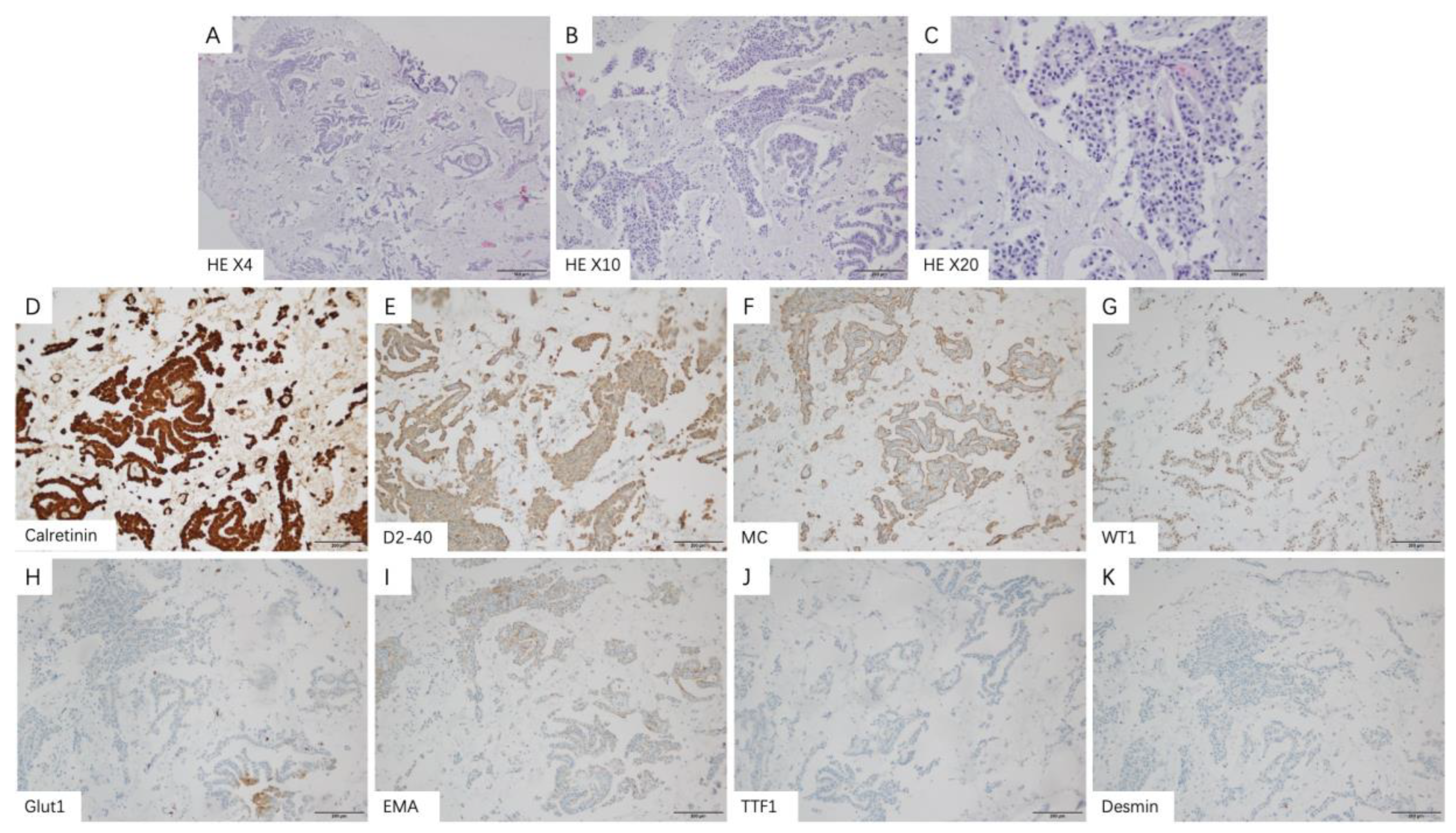
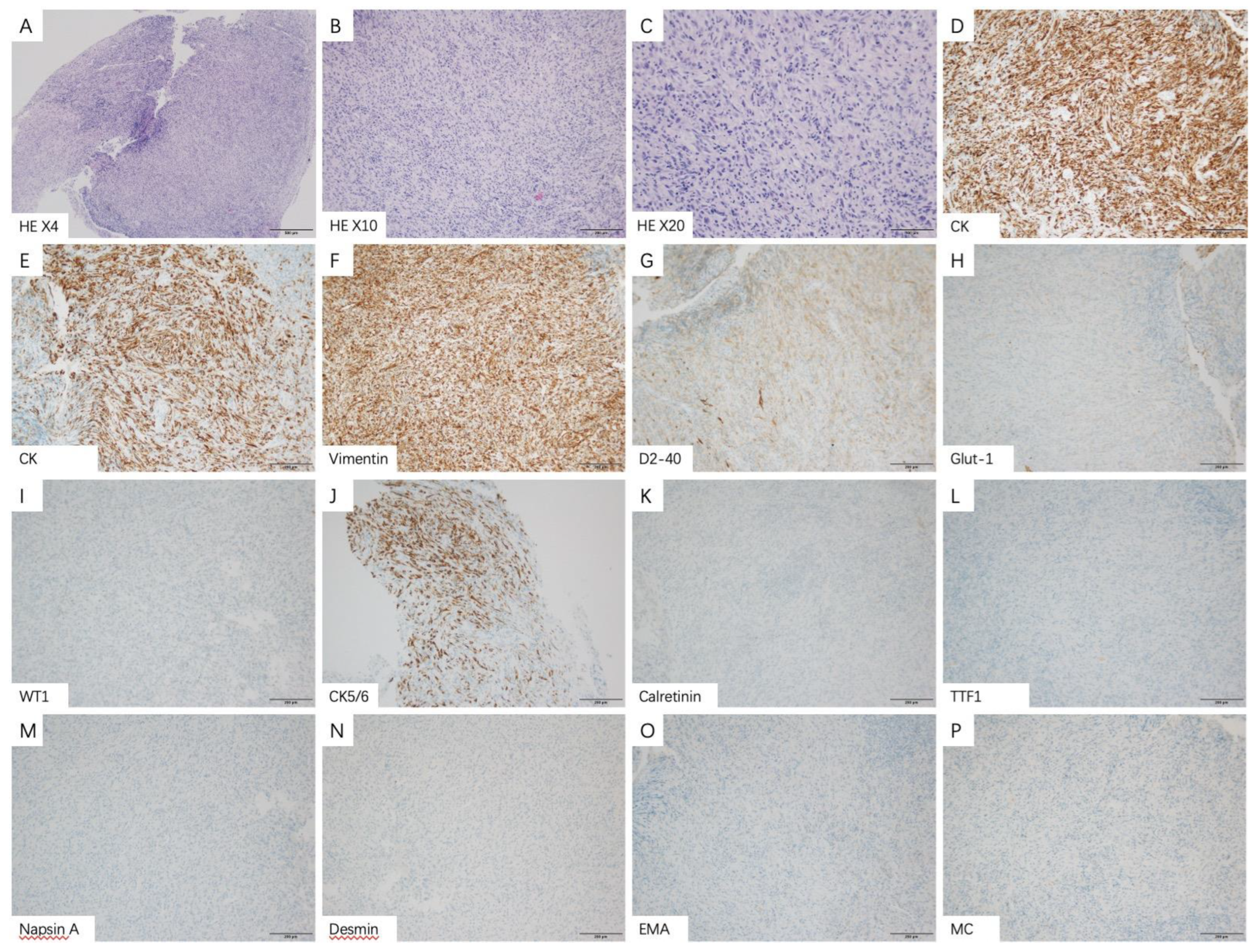
3.3. Thoracoscopic Findings and Pathological Features
3.4. Radiological Features
3.5. PET-CT Metabolic Characteristics
4. Discussion
4.1. Prognostic Risk Factors for Malignant Pleural Mesothelioma
4.2. Pathological Features of Malignant Pleural Mesothelioma
4.3. Radiological Characteristics of Malignant Pleural Mesothelioma
4.4. PET-CT Metabolic Features of Malignant Pleural Mesothelioma
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, E.K.; Takahashi, K.; Hoshuyama, T.; Cheng, T.J.; Delgermaa, V.; Le, G.V.; Sorahan, T. Global magnitude of reported and unreported mesothelioma. Environ. Health Perspect. 2011, 119, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.; Aerts, J.; Popat, S.; Fennell, D. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Migliore, M. Malignant pleural mesothelioma: Between pragmatism and hope. Ann. Transl. Med. 2020, 8, 896. [Google Scholar] [CrossRef]
- Robinson, B.M. Malignant pleural mesothelioma: An epidemiological perspective. Ann. Cardiothorac. Surg. 2012, 1, 491–496. [Google Scholar] [PubMed]
- Hui, D.; Hannon, B.L.; Zimmermann, C.; Bruera, E. Improving patient and caregiver outcomes in oncology: Team-based, timely, and targeted palliative care. CA Cancer J. Clin. 2018, 68, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Brims, F.; Meniawy, T.; Duffus, I.; de Fonseka, D.; Segal, A.; Creaney, J.; Maskell, N.; Lake, R.; de Klerk, N.; Nowak, A. A Novel Clinical Prediction Model for Prognosis in Malignant Pleural Mesothelioma Using Decision Tree Analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 573–582. [Google Scholar] [CrossRef]
- Harris, E.; Kao, S.; McCaughan, B.; Nakano, T.; Kondo, N.; Hyland, R.; Nowak, A.; de Klerk, N.; Brims, F. Prediction modelling using routine clinical parameters to stratify survival in Malignant Pleural Mesothelioma patients undergoing cytoreductive surgery. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 288–293. [Google Scholar] [CrossRef]
- Travis, W.; Brambilla, E.; Burke, A.; Marx, A.; Nicholson, A. Introduction to the 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 1240–1242. [Google Scholar] [CrossRef]
- Blum, Y.; Meiller, C.; Quetel, L.; Elarouci, N.; Ayadi, M.; Tashtanbaeva, D.; Armenoult, L.; Montagne, F.; Tranchant, R.; Renier, A.; et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat. Commun. 2019, 10, 1333. [Google Scholar] [CrossRef]
- Husain, A.N.; Colby, T.V.; Ordonez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Clive, A.; Kahan, B.; Hooper, C.; Bhatnagar, R.; Morley, A.; Zahan-Evans, N.; Bintcliffe, O.; Boshuizen, R.; Fysh, E.; Tobin, C.; et al. Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax 2014, 69, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.; Mauri, F.; Ramakrishnan, R.; Wahab, L.; Lloyd, T.; Sharma, R. Inflammation-based prognostic indices in malignant pleural mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2012, 7, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.; De Rienzo, A.; Gill, R.; Oster, M.; Dao, M.; Dao, N.; Levy, R.; Vermilya, K.; Gustafson, C.; Ovsak, G.; et al. Mesothelioma Risk Score: A New Prognostic Pretreatment, Clinical-Molecular Algorithm for Malignant Pleural Mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, T.; Anraku, M.; Morodomi, Y.; Takenaka, T.; Okamoto, T.; Takenoyama, M.; Ichinose, Y.; Maehara, Y.; Cho, B.C.J.; Feld, R.; et al. Clinical role of a new prognostic score using platelet-to-lymphocyte ratio in patients with malignant pleural mesothelioma undergoing extrapleural pneumonectomy. J. Thorac. Dis. 2015, 7, 1898–1906. [Google Scholar]
