Impact of Depression on Cognitive Function and Disease Severity in Idiopathic Cervical Dystonia Patients: One-Center Data in Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
- Patients had reached 18 years of age or older with a diagnosis of idiopathic focal or segmental dystonia with adult-onset affecting cervical muscles (patients who experienced either long-lasting involuntary contractions or periodic, intermittent spasms of the neck muscles that cause the neck to turn in different ways);
- Symptoms were experienced for at least one year (not newly diagnosed CD);
- Patients had agreed to take part in the study.
- Secondary or pseudodystonia of the neck muscles;
- Patients with low cognitive skills who were not able to fill in the questionnaire, due to other possible comorbidities (dementia, alcohol use disorder, posttraumatic brain injury, metabolic disorders, Parkinson’s disease, Huntington’s disease or patients after stroke).
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.R.; Pagan, F.L. Patient considerations in the treatment of cervical dystonia: Focus on botulinum toxin type A. Patient Prefer. Adherence 2015, 9, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.T.; Burke, R.E.; Greene, P.; Fahn, S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain 1998, 121 Pt 4, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.D.; Groth, C.L.; Sillau, S.H.; Pirio Richardson, S.; Norris, S.A.; Junker, J.; Brüggemann, N.; Agarwal, P.; Barbano, R.L.; Espay, A.J.; et al. Risk of spread in adult-onset isolated focal dystonia: A prospective international cohort study. J. Neurol. Neurosurg. Psychiatry 2020, 91, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.; Bhatia, K. An international survey of patients with cervical dystonia. J. Neurol. 2015, 262, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; McGovern, E.M.; Narasimham, S.; Beck, R.; Killian, O.; O’Riordan, S.; Reilly, R.B.; Hutchinson, M. Temporal Discrimination: Mechanisms and Relevance to Adult-Onset Dystonia. Front. Neurol. 2017, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, M.; Erro, R.; Mascia, M.M.; Esposito, M.; Ercoli, T.; Ferrazzano, G.; Di Biasio, F.; Pellicciari, R.; Eleopra, R.; Bono, F.; et al. Demographic and clinical determinants of neck pain in idiopathic cervical dystonia. J. Neural. Transm. 2020, 127, 1435–1439. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Torkamani, M. The cognitive features of idiopathic and DYT1 dystonia. Mov. Disord. 2017, 32, 1348–1355. [Google Scholar] [CrossRef]
