Utilizing Graphical Analysis of Chest Radiographs for Primary Screening of Osteoporosis
Abstract
1. Introduction
2. Materials and Methods
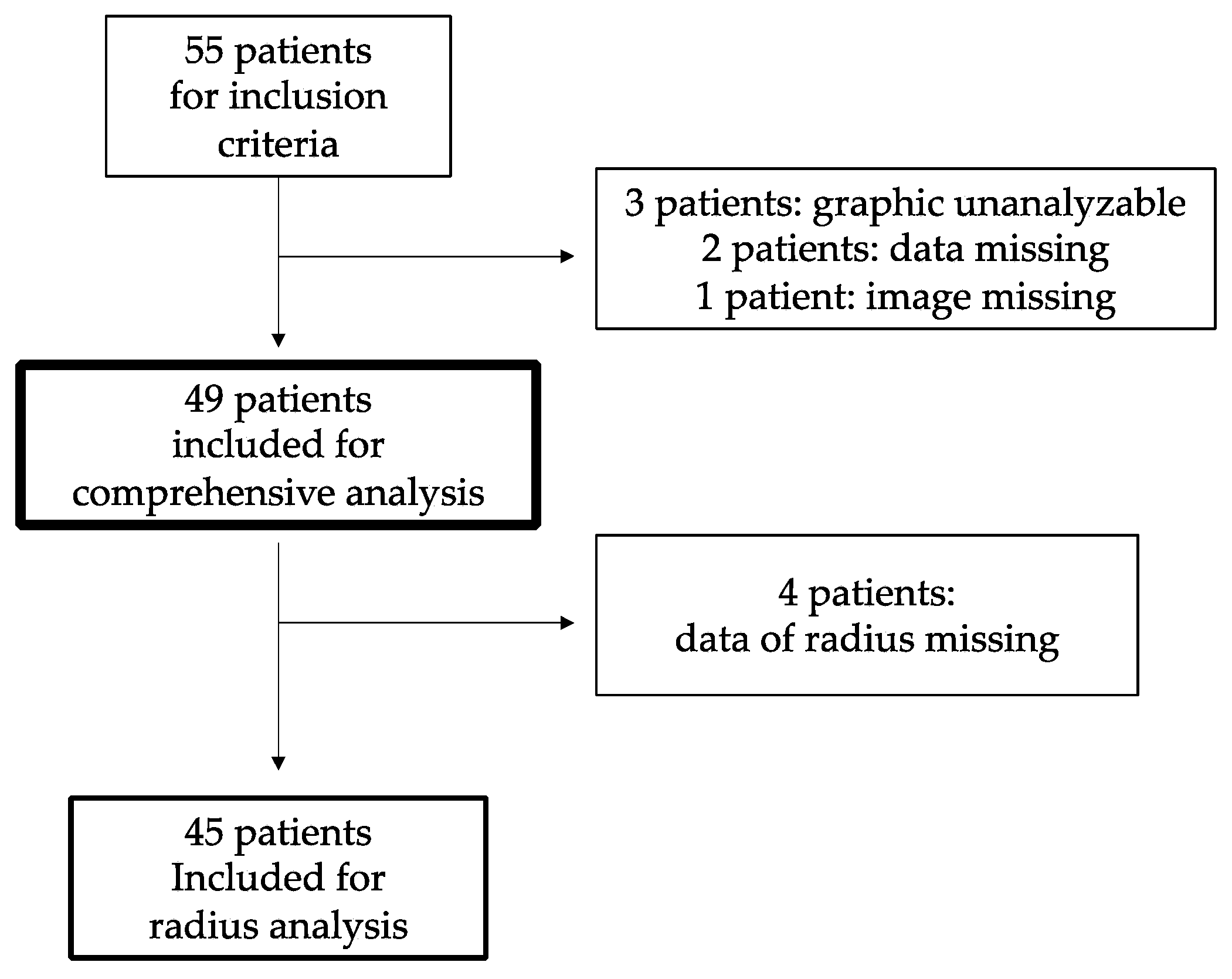
2.1. Subjects, Study Design
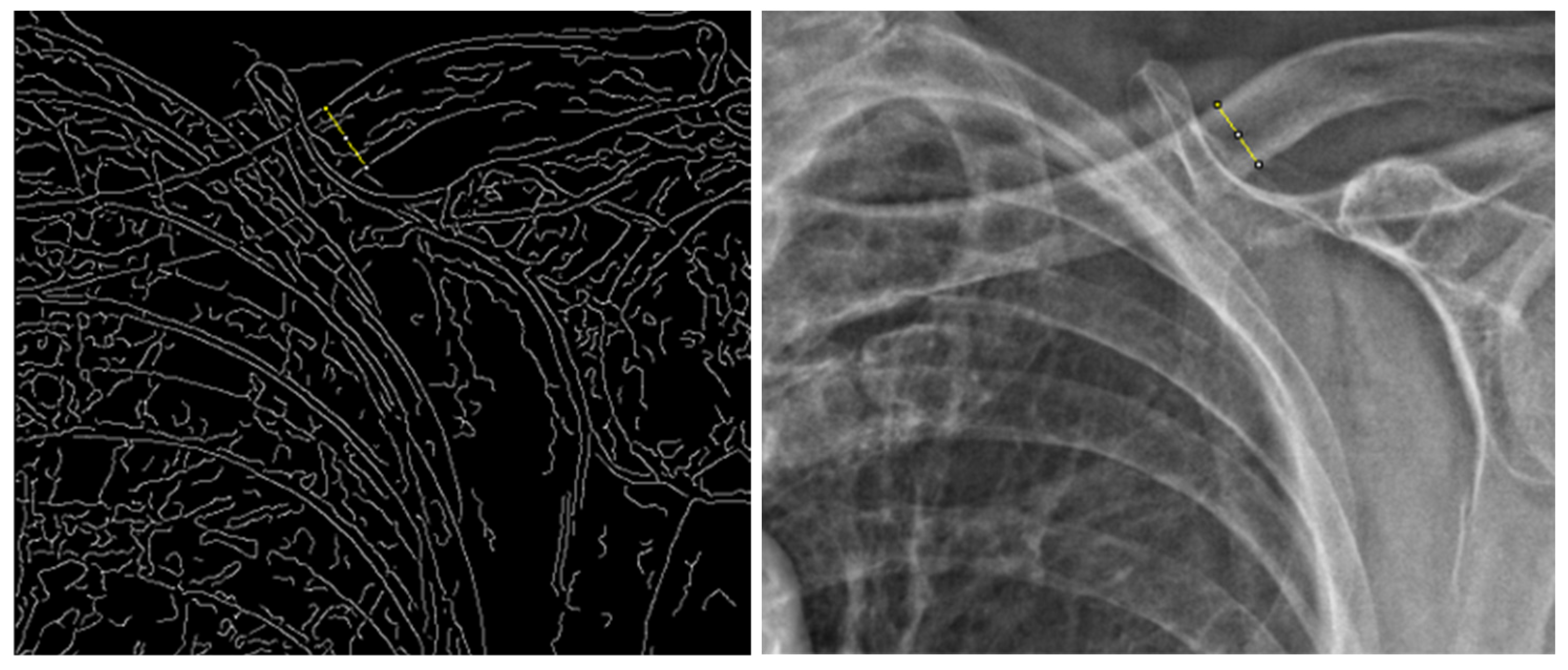
2.2. Graphical Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, J.M.; Russell, L.; Khan, S.N. Osteoporosis. Clin. Orthop. Relat. Res. 2000, 372, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D. Management of severe osteoporosis. Expert Opin. Pharmacother. 2016, 17, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshida, H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos. Int. 2010, 21, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, K.; Nakamura, T.; Suzuki, T.; Gorai, I.; Fujinawa, O.; Ohta, H.; Shiraki, M.; Yoh, K.; Fujiwara, S.; Endo, N.; et al. Validation of the Japanese Osteoporosis Quality of Life Questionnaire. J. Bone Miner. Metab. 2010, 28, 1. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, M.; Kuroda, T.; Shiraki, Y.; Aoki, C.; Sasaki, K.; Tanaka, S. Effects of bone mineral density of the lumbar spine and prevalent vertebral fractures on the risk of immobility. Osteoporos. Int. 2010, 21, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Horii, M.; Ikoma, K.; Goto, T.; Okubo, N.; Kuriyama, N.; Takahashi, K. Hip fractures among the elderly in Kyoto, Japan: A 10-year study. Arch. Osteoporos. 2021, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, J.; Fujimori, K.; Ikehara, S.; Kamiya, K.; Nakatoh, S.; Okimoto, N.; Ogawa, S.; Ishii, S.; Iki, M.; Working Group of Japan Osteoporosis Foundation. Estimates of hip fracture incidence in Japan using the National Health Insurance Claim Database in 2012–2015. Osteoporos. Int. 2019, 30, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.W.; Strader, C.; Fitzpatrick, L.A.; Anthony, M.S.; O’Malley, C.D. Estimating prevalence of osteoporosis: Examples from industrialized countries. Arch. Osteoporos. 2014, 9, 182. [Google Scholar] [CrossRef]