- Yamagishi, T.; Fujimoto, N.; Nishi, H.; Miyamoto, Y.; Hara, N.; Asano, M.; Fuchimoto, Y.; Wada, S.; Kitamura, K.; Ozaki, S.; et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer 2015, 90, 111–117. [Google Scholar] [CrossRef]
- Vogl, M.; Rosenmayr, A.; Bohanes, T.; Scheed, A.; Brndiar, M.; Stubenberger, E.; Ghanim, B. Biomarkers for Malignant Pleural Mesothelioma-A Novel View on Inflammation. Cancers 2021, 13, 658. [Google Scholar] [CrossRef]
- Cannon, N.A.; Meyer, J.; Iyengar, P.; Ahn, C.; Westover, K.D.; Choy, H.; Timmerman, R. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J. Thorac. Oncol. 2015, 10, 280–285. [Google Scholar] [CrossRef]
- Ye, K.; Xiao, M.; Li, Z.; He, K.; Wang, J.; Zhu, L.; Xiong, W.; Zhong, Z.; Tang, Y. Preoperative systemic inflammation response index is an independent prognostic marker for BCG immunotherapy in patients with non-muscle-invasive bladder cancer. Cancer Med. 2022. [Google Scholar] [CrossRef]
- Ma, A.; Wang, G.; Du, Y.; Guo, W.; Guo, J.; Hu, Y.; Bai, D.; Huang, H.; Zhuang, L.; Chen, J.; et al. The clinical relevance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in chronic obstructive pulmonary disease with lung cancer. Front. Oncol. 2022, 12, 902955. [Google Scholar] [CrossRef]
- Larose, F.; Quigley, N.; Lacasse, Y.; Martel, S.; Lang-Lazdunski, L. Malignant pleural mesothelioma: Comparison of surgery-based trimodality therapy to medical therapy at two tertiary academic institutions. Lung cancer 2021, 156, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, M.; Gill, R.; Mazzola, E.; Freyaldenhoven, S.; Swanson, S.; Jaklitsch, M.; Sugarbaker, D.; Bueno, R. Pleurectomy Decortication in the Treatment of Malignant Pleural Mesothelioma: Encouraging Results and Novel Prognostic Implications Based on Experience in 355 Consecutive Patients. Ann. Surg. 2020, 275, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Nardone, V.; Giannicola, R.; Bianco, G.; Giannarelli, D.; Tini, P.; Pastina, P.; Falzea, A.C.; Macheda, S.; Caraglia, M.; Luce, A.; et al. Inflammatory Markers and Procalcitonin Predict the Outcome of Metastatic Non-Small-Cell-Lung-Cancer Patients Receiving PD-1/PD-L1 Immune-Checkpoint Blockade. Front. Oncol. 2021, 11, 684110. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Fushiya, N.; Koike, K.; Nishino, H.; Tajiri, H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br. J. Cancer 2012, 107, 988–993. [Google Scholar] [CrossRef]
- Bugada, D.; Allegri, M.; Lavand’homme, P.; De Kock, M.; Fanelli, G. Inflammation-based scores: A new method for patient-targeted strategies and improved perioperative outcome in cancer patients. Biomed. Res. Int. 2014, 2014, 142425. [Google Scholar] [CrossRef]
- Hannisdal, E.; Engan, T. Blood analyses and survival in symptom- and survey-detected lung cancer patients. J. Intern. Med. 1991, 229, 337–341. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, Y.; Zhou, Y.; Rong, W.; Wang, Y.; Ling, X.; Zhang, L.; Li, J.; Tomita, Y.; Watanabe, S.; et al. A nomogram for predicting hyperprogressive disease after immune checkpoint inhibitor treatment in lung cancer. Transl. Lung Cancer Res. 2022, 11, 607–616. [Google Scholar] [CrossRef]
