Routine Detachment of the Anterior and Septal Tricuspid Leaflets Simplifies VSD Closure and Improves the Outcomes
Abstract
1. Introduction
2. Methods
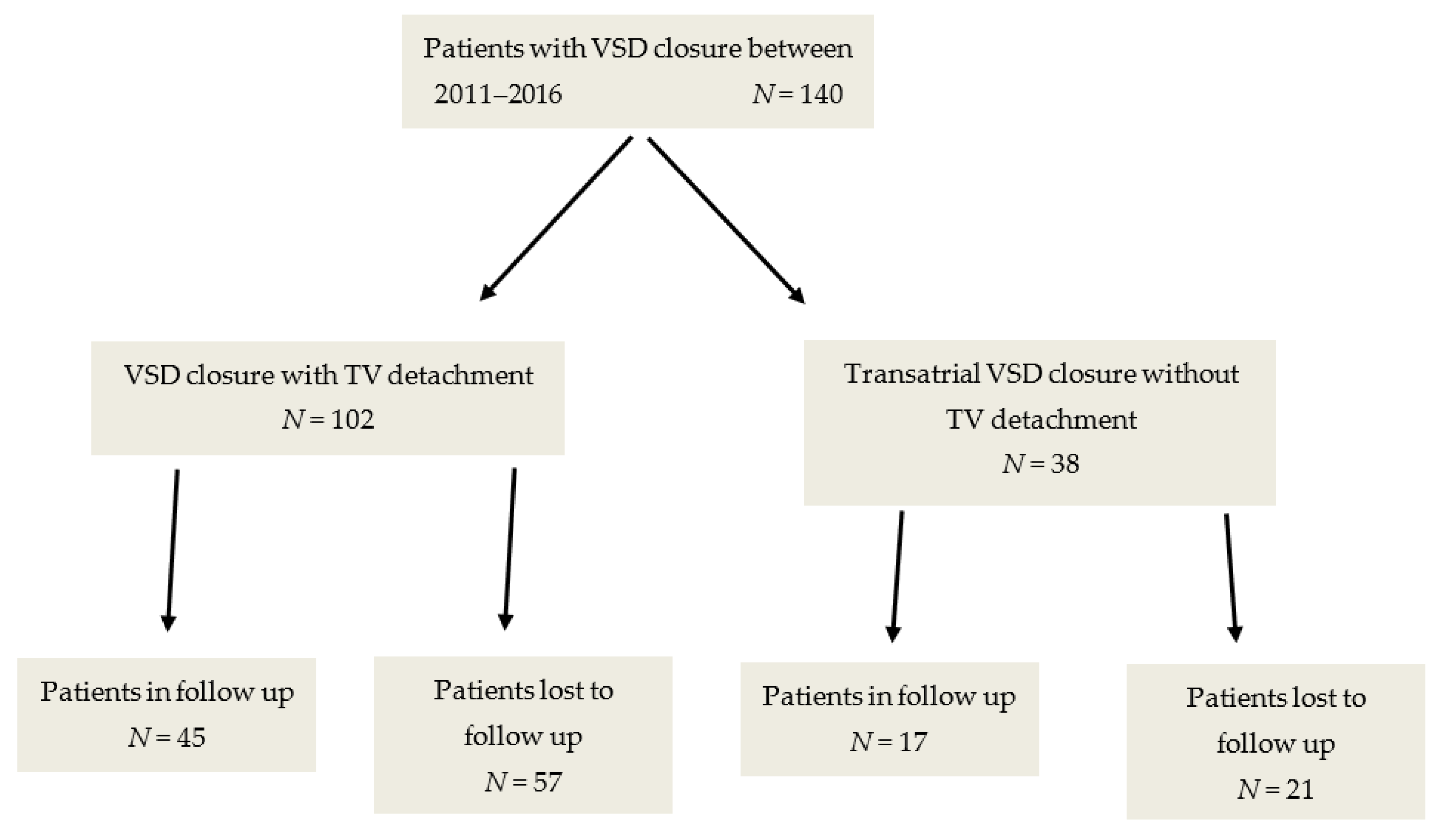
2.1. Patient Selection Process
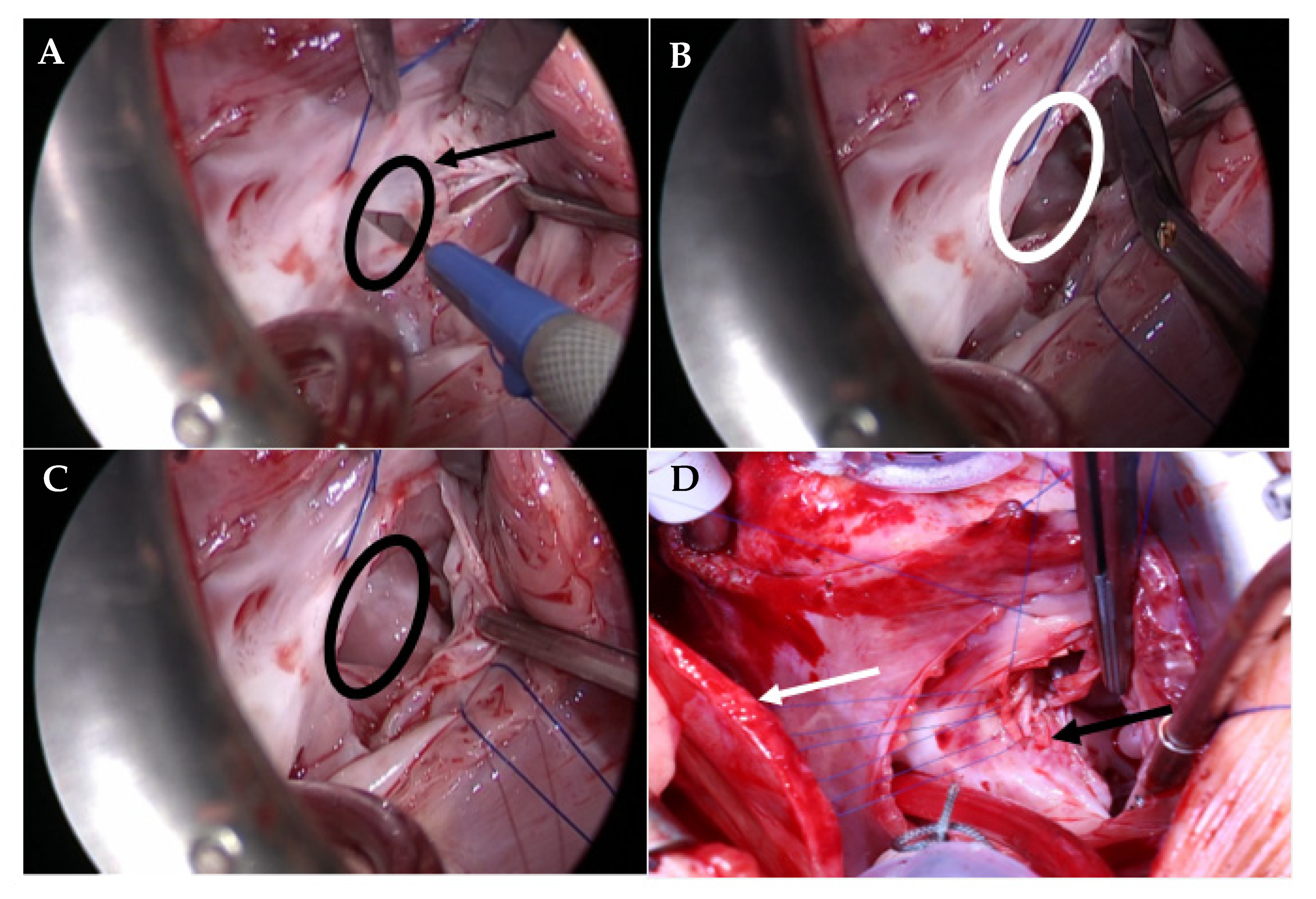
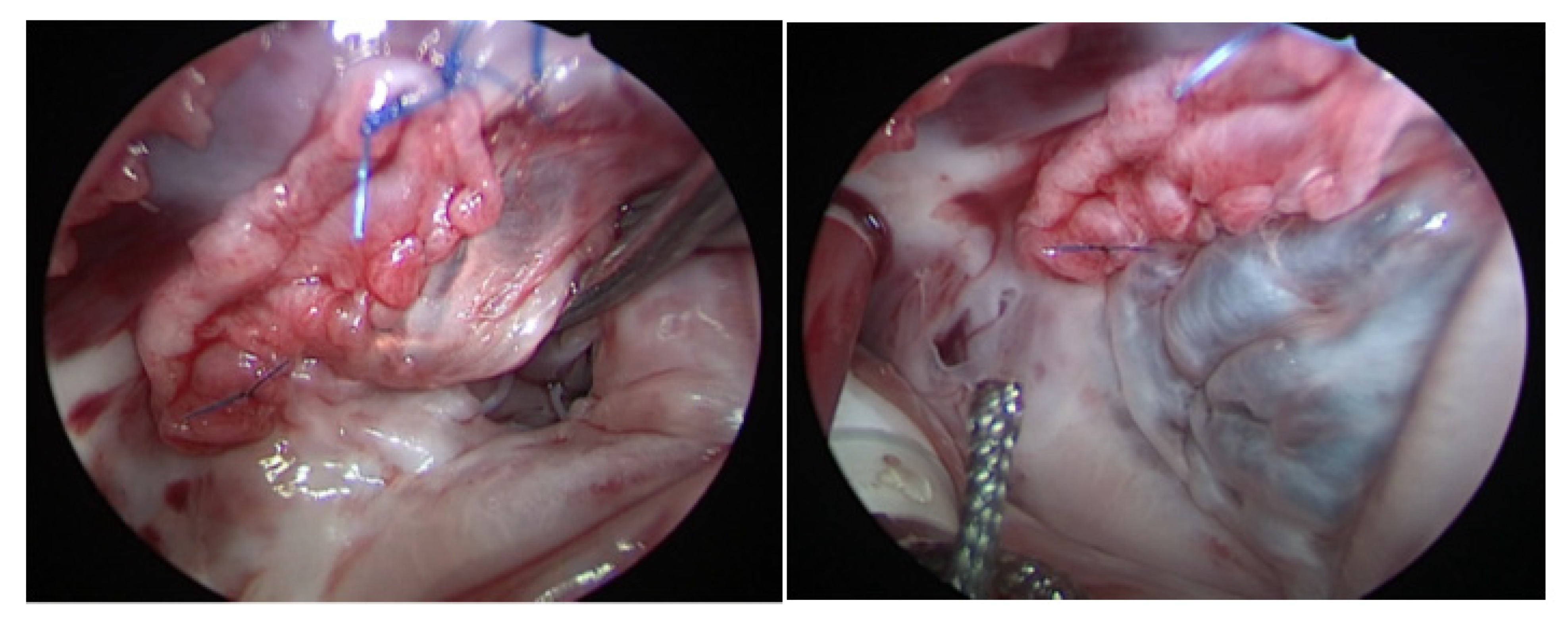
2.2. Operative Technique
2.3. Echocardiographic Studies
2.4. Statistical Analysis
2.5. Written Consent
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindinger, A.; Schwedler, G.; Hense, H.W. Prevalence of congenital heart defects in newborns in Germany: Results of the first registration year of the PAN Study (July 2006 to June 2007). Klin. Padiatr. 2010, 222, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Schwedler, G.; Lindinger, A.; Lange, P.E.; Sax, U.; Olchvary, J.; Peters, B.; Bauer, U.; Hense, H.W. Frequency and spectrum of congenital heart defects among live births in Germany. Clin. Res. Cardiol. 2011, 100, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Roguin, N.; Du, Z.D.; Barak, M.; Nasser, N.; Hershkowitz, S.; Milgram, E. High prevalence of muscular ventricular septal defect in neonates. J. Am. Coll. Cardiol. 1995, 26, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Burke, R.P.; Quintessenza, J.A.; Mavroudis, C. Congenital Heart Surgery Nomenclature and Database Project: Ventricular septal defect. Ann. Thorac. Surg. 2000, 69, S25–S35. [Google Scholar] [CrossRef]
- Warden, H.E.; Cohen, M.; Read, R.C.; Lillehei, C.W. Controlled cross circulation for open intracardiac surgery: Physiologic studies and results of creation and closure of ventricular septal defects. J. Thorac. Surg. 1954, 28, 331–341. [Google Scholar] [CrossRef]