- King, A.B.; Fiorentino, D.M. Medicare payment cuts for osteoporosis testing reduced use despite tests’ benefit in reducing fractures. Health Aff. 2011, 30, 2362–2370. [Google Scholar] [CrossRef]
- Adams, A.L.; Fischer, H.; Kopperdahl, D.L.; Lee, D.C.; Black, D.M.; Bouxsein, M.L.; Fatemi, S.; Khosla, S.; Orwoll, E.S.; Siris, E.S.; et al. Osteoporosis and Hip Fracture Risk From Routine Computed Tomography Scans: The Fracture, Osteoporosis, and CT Utilization Study (FOCUS). J. Bone Miner. Res. 2018, 33, 1291–1301. [Google Scholar] [CrossRef]
- Kumar, D.A.; Anburajan, M. The role of hip and chest radiographs in osteoporotic evaluation among south Indian women population: A comparative scenario with DXA. J. Endocrinol. Investig. 2014, 37, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yamamoto, K.; Matsuzawa, H.; Yamamoto, Y.; Ishida, T. Osteoporosis Screening Using Chest Radiographs. Med. Imaging Inf. Sci. 2018, 35, 30–34. [Google Scholar] [CrossRef]
- Soen, S.; Fukunaga, M.; Sugimoto, T.; Sone, T.; Fujiwara, S.; Endo, N.; Gorai, I.; Shiraki, M.; Hagino, H.; Hosoi, T.; et al. Diagnostic criteria for primary osteoporosis: Year 2012 revision. J. Bone Miner. Metab. 2013, 31, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Melton, L.J., 3rd; Chrischilles, E.A.; Cooper, C.; Lane, A.W.; Riggs, B.L. Perspective. How many women have osteoporosis? J. Bone Miner. Res. 1992, 7, 1005–1010. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Atkinson, E.J.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Bone density and fracture risk in men. J. Bone Miner. Res. 1998, 13, 1915–1923. [Google Scholar] [CrossRef]