- Kornum, J.B.; Farkas, D.K.; Svaerke, C.; Severinsen, M.T.; Thomsen, R.W.; Sorensen, H.T. Cancer Risk and Prognosis after a Hospital Contact for an Elevated Erythrocyte Sedimentation Rate. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 225–232. [Google Scholar] [CrossRef]
- Kantor, E.D.; Udumyan, R.; Signorello, L.B.; Giovannucci, E.L.; Montgomery, S.; Fall, K. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut 2016, 65, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Bille, A.; Krug, L.M.; Woo, K.M.; Rusch, V.W.; Zauderer, M.G. Contemporary Analysis of Prognostic Factors in Patients with Unresectable Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2016, 11, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.; Sahmoud, T.; Therasse, P.; van Meerbeeck, J.; Postmus, P.E.; Giaccone, G. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J. Clin. Oncol. 1998, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.E.; Green, M.R.; Chahinian, A.P.; Corson, J.M.; Suzuki, Y.; Vogelzang, N.J. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group, B. Chest 1998, 113, 723–731. [Google Scholar] [CrossRef]
- Kircheva, D.Y.; Husain, A.N.; Watson, S.; Kindler, H.L.; Durkin, A.; Vigneswaran, W.T. Specimen weight and volume: Important predictors of survival in malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2016, 49, 1642–1647. [Google Scholar] [CrossRef]
- Yin, W.; Zheng, G.; Su, S.; Liang, Y. The Value of COX-2, NF-κB, and Blood Routine Indexes in the Prognosis of Malignant Peritoneal Mesothelioma. Oncol. Res. Treat. 2019, 42, 334–341. [Google Scholar] [CrossRef]
- Toomer, K.H.; Chen, Z. Autoimmunity as a double agent in tumor killing and cancer promotion. Front. Immunol. 2014, 5, 116. [Google Scholar] [CrossRef]
- Desage, A.L.; Karpathiou, G.; Peoc’h, M.; Froudarakis, M.E. The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review. Cancers 2021, 13, 3205. [Google Scholar] [CrossRef]
- Napoli, F.; Listi, A.; Zambelli, V.; Witel, G.; Bironzo, P.; Papotti, M.; Volante, M.; Scagliotti, G.; Righi, L. Pathological Characterization of Tumor Immune Microenvironment (TIME) in Malignant Pleural Mesothelioma. Cancers 2021, 13, 2564. [Google Scholar] [CrossRef]
- Rabinowich, H.; Cohen, R.; Bruderman, I.; Steiner, Z.; Klajman, A. Functional analysis of mononuclear cells infiltrating into tumors: Lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 1987, 47, 173–177. [Google Scholar]
- Hegmans, J.; Hemmes, A.; Hammad, H.; Boon, L.; Hoogsteden, H.; Lambrecht, B. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur. Respir. J. 2006, 27, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Marcq, E.; Siozopoulou, V.; De Waele, J.; van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.; Smits, E.L.J.; et al. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 2017, 6, e1261241. [Google Scholar] [CrossRef] [PubMed]
- Fogar, P.; Sperti, C.; Basso, D.; Sanzari, M.C.; Greco, E.; Davoli, C.; Navaglia, F.; Zambon, C.F.; Pasquali, C.; Venza, E.; et al. Decreased total lymphocyte counts in pancreatic cancer: An index of adverse outcome. Pancreas 2006, 32, 22–28. [Google Scholar] [CrossRef]