- Scully, B.B.; Morales, D.L.; Zafar, F.; McKenzie, E.D.; Fraser, C.D., Jr.; Heinle, J.S. Current expectations for surgical repair of isolated ventricular septal defects. Ann. Thorac. Surg. 2010, 89, 544–549. [Google Scholar] [CrossRef]
- Bibevski, S.; Ruzmetov, M.; Mendoza, L.; Decker, J.; Vandale, B.; Jayakumar, K.A.; Chan, K.C.; Bove, E.; Scholl, F.G. The Destiny of Postoperative Residual Ventricular Septal Defects After Surgical Repair in Infants and Children. World J. Pediatric Congenit. Heart Surg. 2020, 11, 438–443. [Google Scholar] [CrossRef]
- Fraser, C.D., 3rd; Zhou, X.; Palepu, S.; Lui, C.; Suarez-Pierre, A.; Crawford, T.C.; Magruder, J.T.; Jacobs, M.L.; Cameron, D.E.; Hibino, N.; et al. Tricuspid valve detachment in ventricular septal defect closure does not impact valve function. Ann. Thorac. Surg. 2018, 106, 145–150. [Google Scholar] [CrossRef]
- Lucchese, G.; Rossetti, L.; Faggian, G.; Luciani, G.B. Long-Term Follow-Up Study of Temporary Tricuspid Valve Detachment as Approach to VSD Repair without Consequent Tricuspid Dysfunction. Tex. Heart Inst. J. 2016, 43, 392–396. [Google Scholar] [CrossRef]
- Hudspeth, A.S.; Cordell, A.R.; Meredith, J.H.; Johnston, F.R. An improved transatrial approach to the closure of ventricular septal defects. J. Thorac. Cardiovasc. Surg. 1962, 43, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, S.; Kwak, J.G.; Kwon, H.W.; Kwak, Y.; Min, J.; Kim, W.H.; Lee, J.R. Tricuspid valve detachment for ventricular septal defect closure in infants <5 kg: Should we be hesitant? Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 60, 544–551. [Google Scholar]