- Orimo, H.; Nakamura, T.; Hosoi, T.; Iki, M.; Uenishi, K.; Endo, N.; Ohta, H.; Shiraki, M.; Sugimoto, T.; Suzuki, T.; et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis—Executive summary. Arch. Osteoporos. 2012, 7, 3–20. [Google Scholar] [CrossRef]
- Piroska, M.; Tarnoki, D.L.; Szabo, H.; Jokkel, Z.; Meszaros, S.; Horvath, C.; Tarnoki, A.D. Strong Genetic Effects on Bone Mineral Density in Multiple Locations with Two Different Techniques: Results from a Cross-Sectional Twin Study. Medicina 2021, 57, 248. [Google Scholar] [CrossRef]
- Rothmann, M.J.; Möller, S.; Holmberg, T.; Højberg, M.; Gram, J.; Bech, M.; Brixen, K.; Hermann, A.P.; Glüer, C.C.; Barkmann, R.; et al. Non-participation in systematic screening for osteoporosis-the ROSE trial. Osteoporos. Int. 2017, 28, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [CrossRef] [PubMed]
- Helmrich, G. Screening for osteoporosis. Clin. Obstet. Gynecol. 2013, 56, 659–666. [Google Scholar] [CrossRef]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Saeki, S.; Szabo, H.; Tomizawa, R.; Tarnoki, A.D.; Tarnoki, D.L.; Watanabe, Y.; Osaka Twin Research, G.; Honda, C. Lobular Difference in Heritability of Brain Atrophy among Elderly Japanese: A Twin Study. Medicina 2022, 58, 1250. [Google Scholar] [CrossRef] [PubMed]
- Flanagin, A.; Frey, T.; Christiansen, S.L.; AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021, 326, 621–627. [Google Scholar] [CrossRef]
- Saeki, S.; Kusumoto, M. Reporting Race and Ethnicity in Population Health and Clinical Research from Japan. J. Epidemiol. 2022, JE20220244. [Google Scholar] [CrossRef]
- Saeki, S. Impact of the “Amendments to the Act of the Protection of Personal Information” to Global Health Research Conducted in Japanese Medical Facilities. J. Epidemiol. 2022, 32, 438. [Google Scholar] [CrossRef]
| n = 49 (Male 15) | Average | SD | Min | Max |
|---|---|---|---|---|
| Age | 65.3 | 13.4 | 25.0 | 85.0 |