- Terabe, M.; Berzofsky, J.A. NKT cells in immunoregulation of tumor immunity: A new immunoregulatory axis. Trends Immunol. 2007, 28, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Qiu, S.J.; Fan, J.; Zhou, J.; Wang, X.Y.; Xiao, Y.S.; Xu, Y.; Li, Y.W.; Tang, Z.Y. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007, 25, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Korets, L.V.; Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005, 7, 411–423. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Al-Shibli, K.; Donnem, T.; Sirera, R.; Al-Saad, S.; Andersen, S.; Stenvold, H.; Camps, C.; Busund, L.T. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: Emphasis on non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 824–833. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Yamada, N.; Oizumi, S.; Kikuchi, E.; Shinagawa, N.; Konishi-Sakakibara, J.; Ishimine, A.; Aoe, K.; Gemba, K.; Kishimoto, T.; Torigoe, T.; et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol. Immunother. 2010, 59, 1543–1549. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, X.; Ruan, G.T.; Zhang, K.P.; Tang, M.; Zhang, Q.; Song, M.M.; Zhang, X.W.; Ge, Y.Z.; Yang, M.; et al. One-Year Mortality in Patients with Cancer Cachexia: Association with Albumin and Total Protein. Cancer Manag. Res. 2021, 13, 6775–6783. [Google Scholar] [CrossRef]
- Cong, M.; Song, C.; Xu, H.; Song, C.; Wang, C.; Fu, Z.; Ba, Y.; Wu, J.; Xie, C.; Chen, G.; et al. The patient-generated subjective global assessment is a promising screening tool for cancer cachexia. BMJ Support Palliat. Care 2022, 12, e39–e46. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Yang, Y.; Wang, Z.; Wang, X.J.; Tong, Z.H.; Shi, H.Z. Malignant pleural mesothelioma: Diagnostic value of medical thoracoscopy and long-term prognostic analysis. BMC Pulm. Med. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Bruno, R.; Fontanini, G. The pathological and molecular diagnosis of malignant pleural mesothelioma: A literature review. J. Thorac. Dis. 2018, 10, S276–S284. [Google Scholar] [CrossRef]
- Rozitis, E.; Johnson, B.; Cheng, Y.Y.; Lee, K. The Use of Immunohistochemistry, Fluorescence in situ Hybridization, and Emerging Epigenetic Markers in the Diagnosis of Malignant Pleural Mesothelioma (MPM): A Review. Front. Oncol. 2020, 10, 1742. [Google Scholar] [CrossRef]
- Ordonez, N.G. Value of PAX8, PAX2, claudin-4, and h-caldesmon immunostaining in distinguishing peritoneal epithelioid mesotheliomas from serous carcinomas. Mod. Pathol. 2013, 26, 553–562. [Google Scholar] [CrossRef]
- Nasu, M.; Emi, M.; Pastorino, S.; Tanji, M.; Powers, A.; Luk, H.; Baumann, F.; Zhang, Y.A.; Gazdar, A.; Kanodia, S.; et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J. Thorac. Oncol. 2015, 10, 565–576. [Google Scholar] [CrossRef]
- Churg, A.; Sheffield, B.S.; Galateau-Salle, F. New Markers for Separating Benign from Malignant Mesothelial Proliferations: Are We There Yet? Arch. Pathol. Lab. Med. 2016, 140, 318–321. [Google Scholar] [CrossRef]
- Woolhouse, I.; Bishop, L.; Darlison, L.; De Fonseka, D.; Edey, A.; Edwards, J.; Faivre-Finn, C.; Fennell, D.A.; Holmes, S.; Kerr, K.M.; et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018, 73, i1–i30. [Google Scholar] [CrossRef]
- Hallifax, R.J.; Haris, M.; Corcoran, J.P.; Leyakathalikhan, S.; Brown, E.; Srikantharaja, D.; Manuel, A.; Gleeson, F.V.; Munavvar, M.; Rahman, N.M. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015, 70, 192–193. [Google Scholar] [CrossRef]