- Bang, J.H.; Park, C.S.; Park, J.J.; Yun, T.J.; Baek, J.S.; Yu, J.J.; Kim, Y.H.; Ko, J.K. Detachment of the tricuspid valve for ventricular septal defect closure in infants younger than 3 months. J. Thorac. Cardiovasc. Surg. 2016, 152, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Bilen, Ç.; Akkaya, G.; Tuncer, O.N.; Atay, Y. Assessment of Tricuspid Valve Detachment Efficiency for Ventricular Septal Defect Closure: A Retrospective Comparative Study. Acta Cardiol. Sin. 2020, 36, 360–366. [Google Scholar]
- Schittek, J.; Sachweh, J.S.; Arndt, F.; Grafmann, M.; Hüners, I.; Kozlik-Feldmann, R.; Biermann, D. Outcomes of Tricuspid Valve Detachment for Isolated Ventricular Septal Defect Closure. Thorac. Cardiovasc. Surg. 2021, 69, e48–e52. [Google Scholar] [CrossRef]
- Giordano, R.; Cantinotti, M.; Di Tommaso, L.; Comentale, G.; Tozzi, A.; Pilato, E.; Iannelli, G.; Palma, G. The fate of the tricuspid valve after the transatrial closure of the ventricular septal defect. Ann. Thorac. Surg. 2018, 106, 1229–1233. [Google Scholar] [CrossRef]
- Luxford, J.; Bassin, L.; D’Ambra, M. Echocardiography of the tricuspid valve. Ann. Cardiothorac. Surg. 2017, 6, 223–239. [Google Scholar] [CrossRef]
- Pourmoghadam, K.K.; Boron, A.; Ruzmetov, M.; Narasimhulu, S.S.; Kube, A.; O’Brien, M.C.; DeCampli, W.M. Septal leaflet versus chordal detachment in closure of hard-to-expose ventricular septal defects. Ann. Thorac. Surg. 2018, 106, 814–821. [Google Scholar] [CrossRef]