- Gündel, H.; Wolf, A.; Xidara, V.; Busch, R.; Ceballos-Baumann, A.O. Social phobia in spasmodic torticollis. J. Neurol. Neurosurg. Psychiatry 2001, 71, 499–504. [Google Scholar] [CrossRef]
- Ozel-Kizil, E.T.; Akbostanci, M.C.; Ozguven, H.D.; Atbasoglu, E.C. Secondary social anxiety in hyperkinesias. Mov. Disord. 2008, 23, 641–645. [Google Scholar] [CrossRef]
- Gündel, H.; Wolf, A.; Xidara, V.; Busch, R.; Ladwig, K.H.; Jacobi, F.; von Rad, M.; Ceballos-Baumann, A.O. High psychiatric comorbidity in spasmodic torticollis: A controlled study. J. Nerv. Ment. Dis. 2003, 191, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, G.; Berardelli, I.; Moretti, G.; Pasquini, M.; Bloise, M.; Colosimo, C.; Biondi, M.; Berardelli, A. Psychiatric disorders in adult-onset focal dystonia: A case-control study. Mov. Disord. 2010, 25, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef]
- Lencer, R.; Steinlechner, S.; Stahlberg, J.; Rehling, H.; Orth, M.; Baeumer, T.; Rumpf, H.J.; Meyer, C.; Klein, C.; Muenchau, A.; et al. Primary focal dystonia: Evidence for distinct neuropsychiatric and personality profiles. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1176–1179. [Google Scholar] [CrossRef]
- Stamelou, M.; Edwards, M.J.; Hallett, M.; Bhatia, K.P. The non-motor syndrome of primary dystonia: Clinical and pathophysiological implications. Brain 2012, 135 Pt 6, 1668–1681. [Google Scholar] [CrossRef]
- Berardelli, I.; Ferrazzano, G.; Pasquini, M.; Biondi, M.; Berardelli, A.; Fabbrini, G. Clinical course of psychiatric disorders in patients with cervical dystonia. Psychiatry Res. 2015, 229, 583–585. [Google Scholar] [CrossRef]
- Foley, J.A.; Vinke, R.S.; Limousin, P.; Cipolotti, L. Relationship of Cognitive Function to Motor Symptoms and Mood Disorders in Patients With Isolated Dystonia. Cogn. Behav. Neurol. 2017, 30, 16–22. [Google Scholar] [CrossRef]
- Bradnam, L.; Chen, C.S.; Callahan, R.; Hoppe, S.; Rosenich, E.; Loetscher, T. Visual compensation in cervical dystonia. J. Clin. Exp. Neuropsychol. 2019, 41, 769–774. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Joormann, J. Cognition and depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2010, 6, 285–312. [Google Scholar] [CrossRef]