- Seely, J.M.; Nguyen, E.T.; Churg, A.M.; Muller, N.L. Malignant pleural mesothelioma: Computed tomography and correlation with histology. Eur. J. Radiol. 2009, 70, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Cabon, E.; Berbis, J.; Laroumagne, S.; Guinde, J.; Elharrar, X.; Dutau, H.; Astoul, P. Diagnostic Value of Computed Tomography Imaging Features in Malignant Pleural Mesothelioma. Respiration 2020, 99, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Benard, F.; Sterman, D.; Smith, R.J.; Kaiser, L.R.; Albelda, S.M.; Alavi, A. Metabolic imaging of malignant pleural mesothelioma with fluorodeoxyglucose positron emission tomography. Chest 1998, 114, 713–722. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, H.; Ma, J.; Lu, P. The Role of 18F-FDG PET/CT Integrated Imaging in Distinguishing Malignant from Benign Pleural Effusion. PLoS ONE 2016, 11, e0161764. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tamura, K.; Sakata, I.; Ishida, J.; Ozeki, Y.; Tamura, A.; Uematsu, K.; Sakai, H.; Goya, T.; Kanazawa, M.; et al. Clinical implications of 18F-fluorodeoxyglucose positron emission tomography/computed tomography at delayed phase for diagnosis and prognosis of malignant pleural mesothelioma. Oncol. Rep. 2012, 27, 333–338. [Google Scholar] [CrossRef]
- Robinson, B.W.; Musk, A.W.; Lake, R.A. Malignant mesothelioma. Lancet 2005, 366, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Choi, J.Y.; Im, Y.; Yoo, H.; Jhun, B.W.; Jeong, B.H.; Park, H.Y.; Lee, K.; Kim, H.; Kwon, O.J.; et al. Prognostic value of SUVmax on 18F-fluorodeoxyglucose PET/CT scan in patients with malignant pleural mesothelioma. PLoS ONE 2020, 15, e0229299. [Google Scholar] [CrossRef] [PubMed]
- Lococo, F.; Rena, O.; Torricelli, F.; Filice, A.; Rapicetta, C.; Boldorini, R.; Paci, M.; Versari, A. 18F-fluorodeoxyglucose positron emission tomography in malignant pleural mesothelioma: Diagnostic and prognostic performance and its correlation to pathological results. Interact Cardiovasc Thorac. Surg. 2020, 30, 593–596. [Google Scholar] [CrossRef]
- Tan, C.; Barrington, S.; Rankin, S.; Landau, D.; Pilling, J.; Spicer, J.; Cane, P.; Lang-Lazdunski, L. Role of integrated 18-fluorodeoxyglucose position emission tomography-computed tomography in patients surveillance after multimodality therapy of malignant pleural mesothelioma. J. Thorac. Oncol. 2010, 5, 385–388. [Google Scholar] [CrossRef]
- Maggio, A.; Cutaia, C.; Di Dia, A.; Bresciani, S.; Miranti, A.; Poli, M.; Del Mastro, E.; Garibaldi, E.; Gabriele, P.; Stasi, M. Tomotherapy PET-guided dose escalation: A dosimetric feasibility study for patients with malignant pleural mesothelioma. Strahlenther Onkol. 2016, 192, 102–108. [Google Scholar] [CrossRef]
- Sandach, P.; Seifert, R.; Fendler, W.P.; Hautzel, H.; Herrmann, K.; Maier, S.; Plones, T.; Metzenmacher, M.; Ferdinandus, J. A Role for PET/CT in response assessment of malignant pleural mesothelioma. Semin. Nucl. Med. 2022, 52, 816–823. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Patients |
|---|---|
| Age (Median, IQR) | 65.5 (56.0, 70.0) |
| Gender | |
| Male | 71 (55.0) |
| Female | 58 (45.0) |
| Smoke history | |
| Never | 77 (59.7) |
| Current and former | 52 (40.3) |
| Asbestos exposure | |
| No | 102 (79.1) |