| BMI | 26.3 | 5.8 | 17.0 | 39.4 |
| Height (cm) | 161.3 | 10.4 | 144.0 | 188.0 |
| Weight (kg) | 68.3 | 14.7 | 40.0 | 105.0 |
| CLR | 0.25 | 0.067 | 0.15 | 0.41 |
| n | Average | SD | Min | Max | ||
|---|---|---|---|---|---|---|
| L2-4 | (g/cm2) | 49 | 1.04 | 0.24 | 0.50 | 1.60 |
| Z-score | −0.19 | 1.79 | −3.70 | 3.60 | ||
| Femoral neck | (g/cm2) | 0.80 | 0.17 | 0.49 | 1.29 | |
| Z-score | −0.37 | 1.23 | −3.10 | 2.50 | ||
| Total femur | (g/cm2) | 0.84 | 0.21 | 0.43 | 1.44 | |
| Z-score | −0.32 | 1.44 | −4.10 | 3.20 | ||
| Radius | (g/cm2) | 45 | 0.76 | 0.16 | 0.49 | 1.08 |
| Z-score | −0.48 | 1.25 | −4.30 | 1.90 |
| Coefficients | ||||
|---|---|---|---|---|
| Estimate | Std. Error | T Value | Pr (>|T|) | |
| (Intercept) | 3.128 | 2.30 | 1.361 | 0.18 |
| Age (year) | 0.00466 | 0.0027 | 1.725 | 0.09 |
| BMI (kg/m2) | −0.06062 | 0.0435 | −1.392 | 0.17 |
| CLR | 0.824 | 0.511 | 1.614 | 0.11 |
| Height (cm) | −0.01963 | 0.00143 | −1.374 | 0.18 |
| Weight (kg) | 0.03125 | 0.0172 | 1.815 | 0.07 |
| Sex (male = 1) | 0.159 | 0.0841 | 1.886 | 0.06 |
| Coefficients | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | T Value | Pr (>|T|) | ||
| (Intercept) | 1.795 | 1.51 | 1.190 | 0.24 | |
| Age (year) | 0.000306 | 0.00177 | 0.173 | 0.86 | |
| BMI (kg/m2) | −0.0113 | 0.00286 | −0.397 | 0.69 | |
| CLR | 0.933 | 0.335 | 2.785 | 0.008 | ** |
| Height (cm) | −0.0101 | 0.00941 | −1.070 | 0.29 | |
| Weight (kg) | 0.00930 | 0.0113 | 0.824 | 0.41 | |
| Sex (male = 1) | 0.126 | 0.0552 | 2.277 | 0.03 | ** |
| Coefficients | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | T Value | Pr (>|T|) | ||
| (Intercept) | 1.416 | 1.93 | 0.734 | 0.47 | |
| Age (year) | 0.00112 | 0.00168 | −2.794 | 0.00803 | ** |
| BMI (kg/m2) | −0.00679 | 0.0366 | −0.186 | 0.85 | |
| CLR | 0.905 | 0.429 | 2.109 | 0.04 | * |
| Height (cm) | −0.00882 | 0.0120 | −0.733 | 0.4676 | |