- Burt, D.B.; Zembar, M.J.; Niederehe, G. Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychol. Bull. 1995, 117, 285–305. [Google Scholar] [CrossRef]
- Knight, M.J.; Baune, B.T. The Direct and Indirect Relationship Between Social Cognition and Psychosocial Dysfunction in Major Depressive Disorder. Front. Psychiatry 2019, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Bagnato, S.; Altavista, M.C.; Avanzino, L.; Belvisi, D.; Bologna, M.; Bono, F.; Carecchio, M.; Castagna, A.; Ceravolo, R.; et al. Diagnostic and therapeutic recommendations in adult dystonia: A joint document by the Italian Society of Neurology, the Italian Academy for the Study of Parkinson’s Disease and Movement Disorders, and the Italian Network on Botulinum Toxin. Neurol. Sci. 2022, 43, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.K.; Eisen, A.; Mak, E.; Carruthers, J.; Scott, A.; Calne, D.B. A pilot study on the use of botulinum toxin in spasmodic torticollis. Can. J. Neurol. Sci. 1985, 12, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Stell, R.; Thompson, P.D.; Marsden, C.D. Botulinum toxin in spasmodic torticollis. J. Neurol. Neurosurg. Psychiatry 1988, 51, 920–923. [Google Scholar] [CrossRef]
- Comella, C.L.; Buchman, A.S.; Tanner, C.M.; Brown-Toms, N.C.; Goetz, C.G. Botulinum toxin injection for spasmodic torticollis: Increased magnitude of benefit with electromyographic assistance. Neurology 1992, 42, 878–882. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Lemeshow, S.; Hosmer, D.W.; Klar, J.; Lwanga, S.K.; World Health Organization. Adequacy of Sample Size in Health Studies; Wiley: Chichester, UK, 1990. [Google Scholar]
- Defazio, G.; Jankovic, J.; Giel, J.L.; Papapetropoulos, S. Descriptive epidemiology of cervical dystonia. Tremor. Other. Hyperkinet Mov. 2013, 3. [Google Scholar] [CrossRef]
- Soland, V.L.; Bhatia, K.P.; Marsden, C.D. Sex prevalence of focal dystonias. J. Neurol. Neurosurg. Psychiatry 1996, 60, 204–205. [Google Scholar] [CrossRef]
- Lungu, C.; Karp, B.I.; Alter, K.; Zolbrod, R.; Hallett, M. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: Outcome at 10 years or more. Mov. Disord. 2011, 26, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Marsden, C.D. Depression in torticollis: A controlled study. Psychol. Med. 1988, 18, 925–933. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Marsden, C.D. Body concept, disability, and depression in patients with spasmodic torticollis. Behav. Neurol. 