| Yes | 27 (20.9) |
| ECOG PS | |
| 0–1 | 107 (82.9) |
| 2–3 | 22 (17.1) |
| Diagnostic methods | |
| Cell blocks from malignant pleural effusion | 3 (2.3) |
| Percutaneous pleural needle biopsy | 19 (14.7) |
| Video-Assisted Thoracic Surgery | 12 (9.3) |
| Medical thoracoscopy | 95 (73.6) |
| Histology | |
| Epithelioid | 81 (62.8) |
| Non-epithelioid | 48 (37.2) |
| Treatment | |
| Best supportive care | 21 (16.3) |
| Chemotherapy ± anti-angiogenesis therapy | 108 (83.7) |
| ESR (mm/h) (Median, IQR) | 15.0 (8.0, 35.0) |
| Sodium(mmol/L) (Median, IQR) | 141.0 (139.0, 142.3) |
| Potassium(mmol/L) (Median, IQR) | 4.0 (3.7, 4.3) |
| Calcium(mmol/L) (Median, IQR) | 2.2 (2.1, 2.3) |
| Chloride(mmol/L) (Median, IQR) | 104.5 (102.7, 106.5) |
| WBC (109/L) (Median, IQR) | 6.5 (5.1, 8.3) |
| Neutrophil(109/L) (Median, IQR) | 4.2 (3.3, 5.8) |
| Lymphocyte(109/L) (Median, IQR) | 1.5 (1.2, 1.9) |
| Hemoglobin (g/L) (Median, IQR) | 134.0 (120.5, 143.5) |
| Platelet(109/L) (Median, IQR) | 249.0 (194.0, 321.0) |
| Total protein(g/L) (Median, IQR) | 63.9 (60.7,68.8) |
| Albumin(g/L) (Median, IQR) | 36.8 (32.7, 39.7) |
| Glucose(mmol/L) (Median, IQR) Creatinine(umol/L) (Median, IQR) | 5.0 (4.6,6.2) |
| Creatinine(umol/L) (Median, IQR) | 61.2 (52.6,72.7) |
| Total bilirubin(umol/L) (Median, IQR) | 10.6 (7.6, 14.4) |
| Characteristic | No. (%) | HR | 95%CI | p Value |
|---|---|---|---|---|
| Age (years) | ||||
| ≤65.5 | 76 (58.9) | 1 | ||
| >65.5 | 53 (41.1) | 1.263 | 0.825–1.933 | 0.282 |
| Gender | ||||
| Male | 71 (55.0) | 1 | ||
| Female | 58 (45.0) | 0.786 | 0.518–1.194 | 0.259 |
| Smoke history | ||||
| Never | 77 (59.7) | 1 | ||
| Current and former | 52 (40.3) | 1.301 | 0.858–1.975 | 0.216 |
| Asbestos exposure | ||||
| No | 102 (79.1) | 1 | ||
| Yes | 27 (20.9) | 0.979 | 0.599–1.599 | 0.931 |
| ECOG PS | ||||
| 0–1 | 107 (82.9) | 1 | ||
| 2–3 | 22 (17.1) | 1.221 | 0.735–2.027 | 0.441 |
| Diagnostic methods | ||||
| Cell blocks from malignant pleural effusion | 3 (2.3) | 1 | 0.249 | |
| Percutaneous pleural needle biopsy | 19 (14.7) | 2.540 | 0.334–19.306 | 0.368 |
| Video-Assisted Thoracic Surgery | 12 (9.3) | 0.998 | 0.119–8.340 | 0.998 |
| Medical thoracoscopy | 95 (73.6) | 1.875 | 0.259–13.582 | 0.534 |
| Histology | ||||
| Epithelioid | 81 (62.8) | 1 | ||
| Non-epithelioid | 48 (37.2) | 1.450 | 0.955–2.202 | 0.081 |
| Treatment | ||||
| Best supportive care | 21 (16.3) | 1 | ||
| Only Chemotherapy ± anti-angiogenesis therapy | 108 (83.7) | 0.478 | 0.269–0.848 | 0.012 |
| ESR (mm/h) | ||||
| ≤20.5 | 75 (58.1) | 1 | ||
| >20.5 | 54 (41.9) | 2.484 | 1.633–3.777 | <0.001 |
| Sodium (mmol/L) | ||||
| ≤139.4 | 38 (29.5) | 1 | ||
| >139.4 | 91 (70.5) | 0.588 | 0.380–0.911 | 0.017 |
| Potassium (mmol/L) | ||||
| ≤4.5 | 117 (90.7) | 1 | ||
| >4.5 | 12 (9.3) | 2.044 | 1.104–3.785 | 0.023 |
| Calcium (mmol/L) | ||||
| ≤2.1 | 33 (25.6) | 1 | ||
| >2.1 | 96 (74.4) | 0.676 | 0.429–1.065 | 0.091 |
| Chloride (mmol/L) | ||||
| ≤102.9 | 35 (27.1) | 1 | ||
| >102.9 | 94 (72.9) | 0.656 | 0.421–1.023 | 0.063 |
| WBC (109/L) | ||||
| ≤7.8 | 86 (66.7) | 1 | ||
| >7.8 | 43 (33.3) | 1.310 | 0.852–2.014 | 0.219 |
| Neutrophil (109/L) | ||||
| ≤4.0 | 57 (44.2) | 1 | ||
| >4.0 | 72 (55.8) | 1.349 | 0.883–2.062 | 0.166 |
| Lymphocyte (109/L) | ||||
| ≤1.1 | 26 (20.2) | 1 | ||
| >1.1 | 103 (79.8) | 0.531 | 0.329–0.857 | 0.009 |