- Quarti, A.; Iezzi, F.; Soura, E.; Colaneri, M.; Pozzi, M. Anterior and posterior leaflets augmentation to treat tricuspid valve regurgitation. J. Card. Surg. 2015, 30, 421–423. [Google Scholar] [CrossRef]
- Rescigno, G.; Hothi, S.; Bond, C.; Uddin, M.; Bhatti, V.; Billing, J.S. CorMatrix Anterior Leaflet Augmentation of the Tricuspid Valve: Midterm Results. Heart Surg. Forum 2021, 24, E261–E266. [Google Scholar] [CrossRef]
- Holst, K.A.; Dearani, J.A.; Said, S.; Pike, R.B.; Connolly, H.M.; Cannon, B.C.; Sessions, K.L.; O’Byrne, M.M.; O’Leary, P.W. Improving results of surgery for Ebstein anomaly: Where are we after 235 cone repairs? Ann. Thorac. Surg. 2018, 105, 160–168. [Google Scholar] [CrossRef] [PubMed]
| TVD | No TVD | p-Value | |
|---|---|---|---|
| No. of patients | 102 | 38 | |
| Age (years) | 0.41 (0.31–0.85) | 0.38 (0.24–0.82) | 0.72 |
| Gender (m) | 51 (50.0%) | 21 (56.7%) | 0.5 |
| Weight (kg) | 5.95 (4.7–7.2) | 5.7 (3.85–8.65) | 0.45 |
| Cross-clamp time (min) | 70.5 (49–89) ± 74.45 | 68.5 (51–91) ± 66.65 | 0.92 |
| Cardiopulmonary bypass time (min) | 95 (66–123) | 97 (82–122) | 0.37 |
| Diagnoses | |||
| VSD | 62 | 19 | |
| Tetralogy of Fallot | 36 | 15 | |
| VSD, hypoplastic aortic arch | 1 | 1 | |
| TGA, VSD | 0 | 1 | |
| TGA, VSD, ISTA | 1 | 0 | |
| VSD, ISTA | 1 | 0 | |
| PA-VSD | 1 | 2 | |
| Preoperative TR | |||
|---|---|---|---|
| Severity of TR | TVD | No TVD | p-Value |
| 0 | 93 (91.2%) | 34 (89.5%) | 0.75 |
| 1 | 9 (8.8%) | 3 (7.9%) | 1 |
| 2 | 0 | 1 (2.6%) | 1 |
| 3 | 0 | 0 | 1 |
| Intraoperative TR | |||
| 0 | 70 (68.6%) | 33 (86.8%) | 0.03 |
| 1 | 31 (30.4%) | 3 (7.9%) | <0.01 |
| 2 | 1 (1%) | 2 (5.3%) | 0.1 |
| 3 | 0 | 0 | 1 |