1990, 3, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Marsden, C.D. A Longitudinal Follow-up Study of Depression, Disability, and Body Concept in Torticollis. Behav. Neurol. 1990, 3, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Skogseid, I.M.; Malt, U.F.; Røislien, J.; Kerty, E. Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur. J. Neurol 2007, 14, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.; Frank, J.E.; Pereira, C.; Tomaz, C. Sustained attention in cranial dystonia patients treated with botulinum toxin. Acta Neurol. Scand. 2007, 116, 196–200. [Google Scholar] [CrossRef]
- Silberman, E.K.; Weingartner, H.; Post, R.M. Thinking disorder in depression. Arch. Gen. Psychiatry 1983, 40, 775–780. [Google Scholar] [CrossRef]
- Franco-Robles, E.; López, M.G. Agavins Increase Neurotrophic Factors and Decrease Oxidative Stress in the Brains of High-Fat Diet-Induced Obese Mice. Molecules 2016, 21, 998. [Google Scholar] [CrossRef]
- Axelson, D.A.; Doraiswamy, P.M.; McDonald, W.M.; Boyko, O.B.; Tupler, L.A.; Patterson, L.J.; Nemeroff, C.B.; Ellinwood, E.H., Jr.; Krishnan, K.R. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993, 47, 163–173. [Google Scholar] [CrossRef]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef]
- Defazio, G.; Berardelli, A.; Hallett, M. Do primary adult-onset focal dystonias share aetiological factors? Brain 2007, 130 Pt 5, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Corp, D.T.; Joutsa, J.; Darby, R.R.; Delnooz, C.C.S.; van de Warrenburg, B.P.C.; Cooke, D.; Prudente, C.N.; Ren, J.; Reich, M.M.; Batla, A.; et al. Network localization of cervical dystonia based on causal brain lesions. Brain 2019, 142, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Vitanova, K.S.; Stringer, K.M.; Benitez, D.P.; Brenton, J.; Cummings, D.M. Dementia associated with disorders of the basal ganglia. J. Neurosci. Res. 2019, 97, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
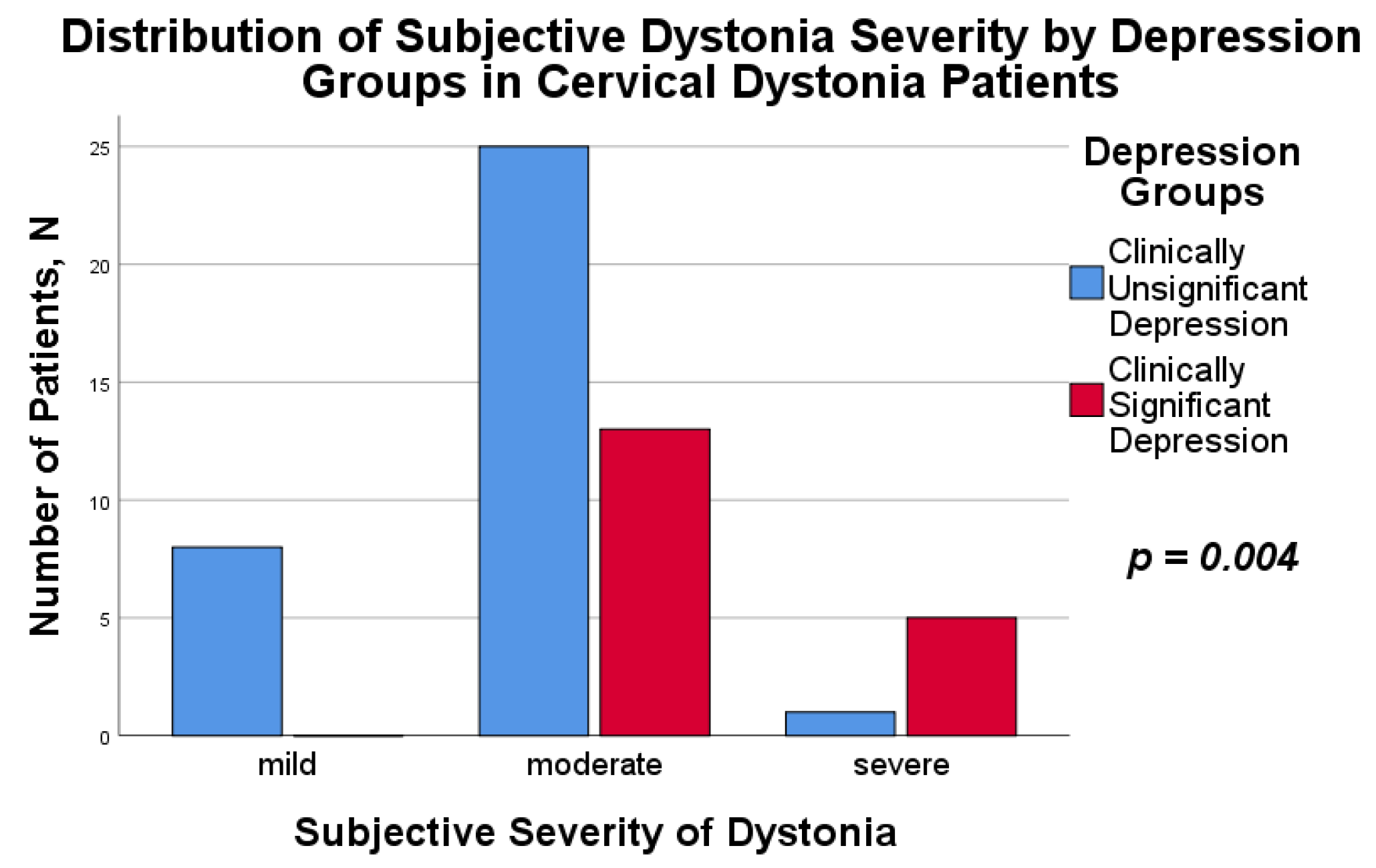
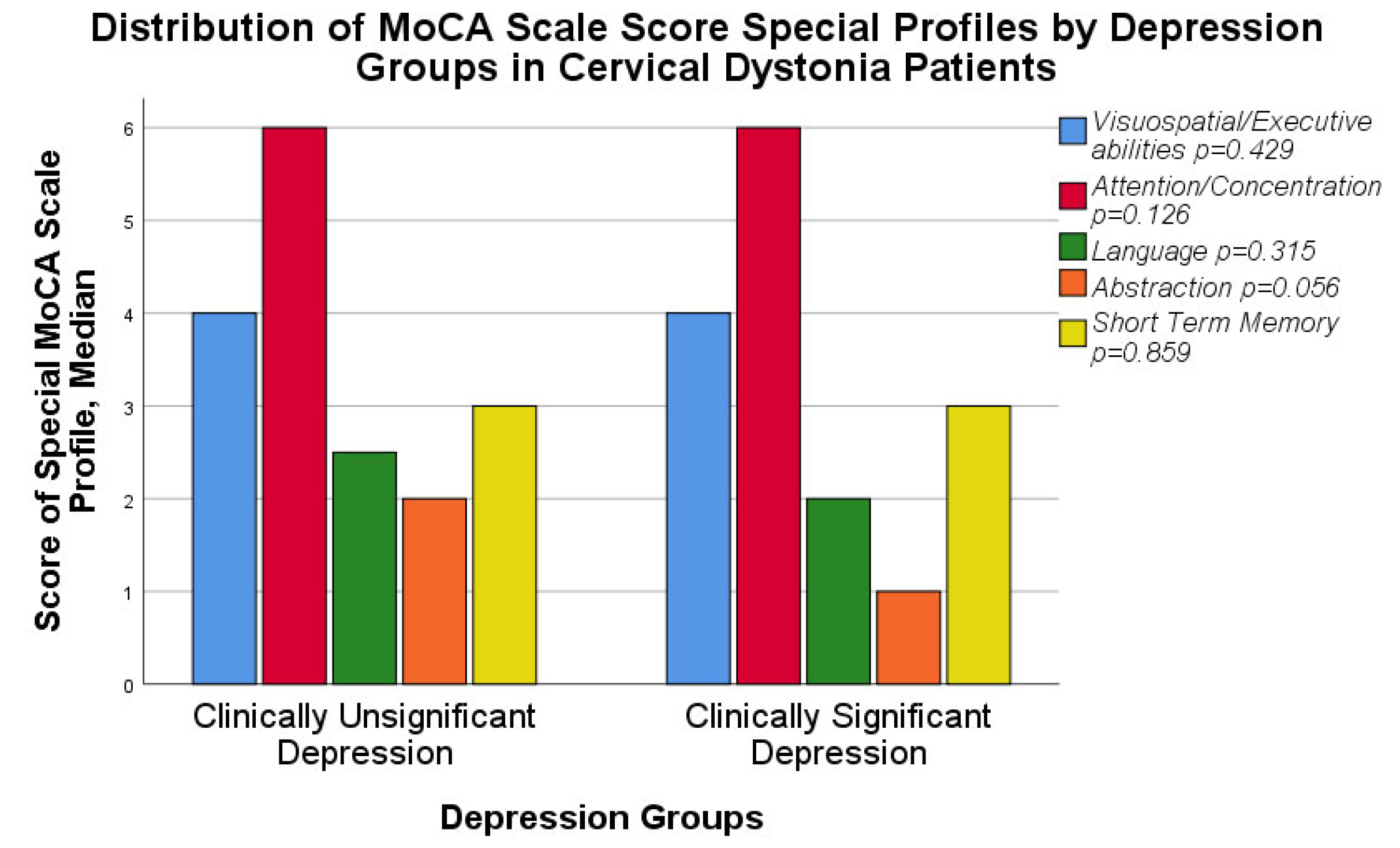
| Dystonia Severity and Cognitive Assessment in Cervical Dystonia (CD) Patients Depending on Depressive Signs | ||||||
|---|---|---|---|---|---|---|
| Nonsignificant Depression | Significant Depression | Test | U Value | Z Test | p Value | |
| Number of Patients, N (%) | 34 (65.4%) | 18 (34.6%) | - | - | - | - |