| Hemoglobin (g/L) | ||||
| ≤124.5 | 40 (31.0) | 1 | ||
| >124.5 | 89 (69.0) | 0.664 | 0.430–1.025 | 0.065 |
| Platelet (109/L) | ||||
| ≤289.5 | 90 (69.8) | 1 | ||
| >289.5 | 39 (30.2) | 1.807 | 1.166–2.801 | 0.008 |
| Total protein (g/L) | ||||
| ≤62.9 | 54 (41.9) | 1 | ||
| >62.9 | 75 (58.1) | 0.651 | 0.430–0.987 | 0.043 |
| Albumin (g/L) | ||||
| ≤37.5 | 75 (58.1) | 1 | ||
| >37.5 | 54 (41.9) | 0.654 | 0.421–1.018 | 0.060 |
| Glucose (mmol/L) | ||||
| ≤5.7 | 86 (66.7) | 1 | ||
| >5.7 | 43 (33.3) | 1.293 | 0.844–1.981 | 0.237 |
| Creatinine (umol/L) | ||||
| ≤65.8 | 77 (59.7) | 1 | ||
| >65.8 | 52 (40.3) | 1.290 | 0.853–1.951 | 0.228 |
| Total bilirubin (umol/L) | ||||
| ≤10.7 | 66 (51.2) | 1 | ||
| >10.7 | 63 (48.8) | 0.700 | 0.459–1.067 | 0.097 |
| Characteristic | HR | 95%CI | p Value |
|---|---|---|---|
| Age (years) | |||
| ≤65.5 | 1 | ||
| >65.5 | 1.824 | 1.159–2.872 | 0.009 |
| Gender | |||
| Male | 1 | ||
| Female | 0.719 | 0.465–1.113 | 0.139 |
| Histology | |||
| Epithelioid | 1 | ||
| Non-epithelioid | 1.007 | 0.624–1.624 | 0.977 |
| Treatment | |||
| Best supportive care | 1 | ||
| Chemotherapy ± anti-angiogenesis therapy | 0.674 | 0.355–1.281 | 0.229 |
| ESR (mm/h) | |||
| ≤20.5 | 1 | ||
| >20.5 | 2.197 | 1.318–3.664 | 0.003 |
| Serum sodium (mmol/L) | |||
| ≤139.4 | 1 | ||
| >139.4 | 0.774 | 0.477–1.257 | 0.300 |
| Serum potassium (mmol/L) | |||
| ≤4.5 | 1 | ||
| >4.5 | 1.397 | 0.710–2.747 | 0.333 |
| Lymphocyte (109/L) | |||
| ≤1.1 | 1 | ||
| >1.1 | 0.436 | 0.258–0.737 | 0.002 |
| Platelet (109/L) | |||
| ≤289.5 | 1 | ||
| >289.5 | 1.802 | 1.084–2.997 | 0.023 |
| Total protein (g/L) | |||
| ≤62.9 | 1 | ||
| >62.9 | 0.625 | 0.394–0.990 | 0.045 |
| IHC Markers | Tested Patients | Patients n (%) | Epithelioid n (%) | Non-Epithelioid n (%) |
|---|---|---|---|---|
| Calretinin | ||||
| Negative | 112 | 9 (8.0) | 3 (4.2) | 6 (14.6) |
| Positive | 103 (92.0) | 68 (95.8) | 35 (85.4) | |
| TTF-1 | ||||
| Negative | 107 | 105 (98.1) | 65 (98.5) | 40 (97.6) |
| Positive | 2 (1.9) | 1 (1.5) | 1 (2.4) | |
| CK5/6 | ||||
| Negative | 98 | 20 (22.5) | 4 (7.1) | 16 (48.5) |
| Positive | 69 (77.5) | 52 (92.9) | 17 (51.5) | |
| D2-40 | ||||
| Negative | 88 | 12 (13.6) | 4 (7.4) | 8 (23.5) |
| Positive | 76 (86.4) | 50 (92.6) | 26 (76.5) | |
| CEA | ||||
| Negative | 84 | 73 (86.9) | 48 (92.3) | 25 (78.1) |
| Positive | 11 (13.1) | 4 (7.7) | 7 (21.9) | |
| Desmin | ||||
| Negative | 81 | 67 (82.7) | 41 (77.4) | 26 (92.9) |
| Positive | 14 (17.3) | 12 (22.6) | 2 (7.1) | |
| MC | ||||
| Negative | 81 | 15 (18.5) | 4 (8.9) | 11 (30.6) |
| Positive | 66 (81.5) | 41 (91.1) | 25 (69.4) | |
| Napsin-A | ||||
| Negative | 72 | 71 (98.6) | 49 (100.0) | 22 (95.7) |
| Positive | 1 (1.4) | 0 (0) | 1 (4.3) | |
| WT-1 | ||||
| Negative | 68 | 8 (11.8) | 3 (6.1) | 5 (26.3) |
| Positive | 60 (88.2) | 46 (93.9) | 14 (73.7) | |
| CK7 | ||||
| Negative | 63 | 13 (20.6) | 9 (27.3) | 4 (13.3) |
| Positive | 50 (79.4) | 24 (72.7) | 26 (86.7) | |
| EMA | ||||
| Negative | 60 | 10 (16.7) | 3 (8.1) | 7 (30.4) |
| Positive | 50 (83.3) | 34 (91.9) | 16 (69.6) | |
| CK | ||||
| Negative | 58 | 1 (1.7) | 1 (3.1) | 0 (0) |
| Positive | 57 (98.3) | 31 (96.9) | 26 (100.0) | |
| Glut1 | ||||
| Negative | 51 | 7 (13.7) | 7 (20.0) | 0 (0) |
| Positive | 44 (86.3) | 28 (80.0) | 16 (100.0) | |
| Vimentin | ||||