| Weight (kg) | 0.01000 | 0.0144 | 0.692 | 0.4925 | |
| Sex (male = 1) | 0.153 | 0.0706 | 2.170 | 0.0357 | * |
| Coefficients | ||||
|---|---|---|---|---|
| Estimate | Std. Error | T Value | Pr (>|T|) | |
| (Intercept) | 3.128 | 2.30 | 1.361 | 0.18 |
| Age (year) | 0.00466 | 0.0027 | 1.725 | 0.09 |
| BMI (kg/m2) | −0.06062 | 0.0435 | −1.392 | 0.17 |
| CLR | 0.824 | 0.511 | 1.614 | 0.11 |
| Height (cm) | −0.01963 | 0.00143 | −1.374 | 0.18 |
| Weight (kg) | 0.03125 | 0.0172 | 1.815 | 0.07 |
| Sex (male = 1) | 0.159 | 0.0841 | 1.886 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, S.; Yamamoto, K.; Tomizawa, R.; Meszaros, S.; Horvath, C.; Zoldi, L.; Szabo, H.; Tarnoki, A.D.; Tarnoki, D.L.; Ishida, T.; et al. Utilizing Graphical Analysis of Chest Radiographs for Primary Screening of Osteoporosis. Medicina 2022, 58, 1765. https://doi.org/10.3390/medicina58121765
Saeki S, Yamamoto K, Tomizawa R, Meszaros S, Horvath C, Zoldi L, Szabo H, Tarnoki AD, Tarnoki DL, Ishida T, et al. Utilizing Graphical Analysis of Chest Radiographs for Primary Screening of Osteoporosis. Medicina. 2022; 58(12):1765. https://doi.org/10.3390/medicina58121765
Chicago/Turabian StyleSaeki, Soichiro, Kouichi Yamamoto, Rie Tomizawa, Szilvia Meszaros, Csaba Horvath, Luca Zoldi, Helga Szabo, Adam Domonkos Tarnoki, David Laszlo Tarnoki, Takayuki Ishida, and et al. 2022. "Utilizing Graphical Analysis of Chest Radiographs for Primary Screening of Osteoporosis" Medicina 58, no. 12: 1765. https://doi.org/10.3390/medicina58121765
APA StyleSaeki, S., Yamamoto, K., Tomizawa, R., Meszaros, S., Horvath, C., Zoldi, L., Szabo, H., Tarnoki, A. D., Tarnoki, D. L., Ishida, T., & Honda, C. (2022). Utilizing Graphical Analysis of Chest Radiographs for Primary Screening of Osteoporosis. Medicina, 58(12), 1765. https://doi.org/10.3390/medicina58121765