| Severity of dystonia | ||||||
| TSUI scale score | 7.5 (6.0; 9.0) | 8.0 (6.8; 8.0) | Mann–Whitney U Test | 261.500 | −0.864 | 0.387 |
| TWSTRS scale score-physical finding part | 12.0 (10.0; 14.3) | 13.0 (10.0; 15.0) | Mann–Whitney U Test | 246.000 | −1.164 | 0.244 |
| Self-assessment of the disease | ||||||
| Mild dystonia | 8 (23.5%) | 0 (0%) | Fisher’s Exact Test | - | - | 0.004 |
| Moderately-severe dystonia | 25 (73.5%) | 13 (72.2%) | ||||
| Severe dystonia | 1 (3.0%) | 5 (27.8%) | ||||
| MoCA scale score, median (Q25; Q75) | 26.0 (23.8; 28.0) | 25.0 (23.0; 26.3) | Mann–Whitney U Test | 224.500 | −1.578 | 0.115 |
| Special MoCA scale profiles, median (Q25; Q75) | ||||||
| Visuospatial/Executive abilities | 4.0 (3.0; 5.0) | 4.0 (3.0; 5.0) | Mann–Whitney U Test | 267.000 | −0.791 | 0.429 |
| Attention/concentration | 6.0 (5.8; 6.0) | 6.0 (5.0; 6.0) | 242.000 | −1.528 | 0.126 | |
| Language | 2.5 (2.0; 3.0) | 2.0 (1.8; 3.0) | 258.000 | −1.005 | 0.315 | |
| Abstraction | 2.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | 221.000 | −1.911 | 0.056 | |
| Short-term memory | 3.0 (2.0; 4.0) | 3.0 (2.8; 4.0) | 297.000 | −0.178 | 0.859 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meļņikova, V.; Valante, R.; Valtiņa-Briģe, S.; Logina, I. Impact of Depression on Cognitive Function and Disease Severity in Idiopathic Cervical Dystonia Patients: One-Center Data in Cross-Sectional Study. Medicina 2022, 58, 1793. https://doi.org/10.3390/medicina58121793
Meļņikova V, Valante R, Valtiņa-Briģe S, Logina I. Impact of Depression on Cognitive Function and Disease Severity in Idiopathic Cervical Dystonia Patients: One-Center Data in Cross-Sectional Study. Medicina. 2022; 58(12):1793. https://doi.org/10.3390/medicina58121793
Chicago/Turabian StyleMeļņikova, Vlada, Ramona Valante, Solveiga Valtiņa-Briģe, and Ināra Logina. 2022. "Impact of Depression on Cognitive Function and Disease Severity in Idiopathic Cervical Dystonia Patients: One-Center Data in Cross-Sectional Study" Medicina 58, no. 12: 1793. https://doi.org/10.3390/medicina58121793
APA StyleMeļņikova, V., Valante, R., Valtiņa-Briģe, S., & Logina, I. (2022). Impact of Depression on Cognitive Function and Disease Severity in Idiopathic Cervical Dystonia Patients: One-Center Data in Cross-Sectional Study. Medicina, 58(12), 1793. https://doi.org/10.3390/medicina58121793