| Negative | 41 | 12 (29.3) | 7 (33.3) | 5 (25.0) |
| Positive | 29 (70.7) | 14 (66.7) | 15 (75.0) | |
| P53 | ||||
| Negative | 19 | 5 (26.3) | 3 (25.0) | 2 (28.6) |
| Positive | 14 (73.7) | 9 (75.0) | 5 (71.4) |
| Radiological Characteristics | Patients (n = 109) (%) | Epithelioid (n = 70) (%) | Non-Epithelioid (n = 39) (%) | p Value |
|---|---|---|---|---|
| Tumor location | 0.376 | |||
| Left | 63 (57.8) | 43 (61.4) | 20 (51.3) | |
| Right | 39 (35.8) | 24 (34.3) | 15 (38.5) | |
| Both | 7 (6.4) | 3 (24.3) | 4 (10.2) | |
| Pleural invasion of interlobar fissure | 0.185 | |||
| No | 73 (67.0) | 50 (71.4) | 23 (59.0) | |
| Yes | 36 (33.0) | 20 (28.6) | 16 (41.0) | |
| Mediastinal pleural invasion | 0.127 | |||
| No | 94 (86.2) | 63 (90.0) | 31 (79.5) | |
| Yes | 15 (13.8) | 7 (10.0) | 8 (20.5) | |
| Mediastinal lymph node invasion | 0.463 | |||
| No | 80 (73.4) | 53 (75.7) | 27 (69.2) | |
| Yes | 29 (26.6) | 17 (24.3) | 12 (30.8) | |
| Thoracostenosis | 0.108 | |||
| No | 92 (84.4) | 62 (88.6) | 30 (76.9) | |
| Yes | 17 (15.6) | 8 (11.4) | 9 (23.1) | |
| Volume of pleural effusion | 0.652 | |||
| None | 2 (1.8) | 2 (2.9) | 0 (0) | |
| Small | 8 (7.4) | 6 (8.6) | 2 (5.1) | |
| Moderate | 70 (64.2) | 44 (62.9) | 26 (66.7) | |
| Large | 29 (26.6) | 18 (25.6) | 11 (28.2) | |
| Pleural thickening | 0.342 | |||
| None | 3 (2.8) | 1 (1.4) | 2 (5.1) | |
| Regular | 20 (18.3) | 13 (18.6) | 7 (17.9) | |
| Annular | 9 (8.3) | 6 (8.6) | 3 (7.7) | |
| Lumpy | 14 (12.8) | 12 (17.1) | 2 (5.1) | |
| Nodular | 63 (57.8) | 38 (60.3) | 25 (64.2) | |
| Pleural calcification | 0.054 | |||
| No | 87 (79.8) | 52 (74.3) | 35 (89.7) | |
| Yes | 22 (20.2) | 18 (25.7) | 4 (10.3) | |
| Parenchymal lung metastasis | 0.234 | |||
| No | 95 (87.2) | 63 (90.0) | 32 (82.1) | |
| Yes | 14 (12.8) | 7 (10.0) | 7 (17.9) | |
| Rib metastasis | 0.672 | |||
| No | 107 (98.2) | 69 (98.6) | 38 (97.4) | |
| Yes | 2 (1.8) | 1 (1.4) | 1 (2.6) | |
| Chest wall involvement | 0.344 | |||
| No | 94 (86.2) | 62 (88.6) | 32 (82.1) | |
| Yes | 15 (13.8) | 8 (11.4) | 7 (17.9) | |
| Tumor necrosis | - | |||
| No | 109 (100.0) | 70 (100.0) | 39 (100.0) | |
| Yes | 0 (0) | 0 (0) | 0 (0) | |
| Pericardial effusion | - | |||
| No | 109 (100.0) | 70 (100.0) | 39 (100.0) | |
| Yes | 0 (0) | 0 (0) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, R.; Gu, Y.; LiZhu, Y.; Liu, X.; Zhang, S. Clinical, Laboratory, Histological, Radiological, and Metabolic Features and Prognosis of Malignant Pleural Mesothelioma. Medicina 2022, 58, 1874. https://doi.org/10.3390/medicina58121874
Zhang Y, Li R, Gu Y, LiZhu Y, Liu X, Zhang S. Clinical, Laboratory, Histological, Radiological, and Metabolic Features and Prognosis of Malignant Pleural Mesothelioma. Medicina. 2022; 58(12):1874. https://doi.org/10.3390/medicina58121874
Chicago/Turabian StyleZhang, Yuan, Ran Li, Yumei Gu, Yuerong LiZhu, Xiaofang Liu, and Shu Zhang. 2022. "Clinical, Laboratory, Histological, Radiological, and Metabolic Features and Prognosis of Malignant Pleural Mesothelioma" Medicina 58, no. 12: 1874. https://doi.org/10.3390/medicina58121874
APA StyleZhang, Y., Li, R., Gu, Y., LiZhu, Y., Liu, X., & Zhang, S. (2022). Clinical, Laboratory, Histological, Radiological, and Metabolic Features and Prognosis of Malignant Pleural Mesothelioma. Medicina, 58(12), 1874. https://doi.org/10.3390/medicina58121874