| TR Prior to Discharge | |||
| 0 | 66 (64.7%) | 22 (57.9%) | 0.46 |
| 1 | 34 (33.3%) | 14 (36.8%) | 0.7 |
| 2 | 2 (1.9%) | 2 (5.3%) | 1 |
| 3 | 0 | 0 | 1 |
| Tricuspid Regurgitation during Follow-Up | |||
|---|---|---|---|
| TR Severity | TVD | No-TVD | p-Value |
| 0 | 32 (71.1%) | 10 (58.8%) | 0.36 |
| 1 | 13 (28.9%) | 6 (35.3%) | 0.62 |
| 2 | 0 | 1 (5.9%) | 1 |
| 3 | 0 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval Boburg, R.; Schlensak, C.; Hofbeck, M.; Magunia, H.; Berger, R.; Jost, W.; Mustafi, M. Routine Detachment of the Anterior and Septal Tricuspid Leaflets Simplifies VSD Closure and Improves the Outcomes. Medicina 2022, 58, 1849. https://doi.org/10.3390/medicina58121849
Sandoval Boburg R, Schlensak C, Hofbeck M, Magunia H, Berger R, Jost W, Mustafi M. Routine Detachment of the Anterior and Septal Tricuspid Leaflets Simplifies VSD Closure and Improves the Outcomes. Medicina. 2022; 58(12):1849. https://doi.org/10.3390/medicina58121849
Chicago/Turabian StyleSandoval Boburg, Rodrigo, Christian Schlensak, Michael Hofbeck, Harry Magunia, Rafal Berger, Walter Jost, and Migdat Mustafi. 2022. "Routine Detachment of the Anterior and Septal Tricuspid Leaflets Simplifies VSD Closure and Improves the Outcomes" Medicina 58, no. 12: 1849. https://doi.org/10.3390/medicina58121849
APA StyleSandoval Boburg, R., Schlensak, C., Hofbeck, M., Magunia, H., Berger, R., Jost, W., & Mustafi, M. (2022). Routine Detachment of the Anterior and Septal Tricuspid Leaflets Simplifies VSD Closure and Improves the Outcomes. Medicina, 58(12), 1849. https://doi.org/10.3390/medicina58121849






